Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Gulzat Nuroldayeva and Version 2 by Fanny Huang.
The application potential of flexible electrochromic materials for wearable devices, smart textiles, flexible displays, electronic paper, and implantable biomedical devices is enormous. These materials offer the advantages of conformability and mechanical robustness, making them highly desirable for these applications.
- flexible electrochromic materials
1. Understanding Electrochromic Materials: Definition and Working Principles
Electrochromism is a remarkable property of certain materials that sets them apart from other substances [1]. These fascinating electrochromic materials have the unique ability to reversibly change their color or optical properties when exposed to an external electrical stimulus. Through reversible redox reactions within the material, the dynamic nature of electrochromism (EC) enables effective modulation of its light absorption and transmission properties across the electromagnetic spectrum [2]. When an electrical voltage is applied to these materials, which contain certain electroactive components, oxidation or reduction reactions are initiated. As a result, the materials significantly change their physical appearance and alternate between different color states. By precisely controlling these changes and the timing of the changes, the desired effects can be achieved [3]. These unique adaptations have found numerous applications in areas such as the design of advanced structures [4], displays [5], and optical devices [6]. The exceptional properties of these materials are particularly valuable for the development of energy-efficient heat and light transfer systems [7]. They can switch between transparent and colored states, which offers advantages in architecture, automotive, and other industries.
An electrochromic system usually consists of several major components. The electrochromic layer, located at the heart of the system, consists of a material that can undergo a reversible redox reaction when electric current flows through it, resulting in a change in its color state. Adjacent to the electrochromic layer is the ion storage layer or counter electrode, which serves as a reservoir for the ions involved in the redox process and facilitates their movement to and from the electrochromic layer. On either side of these layers are two transparent conductive electrodes responsible for applying the electrical potential to the system. These electrodes allow the ions to move and trigger the color change. It is worth noting that the substrate on which the electrochromic system is built can be either rigid or flexible, depending on the application. Examples of commonly used substrate materials include glass, plastic films, and flexible polymers. Finally, the electrolyte plays a crucial role by providing a medium for ion transport and ensuring the overall electrochemical stability of the system [8][9][10][11][8,9,10,11].
The classification of electrochromic devices (ECDs) is based on the solubility of the bleached and colored electrochromic components in the electrolyte layer. There are three types of ECDs: type I, II, and III [1][12][13][1,12,13] (Figure 1). Type I devices are characterized by the fact that both components are soluble in the electrolyte during operation. In contrast, insoluble solids of both components form on the electrodes in type III devices [12]. In these devices, the electrolyte layer does not contain EC materials. Their purpose is to provide counterions for the EC layers to maintain charge neutrality during electrochemical coloration and bleaching. Type II ECDs fall between type I and type III. In type II devices, only the bleached component is dissolved in the electrolyte, while the colored component forms a coating on one of the electrodes [12][13][12,13].
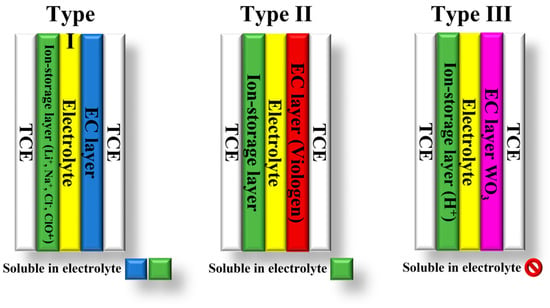
Figure 1. Types of electrochromic devices based on the solubility of the EC components.
By carefully optimizing materials, device configurations, and electrical stimuli, electrochromic materials offer a wide range of applications [14]. One notable example is smart windows [11][15][11,15], which can regulate the amount of light entering an outdoor space to improve energy efficiency and occupant comfort. Zhai et al. [16] investigated dual-band electrochromics, i.e., the independent modulation of visible (Vis) and near-infrared (NIR) light transmittance by EC materials. Dual-band electrochromic devices have unique characteristics, such as bistability, low power consumption, and separate control over the visible and NIR regions. Therefore, the development of dual-band EC devices is of great importance in the pursuit of an energy-saving society.
Previous studies have shown the working mechanism of electrochromic materials in supercapacitors, electric double-layer capacitors (EDLCs), and lithium-ion batteries (LIBs) [17][18][19][20][21][17,18,19,20,21]. The different electrochemical processes that determine the charge storage properties of batteries and supercapacitors can be distinguished through electrochemical measurements [18]. Unlike batteries, EDLCs respond quickly to voltage changes and have high energy density because no redox reactions occur [19][20][19,20]. However, EDLCs have lower energy density because the charges are mainly located on the material surfaces. The main characteristics of EDLCs include a rectangular cyclic voltammogram curve (CV) and a linear discharge curve versus time [21].
Recent research shows that the integration of electrochromic technology with other cutting-edge technologies has significantly accelerated its progress [22][23][24][25][22,23,24,25]. This integration has expanded the applications of electrochromic technology in various fields. Researchers have explored a novel self-structuring electrochromic device that uses a single layer of multicolor polyaniline (PANI) [26]. Unlike conventional symmetric ECDs with five functional layers, this design features a three-layer structure that includes a PANI EC layer, a quasi-solid-state gel electrolyte, and a conductive layer [27]. The self-structuring ECD exhibits a distinct multicolor and gradient pattern and achieves significant transmission contrast of up to 60% when a horizontal current is applied [26]. To understand this self-structuring behavior, a novel electrochromic mechanism was proposed to highlight the planar motion and redistribution of anions in the PANI layer, which is triggered by a current-driven magnetic effect.
To expand the practical applications of electrochromic devices, the introduction of flexible architectures enables new functions [28]. The development of flexible electrochromic devices (FECDs) has led to electronic skins [29], actuators [30], and wearable electronics [31], expanding the range of their potential applications [28]. FECDs are characterized by their compact size, improved mechanical durability, and multifunctionality, and have attracted considerable attention in both academic and industrial fields [32]. With the increasing demand for portable, flexible, and deformable electronic devices, FECDs play a crucial role in meeting these requirements. Their light weight makes them promising candidates for human–machine interfaces that seamlessly integrate with soft biological tissues, such as the surface of the human body [33].
2. The General Structure of Flexible Electrochromic Materials
Multiple layers play an essential role in the overall structure of flexible electrochromic materials and enable the desired electrochromic behavior. While the specific structure varies depending on the material system and device structure (see Figure 2), here is a general framework for flexible electrochromic materials:
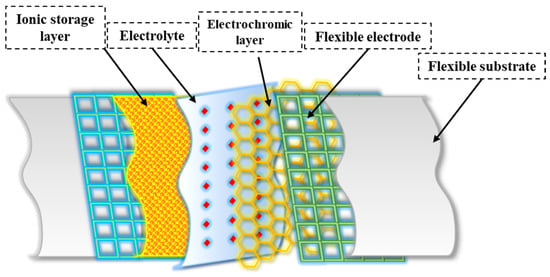
Figure 2. General structure of flexible electrochromic materials.
-
The substrate serves as a flexible base to which other layers are applied. It provides mechanical support and flexibility to the electrochromic device. Compared to rigid substrates, flexible substrates offer several advantages, including increased flexibility, portability, and lower cost [25][34][25,50]. These properties allow flexible electrochromic devices to maintain their electrochromic properties even when bent, twisted, or stretched [25]. For transparent FECDs, an ideal flexible transparent substrate should have high optical transparency, excellent resistance to environmental and chemical factors, and mechanical flexibility. Reflective FECDs, on the other hand, use flexible substrates made of materials such as nylon/Au or PET /Au, which serve as reflectors [35][51[36],52]. In addition, polymers (e.g., polyethylene terephthalate or PEN [37][53], polyimide [38][54], and flexible glasses (e.g., thin soda-lime glass [39][55]) can also be used as flexible substrates.
-
The transparent conductive electrode is usually located on the substrate and serves as a current collector for the electrochromic layer. It allows the electric current to pass through and remains transparent in the visual spectrum. Several critical factors affect the performance of FECDs with transparent conductive electrodes (TCEs). These include low resistivity, high transparency, a wide potential window, improved chemical and electrochemical stability, and increased resistance to deformations such as bending, stretching, and folding [40][56]. Indium tin oxide (ITO) [41][57] and fluorine-doped tin oxide (FTO) [42][58] are widely used for the fabrication of electrochromic devices due to their low resistance and high transparency. However, the adhesion of ITO to flexible substrates often leads to significant degradation of the dyeing efficiency and optical contrast of FECDs after several bending cycles [43][59]. In addition, the brittleness and high cost of ITO pose challenges to its suitability for flexible applications and commercialization of FECDs [44][41]. Therefore, alternative flexible electrode materials such as conducting polymers [45][60], carbon nanotubes [46][61], graphene [47][62], metal nanowires [48][63], and grids [49][64] have been thoroughly investigated as potential replacements for the traditional ITO /FTO to improve the overall flexibility of the devices.
-
The electrochromic layer is the active layer responsible for reversible color change or a change in optical properties. It undergoes redox reactions in response to an applied electrical potential. The electrochromic layer, which consists of an electrochromic material, plays an important role in flexible electrochromic devices [50][65]. This layer enables the reversible electrochemical redox process that allows visual manipulation. The EC films require properties such as strong ionic and electron conductivity, a significant optical difference between the dyeing and bleaching states, high dyeing efficiency, and consistent cycling stability [51][66]. Flexible EC films are particularly valuable for FECDs, and organic EC materials, especially conjugated polymers, have found wide application [52][67]. In addition, hard inorganic materials can be used to fabricate EC films whose flexibility is enhanced by selective morphology and structural design [53][68]. The choice of electrochromic material depends on criteria such as the desired color range, response time, durability, and device compatibility [54][69]. In addition to the primary electrochromic layer, the ion storage layer plays a crucial role in maintaining the stability of FECDs. This layer cooperates with the primary electrochromic layer to facilitate the reversible exchange of small ions and charge-balancing electrons between the electrodes and the electrolyte layer [9]. It effectively reduces the accumulation of small ions on the electrode surfaces and prevents their injection into the electrodes, which is critical for device performance and lifetime. Complementary electrochromic materials are often used as an alternative to the ion storage layer to improve the optical modulation and coloring efficiency of electrochromic devices [2]. This substitution allows the ion storage layer to change color when the devices are dyed, which improves the overall performance of the devices.
3. Overview of the Application and Potential Benefits of Flexible Electrochromic Materials
Electrochromic devices include a range of technologies that use the electrochromic effect to modulate color or optical properties in response to an electric field [55][70]. These devices have attracted considerable attention due to their applications in various fields, including smart windows, displays, and optical filters. Understanding the different types of electrochromic devices provides valuable insight into their unique functions and potential applications.
-
An important application of electrochromic devices is the development of smart windows [56][71]. These windows contain thin films or coatings of electrochromic materials that can switch between transparent and opaque states. By applying a voltage, these windows can dynamically control the amount of light and heat entering a building or vehicle, providing energy-efficient solutions for lighting and air conditioning.
-
Electrochromic displays are another fascinating application of electrochromism [57][72]. These displays use electrochromic materials that can change color or opacity to produce visual information. Electrical signals selectively drive individual pixels or segments to produce the desired image or text information. The advantages of electrochromic displays are their low power consumption and high contrast capability, making them suitable for e-readers and low-power electronic signage.
-
Electrochromic mirrors are used in automotive applications to reduce glare from the headlights of following vehicles [58][73]. These mirrors consist of an electrochromic layer sandwiched between two transparent conductive layers. When an electrical voltage is applied, the mirror darkens, reducing the intensity of reflected light and improving driver visibility and safety.
-
Electrochromic materials can be used to fabricate EC sensors that detect and quantify various analytes [59][74]. In these sensors, a reaction usually occurs between the analyte and a particular electrochromic material, resulting in a color change that can be measured and correlated with the concentration of the analyte. Electrochromic sensors are used in environmental monitoring, food quality control, and medical diagnostics.
-
Recent advances have enabled the integration of electrochromic materials into textiles, resulting in smart fabrics with color-changing capabilities [60][75]. These fabrics can be used for wearable technology, fashion, or artistic installations to achieve dynamic and interactive designs. The color and appearance of the fabric can be changed by applying an electrical potential, allowing for a customized and customizable esthetic experience.
Various flexible electrode materials and design methods have enabled the fabrication of flexible and stretchable electrochromic devices with excellent mechanical and photoelectric properties. Indium tin oxide on polyethylene terephthalate (ITO/PET) is widely used as a flexible conductive material. In addition, new transparent conductive electrodes such as carbon nanotubes, graphene, and metal-based nanomaterials such as Ag/Au nanowires and soft conductive polymers have emerged as viable alternatives for fabricating flexible electronic devices. Through the use of various electrochromic materials and physical or chemical processes, flexible electrochromic electrodes with electrochromic functionality have emerged, building on advances in flexible electrodes. These advances have paved the way for the development of flexible electrochromic devices.
