Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Servet Altay and Version 2 by Camila Xu.
Hypertension (HT) is a worldwide public health issue and an essential risk factor for cardiovascular and cerebrovascular diseases. Obstructive sleep apnea (OSA) is a condition characterized by recurrent episodes of apnea and hypopnea as a consequence of partial or complete obstruction of the upper airways due to anatomic and/or functional disturbances.
- obstructive sleep apnea
- hypertension
1. Introduction
In general, patients with an office systolic blood pressure (SBP) ≥ 140 mm Hg and a diastolic blood pressure (DBP) ≥ 90 mmHg are deemed to have hypertension (HT). The overall prevalence of HT in adults is reportedly around 30–45% [1]. Obstructive sleep apnea (OSA) is a condition with recurrent apnea and hypopnea, frequent arousal, and hypoxemia, which can lead to serious cardiovascular consequences such as HT, heart failure, arrhythmia, and atherosclerosis [2]. As an important cause of morbidity and mortality, HT accounted for the deaths of approximately 10 million people in 2015 and over 200 million disability-adjusted life years [3]. Blood pressure normally drops during sleep. A nocturnal decrease of more than 10% in the mean blood pressure level throughout the day is defined as the “dipping pattern”. The absence of this decrease indicates a nondipping pattern. One of the important causes for the nondipping pattern is OSA [4][5][4,5]. Here, the sympathetic nervous system is activated due to the obstructed airway in patients with OSA, resulting in the disruption of the natural dipping pattern and causing an increase in blood pressure [5]. Traditionally recognized risk factors for cardiovascular diseases (CVDs), such as obesity, insulin resistance, diabetes mellitus, and hyperlipidemia, are common in OSA patients, and the most common CVD in patients with OSA is HT. The coexistence of OSA and chronic obstructive pulmonary disease (COPD) is called overlap syndrome [6], and individuals with overlap syndrome have a significantly increased risk of HT compared with those with COPD alone [7].
2. Epidemiology of OSA and Hypertension
HT and OSA often coexist. Pensukan et al. found a significant relationship between OSA and elevated blood pressure (odds ratio (OR): 2.38; 95% confidence interval (CI): 1.68–3.39), and HT (OR: 2.55; 95% CI: 1.57–4.15) after adjusting for demographic characteristics [8][9]. OSA has been reported among 30–50% of hypertensive patients. This rate can increase to 80% among cases with drug-resistant HT [9][10][11][10,11,12]. It has also been reported that masked HT is 2.7 times more common in OSA patients [12][13][13,14]. There is a bidirectional and causal relationship between HT and OSA. Several studies have revealed a clear dose–response relationship with OSA severity and HT. The meta-analyses on the relationship between OSA and HT are summarized in Table 1 [14][15][16][17][15,16,17,18].Table 1.
Recent meta-analyses regarding the association of OSA with the risk of HT.
| Author (Reference) | Year | Number of Studies | Total Sample Size | OSA (OR (95% CI)) for HT |
|---|---|---|---|---|
| Meng [14][15] | 2016 | 6 | 20,367 | 1.41 (1.29–1.89) |
| Hou [15][16] | 2018 | 26 | 51,623 | 1.80 (1.54–2.06) |
| Han [16][17] | 2020 | 10 | 13,274 | 1.80 (1.36–2.38) |
| Yuan [17][18] | 2021 | 8 | 3484 | 6.44 (5.38–7.71) |
Abbreviations: CI, confidence interval; HT, hypertension; OR, odds ratio; OSA, obstructive sleep apnea.
3. Pathogenesis of Hypertension in OSA
The mechanisms promoting HT in OSA are multifactorial. Sympathetic activity due to intermittent hypoxia is one of the mechanisms triggering the elevation in blood pressure in OSA. Sympathetic activity due to the hypoxemic state causes both vasoconstriction and the stimulation of chemoreceptors. Consequently, the renin–angiotensin–aldosterone system (RAAS) is activated, the endothelin-1 level is increased, and the nitric oxide level is decreased, all of which contribute to the increase in vascular resistance and the development of HT [23][24][24,25]. In addition, RAAS activation increases the amount of angiotensin-2, a strong vasoconstrictor, in the blood and, thus, blood pressure [25][26]. Increased aldosterone levels also contribute to the development of HT by causing fluid and sodium retention [26][27]. Sympathetic hyperactivity leads to a proinflammatory state, resulting in endothelial injury and oxidative stress [27][28][28,29]. The other factors that play a role in the pathogenesis of HT in OSA are obesity, gut dysbiosis, rostral fluid shifts, pharyngeal collapse, nocturnal energy expenditure, and metabolic derangements [23][24] (Figure 1).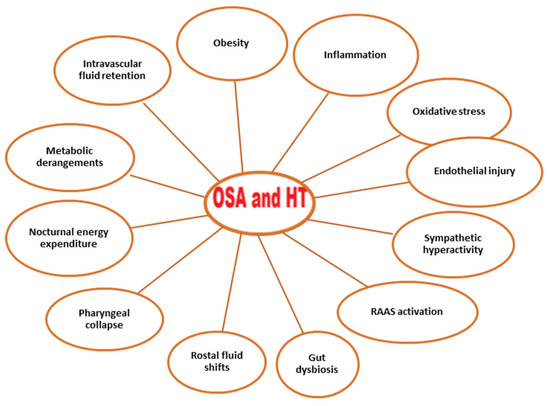
Figure 1. Conditions associated with OSA and hypertension. Abbreviations: OSA, obstructive sleep apnea; RAAS, renin–angiotensin–aldosterone system.
4. Clinical Characteristics of Hypertension in OSA
In patients with OSA, HT is predominantly nocturnal and characterized by a high DBP, masked HT, and a nondipping pattern [4]. Blood pressure is normally the highest during the mid-morning, gradually decreasing as the day progresses, down to 10% of the wakefulness value during sleep, and reaches its lowest value at 3 a.m. This dipping pattern is correlated with the duration of deep sleep. It has been reported that the expected decrease in blood pressure may not occur in the case of several diseases such as OSA. Important cardiovascular consequences may occur in patients who have such diseases who are referred to as “nondippers” [29][30][30,31]. Nocturnal blood pressure elevation is correlated with the severity of OSA [31][32]. The nondipping blood pressure pattern was reported at a rate of 84% in OSA patients who did not receive treatment [32][33]. Nocturnal and nondipping HT is closely associated with target organ damage and the development of cardiovascular diseases [33][34]. Additionally, it has been reported that night-time blood pressure variability, which is related to increased target organ damage, was higher in a OSA group than in a non-OSA group [34][35][35,36]. The pathogenesis of the nondipping pattern and blood pressure variability is multifactorial. Intermittent hypoxia and recurring microarousals are major events leading to sleep fragmentation, reduced slow-wave sleep, and increased sympathetic activity, resulting in elevated blood pressure and increased blood pressure variability [36][37].5. Treatment Modalities
Among the treatment modalities that come to the fore in the treatment of OSA in patients with HT are CPAP, diuretics, renal denervation, use of maxillomandibular advancement devices, and hypoglossal nerve stimulation surgery for restricted airways or tonsillar enlargement. Weight loss, physical exercise, reducing alcohol consumption, and smoking cessation are among the primary lifestyle changes recommended for hypertensive patients with OSA [37][38].6. Pharmacological Therapies of HT in Patients with OSA
6.1. Antihypertensive Medications
Current guidelines for HT do not make specific recommendations on the pharmacological treatment modalities for patients with concomitant OSA and HT. Given the increased sympathetic activity and renin–angiotensin–aldosterone (RAAS) activity in OSA patients, medications that block these pathways are highlights. Among the antihypertensive medications that were initially preferred were angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) [38][39]. It was also demonstrated that beta-blockers mitigate night-time blood pressure rise and apnea-related tachycardias [39][40]. An earlier study conducted by Hedner et al. compared the effects of atenolol, hydrochlorothiazide, amlodipine, enalapril, and losartan on office and ambulatory blood pressures in 40 individuals with HT and OSA [40][41]. Each participant received two of the aforementioned five agents (balanced incomplete block design) for 6 weeks, with a 3-week washout period in-between. Compared with the other four drugs, atenolol lowered the office diastolic BP as well as mean night-time ambulatory SBP and DBP [40][41]. These findings support the hypothesis that overactivity of the sympathetic nervous system is the most important mechanism involved in the development of HT in adults with OSA [40][41].6.2. Diuretics
Two small studies have suggested that the use of spironolactone, an aldosterone antagonist, may efficiently decrease blood pressure in OSA patients with treatment-resistant HT [41][42][42,43]. Similarly, eplerenone, another aldosterone antagonist, was shown to significantly decrease blood pressure in hypertensive OSA patients [43][44]. Recent studies have shown that primary aldosteronism is common in patients with moderate-to-severe OSA, a finding that indicates aldosterone antagonists may be beneficial in this patient group [44][45][46][45,46,47]. There are also studies suggesting that aldosterone antagonists may reduce the frequency of apnea by mitigating laryngeal edema in OSA patients [41][45][42,46]. Taken together, these findings suggest that aldosterone antagonist diuretics, especially spironolactone, may be effective in the treatment of HT in OSA patients. However, large-scale cohort studies are needed to elucidate the efficacy of aldosterone antagonist diuretics in treating HT in OSA patients.6.3. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors
Canagliflozin, one of the SGLT2 inhibitors that has recently been the focus in the treatment of cardiac failure, was shown to provide significant nocturnal blood pressure reductions in adults with diabetes, treatment-resistant HT, and OSA [47][48].7. CPAP Therapy for OSA in Patients with HT
A number of studies have shown that CPAP therapy results in a modest reduction of 2–3 mmHg in SBP and of 1.5–2 mmHg in DBP in OSA patients (Table 2) [48][49][50][51][52][53][54][55][49,50,51,52,53,54,55,56]. On the other hand, this reduction is higher in adults with treatment-resistant HT. Although a 1–2 mmHg decrease in blood pressure may not be considered much, even such a slight decrease in blood pressure was shown to be associated with significant decrease sin cardiovascular mortality and stroke risk [56][57].Table 2.
Recent meta-analyses regarding the effect of OSA treatment on blood pressure values.
| Author (Reference) | Year | Number of Studies | Total Sample Size | OSA Therapy | Main Findings |
|---|---|---|---|---|---|
| Liu [48][49] | 2016 | 5 | 446 | CPAP | MBP reduction: 4.78 mmHg, 95% C:I 1.61–7.95 mmHg SBP reduction: 2.95 mmHg, 95% CI: 0.53–5.37 mmHg DBP reduction: 1.53 mmHg, 95% CI: 0.00–3.07 mmHg |
| Pengo [49][50] | 2020 | 68 | CPAP or MADs | MBP reduction: 2.09 mmHg, 95% CI: 2.78–1.40 mmHg SBP reduction: 1.92 mmHg, 95% CI: 2.40–1.43 mmHg DBP reduction: 1.27 mmHg, 95% CI, 2.34–0.20 mmHg |
|
| Bratton [50][51] | 2015 | 51 | 4888 | CPAP or MADs | DBP reduction: 2.5 mmHg, 95% CI: 1.5–3.5 mmHg DBP reduction: 2.0 mm Hg, 95% CI: 1.3–2.7 mmHg |
| Iftikhar [51][52] | 2013 | 7 | 399 | MADs | MBP reduction: 2.4 mmHg, 95% CI: 0.8–4.0 mmHg SBP reduction: 2.7 mmHg, 95% CI: 0.8–4.6 mmHg DBP reduction: 2.7 mmHg, 95% CI: 0.9–4.6 mmHg |
| Schein [52][53] | 2014 | 16 | 1166 | CPAP | Office SBP reduction: 3.20 mmHg, 95% CI: 1.72–4.67 mmHg Office DBP reduction: 2.87 mmHg, 95% CI: 0.55–5.18 mmHg Night-time SBP reduction: 4.92 mmHg, 95% CI: 1.14–8.70 Mean 24 h BP reduction: 3.56 mmHg, 95% CI: 0.33–6.79 mmHg Mean night-time BP reduction: 2.56 mmHg 95% CI: 0.68–4.43 mmHg |
| Fava [53][54] | 2014 | 29 | 1820 | CPAP | 24- h SBP reduction: 2.6 ± 0.6 mm Hg 24 h DBP reduction: 2.0 ± 0.4 mm Hg |
| Labarca [54][55] | 2021 | 10 | 606 | CPAP | 24 h SBP reduction: 5.06 mmHg, 95% CI: 2.13–7.98 mmHg 24 h DBP reduction: 4.21 mmHg, 95% CI: 1.93–6.50 mmHg Daytime SBP reduction: 2.34 mmHg, 95% CI: 2.27–6.94 mmHg Daytime DBP reduction: 2.14 mmHg, 95% CI: 0.67–4.96 mmHg Night-time SBP reduction: 4.15 mmHg, 95% CI: 1.29–7.01 mmHg Nighttime DBP reduction: 1.95 mmHg, 95% CI: 0.57–3.32 mmHg |
| Shang [55][56] | 2022 | 19 | 1904 | CPAP | SBP reduction: 5.01 mmHg 95% CI: 3.08–6.94 mmHg |
Abbreviations: CI, confidence interval; CPAP, continuous positive airway pressure; DBP, diastolic blood pressure; HT, hypertension; MAD, mandibular advancement device; MBP, mean blood pressure; OR, odds ratio; OSA, obstructive sleep apnea; SBP, systolic blood pressure.
The efficacy of CPAP therapy in the treatment of HT in patients with OSA has been extensively investigated in the literature. Earlier meta-analyses demonstrated a good effect of CPAP therapy in lowering blood pressure in OSA patients. Studies have shown significant reductions in day- and night-time blood pressure values, especially in OSA patients with treatment-resistant HT [57][58][59][60][58,59,60,61].
Kartali N. et al. reported that SBP decreased from 141.5 ± 12.1 mmHg to 133.5 ± 9.7 mmHg (p = 0.007) and that DBP decreased from 87.8 ± 6.8 to 83 ± 5.4 mmHg (p = 0.004) after three months of CPAP treatment [61][62]. Interestingly, the decrease in SBP was observed only during the night (p = 0.031), whereas the decrease in DBP was both during the day and night (p = 0.024 and p = 0.007, respectively). Of note, all the hypertensive participants included in the study were initially nondippers, and the dipping status was significantly improved after CPAP therapy (from 7.9 to 10.4% for SBP, p = 0.014; and from 8.4 to 10.5% for DBP, p = 0.029) [61][62].
In a randomized controlled trial (RCT) by Hoyos C. et al., a significant decrease was detected in blood pressure after CPAP therapy. The magnitude of the decrease was 4.1 mmHg regarding the mean central SBP (p = 0.003), 3.9 mmHg in mean central DBP (p = 0.0009), 4.1 mmHg in mean peripheral SBP (p = 0.004), and 3.8 mmHg in mean peripheral DBP (p = 0.001) [62][63].
In a four-year retrospective study by Yang M.C. et al., the mean blood pressure decreased from 100.8 ± 13.6 mmHg to 96.6 ± 10.8 mmHg (p = 0.004) in OSA patients who were adherent with CPAP [63][64].
A 24-week follow-up study by Campos-Rodriguez F. et al. demonstrated that dose-related beneficial effects were achieved in the long term, even in hypertensive OSA patients who were initially undertreated [64][65].
In an RCT comparing three months of CPAP therapy with sham-CPAP in adults with moderate-to-severe OSA and nocturnal HT, a slight decrease in 24 h SBP/DBP by 2.8/2.5 mmHg was observed, but this was not statistically significant [65][66]. The blood-pressure-lowering effect of CPAP was shown to depend on the baseline daytime pulse rate; and, accordingly, the reduction was significantly greater (10.1 mmHg or more) in patients with a greater daytime pulse rate [65][66].
A multicenter RCT comparing CPAP with sham-CPAP in 272 patients with new-onset systemic HT and moderate-to-severe OSA revealed a significant effect of CPAP on the dipping pattern [64][65]. CPAP therapy was associated with reductions in 24 h ambulatory blood pressure variables and night-time ambulatory blood pressure measurements only in the nondipper group, whereas no significant difference was detected in the dipper group [66][67].
In a recent 8-week-long parallel-group RCT conducted with 92 patients with treatment-resistant HT and OSA, the participants were randomized to CPAP and no-CPAP groups [67][68]. Significant decreases were observed in 24 h SBP by 4.4 mmHg, in 24 h DBP by 2.9 mmHg, in daytime SBP by 5.4 mmHg, and in daytime DBP by 3.4 mmHg, yet only in nondippers, not in dippers [67][68].
Other studies have evaluated the effect of antihypertensive medications vs. CPAP on blood pressure in patients with OSA, suggesting that antihypertensive drugs reduce the blood pressure better than CPAP therapy alone and that the combined use of CPAP therapy and antihypertensive medications provide better results than the stand-alone use of either treatment method [68][69][69,70].
The application of CPAP therapy for one more hour per night reportedly reduced SBP and DBP 1.5 mmHg and 0.9 mmHg more, respectively [70][71]. CPAP therapy reduced blood pressure more in adults with severe OSA and in those with more complaints of insomnia during the day [71][72].
It has been proposed that the blood-pressure-lowering effect of CPAP therapy is more prominent in patients under 60 years of age, in those with higher pretreatment blood pressure, in untreated HT, treatment-resistant HT, nocturnal or nondipping HT, severe OSA, and in those who are adherent to CPAP therapy [12][49][51][70][72][13,50,52,71,73]. Nonetheless, given that CPAP therapy does not correct all factors that increase blood pressure (e.g., volume overload, high salt production, etc.), CPAP alone cannot produce a significant improvement in blood pressure. According to the 2017 American Heart Association (AHA)/American College of Cardiology (ACC) blood pressure guidelines, CPAP therapy is yet not a well-established antihypertensive treatment in adults with HT and OSA (class IIb) [73][74].
