1. Endocytosis
Endocytosis is the process by which cells internalize proteins, lipids and other large molecules
[49][1]. When nanoparticles reach the extracellular membrane, they can interact with the components of the plasma membrane or extracellular matrix, causing plasma membrane-coated nanoparticles to be entrapped in vesicles, which are subsequently pinched to form endocytic vesicles, and they are transported to specialized intracellular compartments
[50][2]. Depending on the nanoparticle type and surface modification, there are several different routes of entry into the epithelium. In this
resear
ticlech, according to the structural characteristics of intestinal epithelial cells, four endocytic pathways into epithelial cells are discussed. As shown in
Figure 1, they are clathrin-mediated endocytosis (CME), caveolae-mediated endocytosis (CavME), clathrin/caveolae-independent endocytosis (CIE) and macropinocytosis.
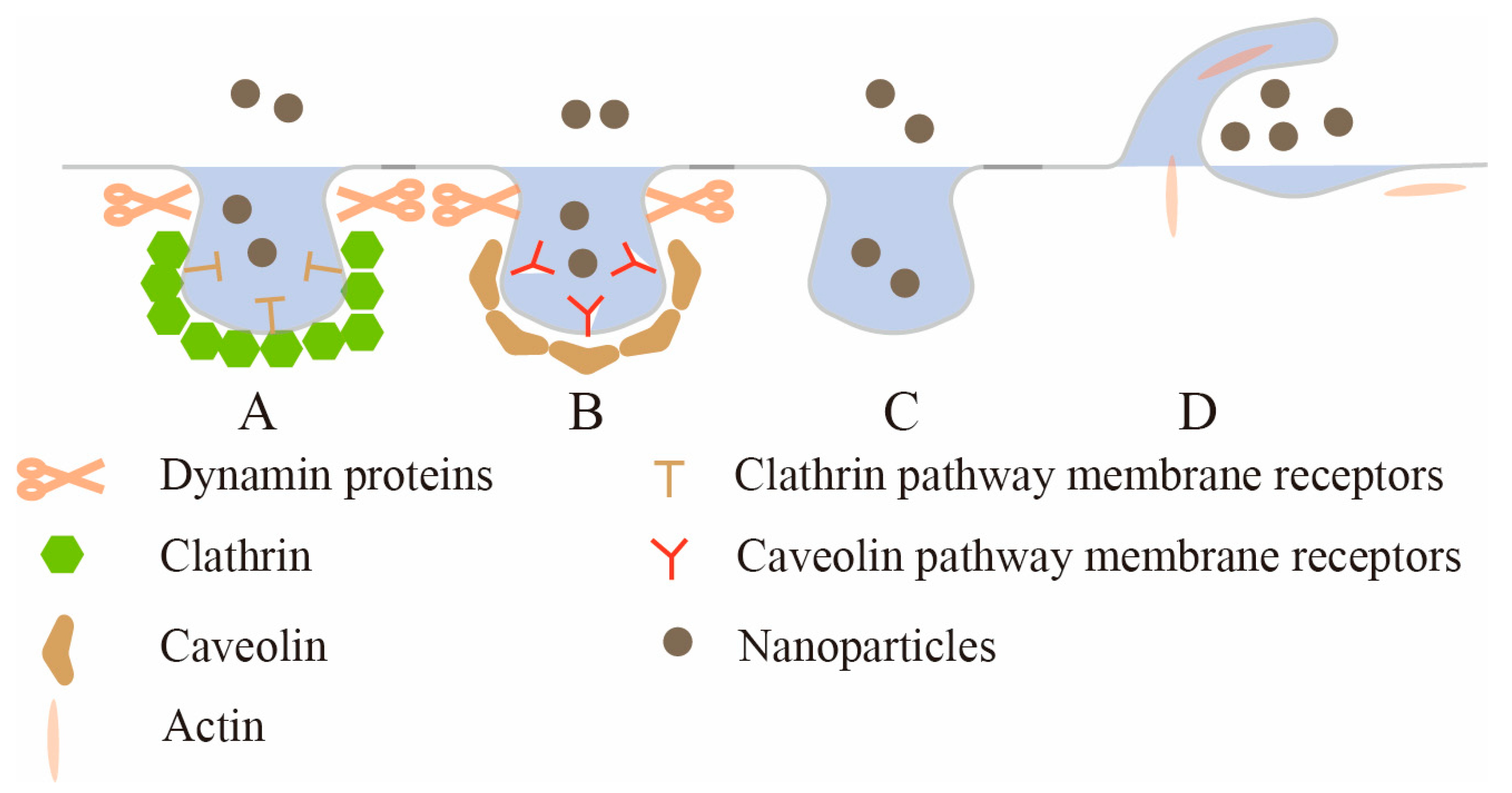
Figure 1. The uptake mechanism of intestinal epithelial cells. A: Clathrin-mediated endocytosis (CME); B: caveolae-mediated endocytosis (CavME); C: clathrin/caveolae-independent endocytosis (CIE); D: macropinocytosis.
Clathrin-mediated endocytosis (CME) is the major route for nanoparticles to enter cells, and many receptors on the epithelial membrane, such as transferrin receptor, low-density lipoprotein receptor, etc., can initiate CME
[51][3]. Some investigators have previously referred to CME as receptor-mediated endocytosis, but this statement is not accurate because most pinocytic pathways also involve specific receptor–ligand interactions
[52][4]. CME generally occurs in clathrin-rich plasma membrane regions, and the entire process can be divided into several stages: the initiation of clathrin-coated pits (CCP), cargo selection, CCP growth and maturation, scission and the release of clathrin-coated vesicles (CCV)
[53][5]. CCP assembly is initiated by heterotetrameric adaptor protein 2 (AP2). First, abundant phosphatidylinositol lipid PI (4,5) P
2 on the plasma membrane recruits the AP2 complex
[54][6]; then, the AP2 complex rapidly recruits clathrin
[55][7]. Clathrin has a three-legged structure comprising three heavy chains and three light chains, and this unique protein and other proteins (such as Eps15 and intersectin) spontaneously co-assemble into a complex structure that generates a curvature that stabilizes the membrane and enables vesicle budding: that is, CCP priming. As nascent pits grow, AP2 and other specific adaptor proteins recognize and recruit cargo, and adaptor and accessory proteins coordinate clathrin protein nucleation at the site of membrane internalization
[56][8]. Clathrin nucleation would induce membrane invagination and stabilize the curvature of the pits. The Bin-Amphiphysin-Rvs (BAR) protein subsequently recruits the membrane scission protein dynamin, which aggregates to the neck of the budding vesicle. Finally, GTPase is hydrolyzed to drive membrane division
[57][9], releasing the mature CCV from the plasma membrane. Huang et al.
[58][10] modified TiO
2 nanoparticles using transferrin and found that the nanoparticles would be taken up by cells through the CME pathway, and transferrin-modified TiO
2 nanoparticles entered the cells faster than the unmodified TiO
2 nanoparticles. In addition to surface ligand modification, some cellular factors such as epidermal growth factor (EGF) can also activate the CME process. Phuc et al.
[59][11] found that the CME pathway was not involved in the uptake of polystyrene nanoparticles in the absence of EGF. However, in the presence of EGF, EGF was able to activate the CME pathway to enhance the cellular uptake of polystyrene nanoparticles.
Caveolae-mediated endocytosis (CavME) is another important pathway for receptor-specific nanoparticle internalization
[60][12]. Unlike CME processes, which involve dynamic and sequential maturation, caveolae are 50–100 nm diameter bottle- or Ω-shaped pits located in the plasma membrane, where they exhibit a defined shape with consistent curvature and proportion in the neck region
[61][13]. The outer surface of caveolae is usually covered with a layer of caveolin, a dimeric protein that binds to cholesterol, is inserted into the inner leaflet of the plasma membrane in a circular manner and binds to the surface of the membrane indentation to form a caveolin coating, which effectively stabilizes the bottle structure of caveolae
[62][14]. Existing studies have shown that CavME is highly regulated and that caveolin stabilization at the plasma membrane is closely linked to actin stress fibers from Filamin A proteins that link caveolin to actin fibers and anchor caveolin to the plasma membrane. The germination of caveolae is regulated by kinase and phosphatase
[63][15]. Studies have shown that kinase inhibitors can inhibit CavME, and phosphatase inhibitors can enhance CavME, which is caused by the loss of connection between caveolae and actin fibers after the rapid phosphorylation of Filamin A protein that is mediated by protein kinase Cα
[64][16]. Once caveolae are detached from the plasma membrane, compared with vesicles produced by the CME pathway, vesicles produced by the CavME pathway are more likely to be transported to the Golgi apparatus and endoplasmic reticulum to avoid lysosomes and protect contents from the degradation of hydrolase in lysosomes
[65][17]. Therefore, if intracellular or organellar targeting is desired, caveolae-mediated endocytosis is an exploitable route. For example, Cao et al.
[66][18] prepared a kind of PEG nanoparticles and connected divalent folic acid to PEG. It was found that with the increase in divalent folic acid, the endocytosis pathway of nanoparticles changed from clathrin-mediated to a caveolin-mediated endocytosis pathway, and the localization of nanoparticles in lysosomes also decreased. Xin et al.
[67][19] prepared a rod-shaped active pure drug nanoparticle, which was found to enter the cell using caveolae-mediated endocytosis, bypass the lysosome and enter the cytoplasm so that the drug is protected from lysosomal degradation. In summary, caveolae-mediated endocytosis can render nanoparticles prone to bypass lysosomes after entering cells and protect drugs from degradation by the action of hydrolytic enzymes in lysosomes. This makes it possible for more nanoparticles to be exocytosed out of the epithelium, reducing their retention inside the cell and, to some extent, increasing their transcellular efficiency.
Clathrin/caveolae-independent endocytosis (CIE), another important cell endocytic pathway, involves neither the formation of clathrin coats nor caveolae formation
[68][20]. And endocytic vesicles involved in CIE have no obvious outer shell
[69][21], so observing them using electron microscopy is difficult. This pathway was first discovered because nanoparticles were still found to be taken into cells after using inhibitors that block CME and CavME, indicating that other endocytosis pathways independent of CME and CavME exist in cells and such endocytosis pathways are resistant to CME and CavME inhibitors, namely CIE
[70][22]. The CIE pathways reported so far can be classified according to whether dynamin proteins are used for membrane separation. RhoA (Ras Homolog Family Member A)-mediated endocytosis, fast endophilin-mediated endocytosis, Shiga-toxin-induced endocytosis, and ARF6 (ADP-ribosylation factor 6)-mediated endocytosis are all CIEs that require dynamin proteins for membrane separation. CDC42 (Cell Division Control protein 42)-dependent endocytosis and Flotillin-mediated endocytosis are CIEs that do not require dynamin proteins for membrane separation
[71][23]. Among them, RhoA-mediated endocytosis, initially thought to be initiated by the activation of the interleukin 2 receptor, is also currently demonstrated to mediate the uptake of many cytokine receptors and their components
[72][24]. ARF6-mediated endocytosis is currently demonstrated to activate phosphatidylinositol-4-phosphate-5-kinase by ARF6, generating phosphoinositide PI (4,5) P
2, stimulating actin assembly and driving endocytosis
[73][25]. CDC42-dependent endocytosis is a clathrin-independent and dynamin-independent pathway involving small GTPase enzymes Rac1 and CDC42, resulting in clathrin-independent carriers (CLICs). These CLICs fuse to form a special early internal compartment, called glycosylphosphatidylinositol-anchored protein-enriched internal compartments (GEECs), so this process is also known as the CLIC/GEEC pathway
[74][26]. However, the degree of overlap between these pathways remains incompletely understood, and more molecular mechanistic studies are currently needed to address these questions and better define these pathways.
Macropinocytosis is a type of endocytosis in which cells nonspecifically take up extracellular fluid and extracellular macromolecules into large intracellular vesicles
[75][27]. Some studies have shown that macropinocytosis is a process driven by actin, and the actin polymerization ring first forms protrusions on the plasma membrane, which are known as membrane folds. As the membrane fold continues to develop, the fold bends inwardly back to the plasma membrane to form a large vesicle
[76][28]. The large vesicles formed in this process are also called macropinosomes, with a diameter of about 0.2–10 μm, which is significantly larger than that of RME (retromer-mediated endosome)
[77][29]. Therefore, 70 KD glucan molecules are commonly used to mark macropinosomes with large sizes for studying macropinocytosis because these molecules are too large to enter cells via other endocytic mechanisms
[78][30]. In addition, the entire endocytic process is regulated by a series of small GTPases, such as Ras GTPases that activate phosphatidylinositol 3-kinase to generate a membrane domain rich in phosphoinositol PIP
3. These domains serve as docking sites for Rho GTPases, regulating actin remodeling and driving the formation of membrane ruffles
[79][31]. After the formation of macropinosomes on the plasma membrane, Rab GTPases and phosphoinositides regulate a series of steps in their maturation. For example, Rab5 and Rab34 participate in the early stages of macropinosome formation, facilitating their fusion with early endosomes. After the maturation of the macropinosome, Rab7 replaces Rab5 to promote its fusion with late endosomes or lysosomes
[80][32]. Nanoparticles tend to exhibit different uptake mechanisms depending on the material and particle size.
2. Intracellular Transport
2.1. Endosomal Circulatory System
After nanoparticles are internalized into cellular endosomes by various uptake pathways, their ultimate intracellular fate is usually determined by intracellular sorting and transport mechanisms, which mainly consist of an endosomal network with the Golgi apparatus, endoplasmic reticulum and lysosomes
[102][33]. Among them, endosomes are initially generated from the plasma membrane, and after being regulated by various enzymes at different stages of development, they can fuse with the Golgi apparatus, endoplasmic reticulum or lysosomes. Endosomes are normally found in the cytoplasm of most human cells and can be classified into three main types: early endosomes, recycling endosomes, and late endosomes, which together form an intracellular endosome network
[103][34]. In general, after leaving the plasma membrane, endosomes can fuse with early endosomes. The particles in the endosome will also become part of the early endosomes, which then serve as a transit station to guide the further trafficking of nanoparticles to different cellular destinations
[104][35]. Some nanoparticles are transported by vesicles into recycling endosomes and finally to the plasma membrane. As early endosomes mature into late endosomes, the remaining nanoparticles are sent to lysosomes for degradation as late endosomes associate with lysosomes or can fuse with the plasma membrane to deliver nanoparticles out of the enterocyte
[105][36].
2.2. Enzyme Regulation of Endosomes
The endosomal circulation network is a highly dynamic system in which transport and transformation between endosomes occur constantly. Therefore, to ensure that the right goods arrive at the right place at the right time, this highly dynamic system must be tightly regulated and regulated. The Rab enzyme family of the small GTPase family plays an important role in ensuring this. Rab enzymes control many aspects of membrane transport, including early endosomal to late endosomal transformation, transport between various endosomes, the fusion of endosomes with organelles and plasma membranes, etc.
[118][37]. Early endosomes can be labeled by Rab5, which plays an important role in the biogenesis of the endosomal system by regulating the fusion of small vesicles entering cells with early endosomes. In addition, its Rab5A isoform also plays a key catalytic role during the progression of early endosomes to late endosomes. Late endosomes can be marked by Rab7, which is directly or indirectly involved in the development of the endosome lysosome system, including the transition of early endosomes to late endosomes, the transport of late endosomes to lysosomes, lysosome biogenesis and the fusion of late endosomes with lysosomes
[119][38], whereas Rab5-Rab7 turnover is a hallmark of the early endosome to late endosome turnover. In this process, Rab5 together with phosphatidylinositol 3-phosphate (PtdIns3P) recruits a complex containing Mon1/SAND1 and Ccz1/CCZ1 proteins into the early endosomes. This complex has two important roles: On the one hand, the Mon1/SAND1 protein inhibits its activity by displacing guanine nucleotide exchange factor rabex-5 from Rab5; Ccz1/CCZ1 on the other hand functions as a guanine nucleotide exchange factor in Rab7, leading to the activation of Rab7. The activation of Rab7 in turn recruits TBC2, a GTPase-activating protein that inactivates Rab5, further reducing Rab5 activity and leading to the dissociation of Rab5 effectors such as EETA
[120][39]. Thereafter, Rab7 formally displaces Rab5, and early endosomes transition into late endosomes
[121][40]. In addition to Rab5 and Rab7, at least 20 different Rab enzymes are involved in the endosomal maturation process, such as Rab7b, Rab6 and Rab9, which are involved in endosomal transport to the Golgi apparatus
[122][41], and Rab10 may act upstream of Rab5 to promote the recycling of glycosyl phosphatidylinositol (GPI)-anchored proteins
[123][42]. Many other Rab enzymes require further investigation to define their mechanism of action.
2.3. Intracellular Trafficking Pathways
Based on the above review of the endosomal recycling network,
wresearche
rs know that transport vesicles containing nanoparticles first fuse with the apical-side early endosome (EE), and then there are three pathways to transport nanoparticles according to the sorting transport mechanism within the cell
[124][43]. As shown in
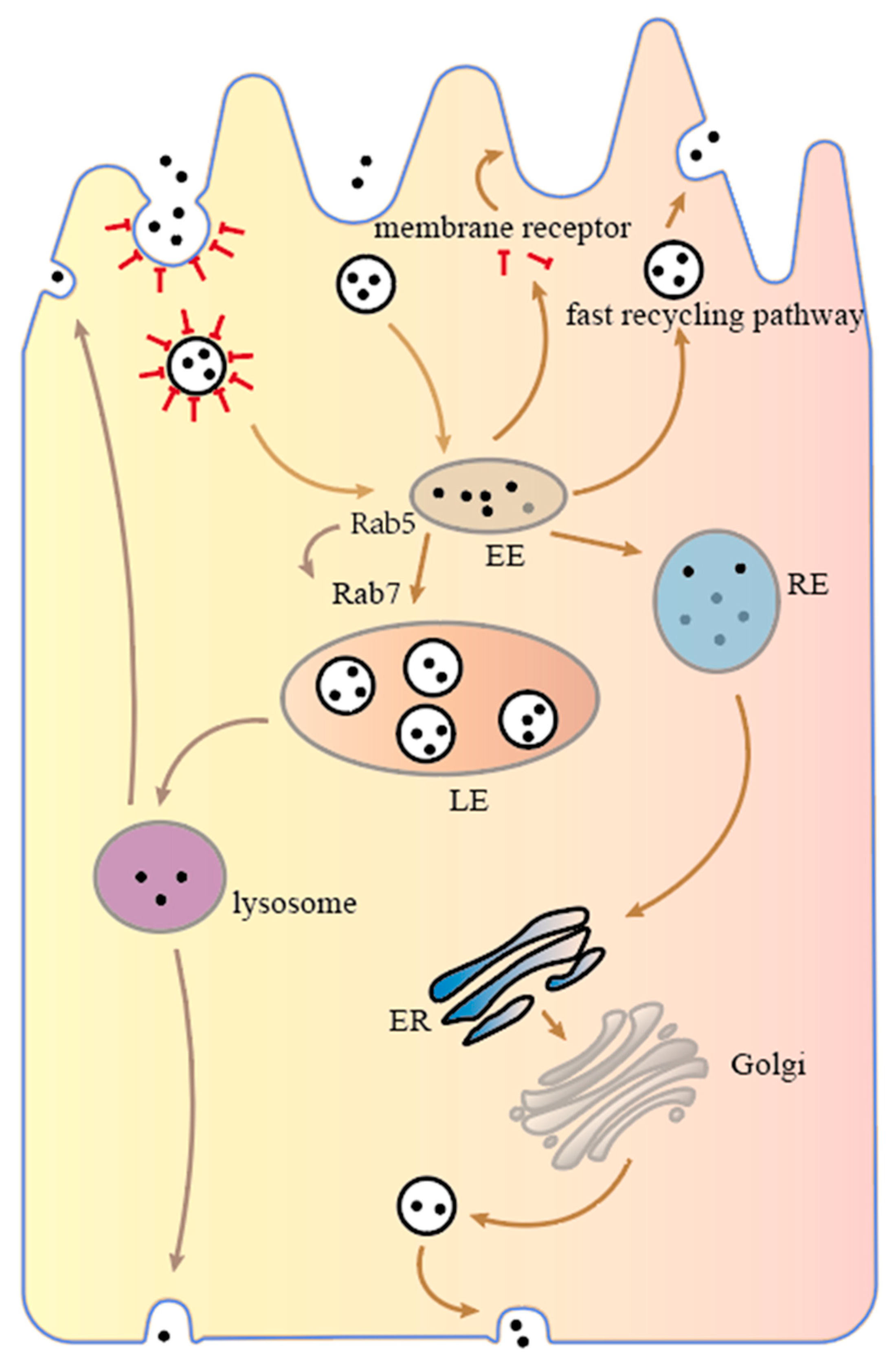
Figure 2, (I) depending on the rapid recycling pathway, nanoparticles are sent back from the early endosome to the apical side of the intestinal epithelial cell. In addition, nanoparticles bound to internalized receptors can be released into the cytoplasm, where receptors return to the cell membrane in a rapid recycling pathway, and the nanoparticles continue to spread in the cytoplasm towards the basal side until they are degraded. (II) The nanoparticles are transported from early endosomes to late endosomes and eventually to lysosomes, which contain a wide range of hydrolytic enzymes capable of degrading almost all types of cellular components, such as proteins, fats, carbohydrates and even organelles. Nanoparticles that enter the lysosomes may be degraded or sent to both sides of the cell. (III) The nanoparticles are sent to recycling endosomes (REs), which then proceed to the ER, Golgi apparatus or late endosomes depending on the slow recycling pathway and eventually reach the basal side of the enterocyte where they are sent out of the cell, completing transcellular transport.
Figure 2.
Intracellular transport mechanism of intestinal epithelial cells.
Based on the possible transport pathways of nanoparticles,
wresearche
rs can summarize two important factors that need to be considered in the transcellular transport of nanoparticles. One is that when nanoparticles are transported from the apical side to the basal side, they are sent back to the apical side and then excreted back to the intestinal lumen, greatly reducing the transmembrane rate of nanoparticles
[125][44]. The other is that the nanoparticles undergo degradation after they are transported to lysosomes during transport, which also greatly reduces the number of nanoparticles across the membrane
[126][45]. Therefore, to improve transmembrane efficiency, inhibiting nanoparticles from refluxing to the apical side or being sent to lysosomes could be an important method.
3. Cellular Exocytosis
The ability of nanoparticles to enter cells does not equal the ability to cross cells. “Easy entry, difficult to cross cells” is a typical phenomenon in which cells cross intestinal epithelial cells
[127][46]. The reasons for this phenomenon are numerous and complex. There are two possible reasons: First, internalized nanoparticles reflux to the apical side of intestinal epithelial cells, which are polarized cells, and the microstructure and proteins at the two ends of cells are not the same. Compared with the basal side, many nanoparticles have a greater tendency to egress from the apical side. The second is the intracellular degradation of nanoparticles. A typical example is the transport of nanoparticles to lysosomes, where they are degraded. Some studies have shown that the exocytosis rate of nanoparticles is slower than the internalization rate. Chai et al.
[125][44], in their experiments studying solid lipid nanoparticles across Caco-2 cells, found that the exocytosis rate was only one-third of the internalization rate over a certain period. In general, exocytosis is even more difficult than internalization. Therefore, to improve the transcellular efficiency of nanoparticles,
wresearche
rs need to pay more attention to exocytosis. However, unfortunately, current research studies on cellular exocytosis number far fewer than studies on cellular uptake. This
paper research focuses on two aspects of exocytosis combined with the analysis of the intracellular transport process of the upper segment. The two aspects are apical-side exocytosis and endoplasmic reticulum/Golgi exocytosis.
3.3.1. Apical Exocytosis
3.1. Apical Exocytosis
The exocytosis of nanoparticles to the apical side is one of the important reasons affecting transcytosis efficiency. Although there is little research on this phenomenon, apical-side reflux can affect the transmembrane efficiency of nanoparticles. Some studies have reported that exocytosis on the apical side of nanoparticles is even greater than that on the basal side. For example, Wu et al.
[128][47] modified polyethylene-glycol-coated nanoparticles using butyrate and found that the bilateral exocytosis of nanoparticles occurred, and significantly more nanoparticles were exocytosed from the apical side than from the basal side, which make up about 80% of the exocytosis from the apical side. Apical exocytosis greatly inhibits the transcellular effect of nanoparticles, which is not
our ideal mode of exocytosis. More studies are needed to explore new strategies for apical exocytosis inhibition. Zhuang et al.
[129][48] explored the effect of nanoparticle shape on their exocytosis on both sides of Caco-2 cells and found that rod-shaped nanoparticles not only had a greater tendency to enter cells than spherical nanoparticles but also had a greater tendency toward exocytosis on the basal side. In addition, Liu et al.
[130][49] modified the surface of nanoparticles with angioproteinin-2 (Ang-2), which can target low-density lipoprotein receptor-associated protein 1 (LRP-1) expressed in the intestine, and Ang-2 nanoparticles were able to increase the expression of LRP-1 on the apical side of epithelial cells and further induce their redistribution to the basal side such that exocytosis on the apical side of nanoparticles decreased and exocytosis on the basal side increased, thus leading to increased transepithelial membrane trafficking. Overall, there are still very few studies on apical-side exocytosis at present, and more studies are needed to explore the involved mechanisms and influencing factors.
3.3.2. Endoplasmic Reticulum/Golgi Exocytosis
3.2. Endoplasmic Reticulum/Golgi Exocytosis
The trans-endoplasmic Golgi network is an important pathway for the outward transport of substances from the cell, and the entire process involves a series of organelles, such as the endoplasmic reticulum (ER), Golgi apparatus, etc. Thus, this pathway of exocytosis is also called the ER/Golgi pathway. In a study on the transcellular mechanism of solid lipid nanoparticles with brefeldin A, Chai et al.
[125][44] evoked the retrograde transport of Golgi enzymes and the retrograde transport of nanoparticles back to the ER to block the ER/Golgi pathway, and they used monensin to disrupt the Golgi apparatus and inhibit the transport of cargo from the Golgi apparatus to the plasma membrane. It was observed that the intracellular residence of nanoparticles increased, and exocytosis was inhibited, indicating that the ER/Golgi pathway plays an important role in the exocytosis of nanoparticles. In addition, the exocytosis of nanoparticles via the ER/Golgi pathway can circumvent lysosomes, decrease the intracellular degradation of nanoparticles and increase their rate of egress.