Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yao Ntifafa and Version 2 by Jason Zhu.
Alkenyl Succinic Anhydride (ASA) is a sizing agent used in papermaking to increase the water repellency of paper. Almost 60 years after the introduction of the chemical in papermaking, scientists still have differing views on how ASA interacts with cellulose. Several experiments were conducted to bring more clarity to the ASA sizing mechanism, especially on the contentious question of ASA-cellulose covalent bonding or the esterification reaction between ASA and cellulose during papermaking.
- Alkenyl Succinic Anhydride (ASA)
- cellulose
- paper sizing
1. Introduction
The objective of paper sizing is to delay wetting by reducing the fiber absorbency. Alkenyl Succinic Anhydride (ASA) was introduced as a sizing agent in papermaking in 1963 by Wurzburg and Mazzarella [1]. ASA is an organic compound with cyclic dicarboxylic anhydride and a tetrafurandione (Figure 1). The chemical is light yellow color, oil-like, non-soluble in water, and liquid at room temperature [2][3][2,3].
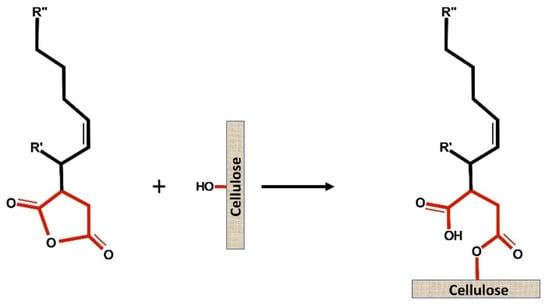
Figure 1. Scheme of ester bond formation between ASA and cellulose.
Today, common internal sizing chemicals (e.g., alum/rosin, AKD [Alkyl ketene dimer] and ASA) are introduced into a pulp slurry at the wet end during the papermaking process. Alum/rosin sizing is commonly used for acidic papermaking with a typical pH range from 4.0 to 5.5. In this pH range, alum forms the right aluminum species to either react with soap rosin or to retain dispersed rosin (DRS) for sizing development. AKD and ASA are used in alkaline papermaking processes, where ASA has a higher reactivity to cellulosic fiber compared to AKD. Moreover, ASA has also been reported to be applied in acidic paper-making conditions [4][5][6][7][8][4,5,6,7,8].
Due to the insolubility of ASA in water, ASA oil needs to be emulsified before adding to the wet end for homogenous distribution in pulp slurries. The emulsifier can be starch-based (cationic starch) or polymer-based (polyacrylamide). Typical ASA emulsion particle size is around 1 µm. The ASA emulsion has a short shelf life, and thus the chemical is typically emulsified on-site in the mill and dosed to the wet end as soon as possible. The emulsion particles are retained in the forming paper sheet at the wet end and forming section. Sizing development mostly happens in the dryer section, where the particles break down to release ASA in contact with the fibers [9][10][9,10].
2. Debate on ASA Sizing Mechanism in Papermaking
Prior to the introduction of ASA to the papermaking process in the 1960s, the additive was used in the textile industry to impart water repellency to cellulosic fabrics. Patent 2,903,382 by Robert Berls [11][20] in 1959 provided details on how to make hydrophobic cellulosic fabric using ASA. Different types of ASA with chains from 19 to 35 carbon atoms were dissolved in solvent such as isopropanol, benzene, toluene, chloroform, carbon tetrachloride, ammonia, morpholine, and water emulsions. The fabric was dipped into the resulting solution then heat cured. The recommended ASA concentration was from 0.7% to 2.5% (14 to 50 lb/t) of the weight of the fabric. The spray test method of the American Association of Textile Chemists and Colorists A.S.T.M. Designation: D583-54 was used to evaluate water repellency. There was no mention of esterification reaction nor covalent bonding in the claim. In addition, the ASA application is like wax application to textile fiber for hydrophobicity, as claimed in patent 2,759,851 in 1956 by Fluck, Pluckemin, and Logan [12][21]. In 1963, Wurzburg and Mazzarella [1] mixed ASA with different derivatives of starch to form an emulsion. It was claimed that the application of the ASA emulsion on fiber made the sheet hydrophobic. The patent demonstrated the sizing process with the use of the ASA emulsion and the addition of alum, aluminum chloride, long chain fatty amines, sodium aluminate, polyacrylamide, animal glue, polyamide polymers, primary amine starch derivatives, secondary amine starch derivatives, tertiary amine starch derivatives, and quaternary amine starch derivatives. The author recommended to use 0.5 to 2 parts by weight of cationic starch per 1 part of the sizing agent to obtain adequate results. Different dosages from 0.25% to 2% (5 to 40 lb/t) of the sizing agent were used in 14 different examples to support the claim. Uranine dye and ink dip tests were used to prove the hydrophobicity of the sheets. There was no disclosure of covalent or ester bonding between ASA and cellulose in the patent document. Cuculo [13][22] at NC State University tried to esterify succinic anhydride with cellulose in 1971. First, successful results were obtained from the reaction between viscose rayon cellulose and succinamic acid. The samples were baked in succinamic acid at 136 °C, 183 °C, and 207 °C, water-washed, and then treated with 3% sodium sulfate to form sodium cellulose-hemisuccinate. The degree of substitutions for the reactions were, respectively, 0.03, 0.24, and 0.25. The author concluded that the degree of the substitution of reaction depends strongly on the reaction temperature. Second, when succinic anhydride in water was used under comparable conditions to those of the succinamic acid, the author stated that there was no evidence of ester formation. The reported reaction yield with succinamic acid was 36%, and the author mentioned that ammonia copiously evolved during the reaction. The recommended temperature using the succinamic acid is above 150 °C. McCarthy and Stratton [14][23] studied the reaction between cotton linters pulp and ASA in 1986. In one study, the cotton linters and ASA were reacted in N,N-dimethyl formamide with triethyl amine as a catalyst. In another study, the cotton linter pulp (washed in chloroform-ethanol solution for 48 h and air dry for several hours) and a high concentration of ASA (1.5% or 30 lb/ton) were reacted. Poly(1,2-dimethyl-5-vinylpyridinium bromide), or DMVPB, was used as ASA retention aid. In both studies, FTIR data showed the formation of ester bonds, however the efficiency of these reactions was not reported. In McCarthy’s thesis in 1987 [15][24], the author showed by FTIR that ASA reacted with ethanol to form ester bonds. It is not known if part of the ASA formed ester bonds with ethanol rather than cellulose since the pulp was washed 48 h with chloroform-ethanol solution. Wan [16][25] studied the mechanism of ASA sizing in 1988 using C 14-labelled tetradecenyl succinic anhydride (TDSA) and tetradecenyl succinic acid (TDSAcid). TDSA and TDSAcid emulsions were made using starch as an emulsifier at a 1:3 ratio. The emulsions containing 1.3% total solid was charged to the pulp to make 60 g/m2 hand-sheets. The C14-labelled technique was used to quantify the ASA component in the sheets after chloroform extraction. The results showed that unreacted ASA is predominantly found in the sheet and about 25% of ASA can produce sizing and is not extractable with chloroform. According to the author, the retained ASA could be explained by covalent bonding due to three reasons. First, the continuous increasing in sizing at room temperature suggests the reduction in moisture allows the ASA to react with the hydroxyls of cellulose. Second, the ASA molecule, which is under constant reorientation during the sizing process, would undergo hydrolysis if at any time the hydrophilic part of the molecule is exposed to moisture. The retained molecule can be assumed to be held by stronger irreversible bond forces. Third, the inability of the ASAcid (hydrolyzed form of ASA in the dicarboxylic acid form) to size hand-sheets demonstrates that hydrogen bonding is not the mechanism of ASA sizing, thus covalent bonding must be the mechanism. The author concluded that this evidence suggests that unextractable ASA is bound to cellulose via covalent bonding. In addition, according to the author, solvent sized sheets gave better sizing than conventional emulsion sized sheets due to the absence of water that induces hydrolysis. Nishiyama et al. [17][26] used a series of extraction, impregnation, and cellulase treatment experiments to study the ASA bonding mechanism under common papermaking conditions in 1996. Pulp and ASA-starch emulsion containing 0.2% (4 lb/ton) ASA were used to make 60 g/m2 hand-sheets. Chloroform, water-acetone, and dimethyl sulfoxide (DMSO) were used to extract the hand-sheets and cellulase was used to isolate the ASA residues. The measurement of the extracts by gas chromatography showed that not all ASA was extractable, and the NMR data showed no presence of formed ester linkage between the ASA and the hydroxyl group of cellulose. When an old ASA emulsion, which has no chance to form ester linkages with cellulose, was used, the extraction results also showed not all ASA was extractable. The authors concluded that a small amount of ASA is physically entangled in the cellulose network without forming covalent bonds. Impregnation of filter papers with acetone solutions of ASA, non-reactive ASAcid, and non-reactive ASAcid methyl esters were also studied. Sizing occurred in all cases, but no ester linkages between ASA and cellulose-OH were found to exist. To ensure that ester bonds were not formed then destroyed by the cellulase isolation and analytical techniques employed, the tests were repeated using cellulase treatment stable conditions. These conditions ensured any ester linkages between cellulose-OH and ASA would not be cleaved during isolation and analysis. These tests were repeated using sample A which is ASA-alum-sized hand-sheets, sample B which is ASA-PAE-sized hand-sheets, and sample C which is PAE-treated hand-sheets. The residues after the enzymatic treatment were 1.4, 0.7, and 1.1% for the samples A, B, and C, respectively. The analysis of the residues showed the samples A and B contain 10% and 15% ASA, respectively. Further analysis of the residues of samples A and B was conducted; the FTIR data showed that most ASA components do not form ester bonds with cellulose, and the predominant components of ASA in the sheets A and B are in the form of ASAcid. The author concluded after the extraction, impregnation, and cellulase treatment studies that the ASA sizing mechanism should not be explained by the formation of ester linkages. In 2000, Akira [18][27] studied the extracts of ASA-sized hand-sheets with ASA content of 2.2 mg/g. Pulp and ASA-starch emulsion containing 0.2% ASA was used to make the 60 g/m2 hands-sheets. A series of extractions using water, chloroform, 1% Tween 80 at 20 °C, and 1% Tween 80 at 70 °C were performed. The extracted sheets were analyzed with pyrolysis gas chromatography–mass spectrometry (GCMS). The amount of ASA retained in the sheet was respectively 1.7 mg/g, 0.6 mg/g, 0.3 mg/g, and almost zero for the listed extraction conditions, respectively. According to the author, the extraction results showed that virtually no covalent bonds between ASA and hydroxyl groups of cellulose were present in the hand-sheet. These results also indicated that chloroform is not a suitable solvent to completely extract ASA even though the ASA is present only by physical interactions without forming covalent bond with cellulose. Moreover, to understand the efficient state of the ASA, three types of hand-sheets containing 0.4% (8 lb/t) ASA were made with different ASA emulsions: fresh emulsion, fresh emulsion and pulp stirred for 3 days, and 3-days-old emulsion. Only the fresh emulsion paper exhibited a sizing effect. SEM data showed large flocs of ASAcid in the two sets of sheets made with either stirred or old emulsion. The set made with fresh emulsion had well dispersed ASA contained within the sheet. The author concluded that fresh ASA emulsion spread over the fiber surface and promotes hydrophobicity. In 2002, Yu and Garnier [19][28] concluded from their work that ASA and cellulose are covalently bonded. In the experiment, cellulose film was regenerated from cellulose acetate on glass. ASA vapor was deposited on the substrate in an airtight adsorption cell, which was heated in an oven at a preset temperature and then quenched. The contact angle was measured immediately, and the measurement revealed the substrate was hydrophobic. According to the authors, the presence of the OH groups, the temperature dependence of the reaction, the non-decrease in hydrophobicity (even after chloroform extraction), and the time frame (hour) of the reaction are typical for the formation of covalent bond by esterification. Acha et al. [20][29] studied the reaction between ASA and wood flour in 2003. The esterification reaction was carried out by immersing the wood flour with an average diameter of 57 μm in 96 g/L (9.6% or 192 lb/ton) ASA in acetone solution; 4-dimethylaminopyridine was used as a catalyst. The mixture was refluxed at 56.5 °C for 4 h. The product was dried at 70 °C in an oven and later washed intensively with distilled water to remove unreacted reagents before it was dried again at 70 °C under vacuum to constant weight. The esterified product was later mixed with unsaturated polyester (UPE) based on bisphenol A-fumarate cross-linked with styrene for molding; benzoyl peroxide was used as an initiator, and polymethyl methacrylate was used as a thermoplastic modifier. Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), a saponification test, and an acid value test were used to analyze qualitatively and quantitatively the esterified products. The results showed that esterification did occur. The yield was 166 g ASA for 1 kg treated wood flour (16.6%). In 2006, Wang [21][30] studied ASA sizing mechanism by comparing FT-IR spectra between ASA-sized hand-sheets, synthesized ASA-cellulose compound, hydrolyzed ASA, hydrolyzed ASA-sodium salt, hydrolyzed ASA-calcium salt, and hydrolyzed ASA-aluminum salt. The amount of ASA charged to the studied hand-sheets was 0.5% or 10 lb/t oven-dried pulp. The results showed no covalent bonding between the ASA and the cellulose. In addition, the results suggested that the sizing material found in ASA-sized hand-sheet was mainly hydrolyzed ASA and its salt. The paper also demonstrated that to size hand-sheets directly with hydrolyzed-ASA emulsion resulted in low sizing degree. Nevertheless, after dipping hydrolyzed-ASA-sized hand-sheets in an alum solution, the sizing degree could be brought up to the level close to ASA emulsion sized paper. Hundhausen et al. [22][31] studied the reaction of ASA and particleboard chips in 2010. The objective was to investigate if ASA bonds to the surface of the chips to repel water. The chips were dried to 1% moisture content and then were wetted with 3% or 60 lb/t ASA (based on the oven dry wood). The samples were processed in a rotating reactor at 130 °C and 1013 mbar for 1 h at 12 rpm. FTIR analysis before and after the treatment revealed that esterification did not occur and both hydrolyzed and non-hydrolyzed ASA were located on the chip surface after curing in the reactor. In addition, FTIR measurement of the chips after Soxhlet extraction did not show any ASA signal, which indicated that the ASA did not esterify and was completely removed by the solvent during the extraction. Furthermore, ASA-ethyl acetate and ASA dimethyl sulfoxide (DMSO) solutions were applied to veneers. It turned out that the ASA was completely removed after extraction when it was applied in ethyl acetate but not completely in DMSO; the covalently bound ASA could not be extracted. The unextractable amount, or the yield of the reaction, is not known. According to the authors, the ASA esterification with cellulose depends on the solvent that provides certain mobility and orientation to the sizing agent. DMSO leads to esterification but its high boiling point (189 °C) and its poor miscibility with ASA make it disadvantageous. Water emulsions would hamper the ASA penetration into the wood; xylene is a better candidate but harmful for human health. Masumi et al. [23][32] used Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) to study the ASA sizing mechanism in 2012. ToF-SIMS is a surface-sensitive analytical method that uses a pulsed ion beam to remove molecules from the very outermost surface of the sample. ToF-SIMS collected on an ASA-sized paper sample was compared to the spectra collected from ASA, ASA/water mixture, ASA/NaOH solution, cellulose, and synthesized ASA-cellulose compound. The results indicated that the form of ASA in ASA-sized paper was different from that in ASA-cellulose compound, which did not support an ester bonding hypothesis. The ToF-SIMS from ASA/NaOH solution was close to that from ASA-sized paper. This suggested the ASA in ASA-sized paper was likely hydrolyzed ASA or its salt. In 2015, Li et al. [24][33] studied the anchorage of ASA on cellulose by dipping cellulose film and filter paper in both acetone-ASA solution and ASA emulsion. The cellulose film was generated by immersing cellulose acetate film in 0.5% sodium methoxide in methanol for 12 h. The film was repeatedly rinsed with running deionized water and methanol, air-dried, and stored in a desiccator. The filter paper used was a commercial ash-free filter paper. The ASA-acetone dipping solution contained 1% (20 lb/ton) of ASA and the ASA emulsion, which also contained 1% ASA, was prepared by using laponite as particle stabilizers after being modified by urea, alanine, tetramethylammonium chloride and melamine or in combination with poly-aluminum sulfate (PAS). The FTIR and the XPS results showed the presence of covalent bonds between ASA and cellulose. The authors concluded that sizing is due to the formation of the ester bond. The ASA-acetone sizing solution performed better than the ASA emulsion co-stabilized by laponite and PAS due to the absence of water, which tends to produce hydrolyzed ASA more easily. Although the yield of the esterification is not known, the authors suggest that the amount of extractable ASA species in the paper is higher than the amount anchored. In addition, laponite and PAS co-stabilized emulsions lead to high sizing performance but greatly depend on the use of aluminum sulfate. According to the author, the added aluminum sulfate may improve the retention of ASA or anchor the hydrolyze ASA to the cellulose. Lackinger et al. [25][34] attempted to provide proof in favor or against covalent binding of ASA to cellulose in 2015. To avoid the interaction of starch, filter paper was soaked in acetone solutions with various levels of ASA (blank, 1, 5, 10, and 25%) and dried under different conditions (room temperature, drum dryer at 115 °C, drum dryer at 115 °C, and oven at 125 °C). The COOH-selective fluorescent labeling for cellulosic material and the gel permeation chromatography (GPC) with a multi-detector setup for covalently bound sizing agent detection approach was used to provide profiles of carboxyl groups along the molecular weight distribution. The result showed that only about 0.5% of the total ASA covalently bonded to cellulose. Furthermore, hand-sheets were made with 0.2% ASA emulsion in starch, and the correlation between the small amount of covalently bonded ASA and the sizing efficiency of the sheets were studied. The results indicated that there was no correlation between the amount of covalently bonded ASA and sizing efficiency. The authors concluded from the two sets of experiments that ester bonds between ASA and cellulose were a small fraction and were not a prerequisite for sizing. In 2016, Porkert [26][35] worked on the localization of ASA in the sheet using confocal white light microscopy and the dye Sudan Red 7B. Preliminary experiments using thin layer chromatography and FTIR were conducted to ensure that the dye does not affect the processability and the performance of ASA and ASA emulsions. Dyed ASA dosages of 0%, 0.05%, 0.1%, 0.2%, 0.3%, and 0.4% were applied to 100 g/m2 hand-sheets and the shades were correlated to each value. Different sheets were mapped to study the relation between size performance and the homogeneity of the ASA distributions under different dosages and conditions. Some of the conditions are reactive ASA emulsion, cationic and anionic hydrolyzed ASA emulsions, ASA-Starch ratio, and emulsion age. The study revealed that the ASA sizing mechanism depends on the distribution of the ASA in the sheet. There is a direct correlation between the agglomeration of ASA and sizing performance: the more agglomerates, the weaker the sizing. The application of reactive ASA led to uniform distribution, which results in better sizing performance. Cationic and anionic hydrolyzed ASAs have higher agglomerates thus low sizing performance. Better size distribution and performance were obtained at ASA/starch ratio of 1:1 for low dosage of 0.05% (1 lb/ton) ASA. Concerning the aging, the first 20 min gave the best performance for low and high dosages. According to the author, the distribution of ASA supports the sizing mechanism based on homogenous molecular distribution and orientation. Hydrophobization is solely based on the physical distribution and orientation of the ASA molecules within the sheet. In addition, the application of reactive ASA is the key element to obtain homogenous distribution as the application of the hydrolyzed ASA led to agglomerations. Furthermore, the esterification reaction between ASA and cellulose is very unlikely to happen during the papermaking process, and the phenomena of size reversion, size reactivation, and size migration exclude any significant extent of esterification but are only explainable by intra- and inter-molecular mobilities. The objective of Wulz [27][36] in 2020 was to hydrophobize the surface of paper by vapor deposition of ASA, palmitoyl chloride, TFAA/Ac2O (trifluoroacetic anhydride/acetic anhydride), and TFAA/Acetic acid mixtures and hexamethyldisilazane (HMDS). Unsized and untreated papers were used for the experiment. The papers were stored at 23 °C and 50% humidity for at least 24 h. The gas phase ASA deposition was performed at 50 °C and 100 °C at 20 mbar for 2 h. The experiment with ASA did not lead to hydrophobicity, however the experiment with other additives led to hydrophobicity via formation of ester bonds. Due to the poor hydrophobization of the ASA gas phase deposition, no further research was carried out with ASA, and no FTIR data were collected. There is no doubt that ASA sizes paper. However, the sizing mechanism, especially the covalent bond formation between the ASA and cellulose is a divergence point among scientists, researchers, and papermakers. The covalent bond theory is often used by vendors to explain sizing development. However, the assessment of the available data over the last 60 years shows that the formation of covalent bond is insignificant in ASA-sized paper. Hydrolyzed ASA and or ASA salts are the fundamental elements found in sized paper. Catalysts and or organic solvents can be used to initiate esterification, but such conditions are unrealistic in papermaking.3. Overview of the ASA Mechanism
A summary of the proposed reaction mechanisms for ASA sizing suggest that multiple potential pathways exist. The first proposed sizing mechanism is bonded ASA molecules to cellulose via esterification. Though some research using organic solvent, high temperature, or catalyst showed the evidence of ester bond formation, most studies conducted close to commercial papermaking conditions yielded scientific evidence supporting hydrolyzed ASA as the major sizing material in a sized paper. However, the direct application of hydrolyzed ASA to the pulp or the application (coating) of hydrolyzed ASA to the paper does not achieve sizing. Thus, the type of sizing material and the uniform distribution or structure of the sizing material significantly contributes to sizing development [17][18][26][28][26,27,35,37]. In commercial paper mills, common ASA application consists of preparing the ASA emulsion, which is later added to the pulp at a point close to the fan pump. The mixture is moved to the headbox where the average consistency of the pulp is about 1%, and the predominant component is water, not organic solvent. Although other materials such as alum, PAE (Polyamide Epichlorohydrin), GCC (Ground Calcium Carbonate), CPAM (cationic polyacrylamide), etc., are added to the furnish depending on the paper grade and the papermaker, the predominant component remains water, and the chance of the formation of hydrolyzed ASA is high. The slurry is dewatered then dried in the dryer section of the machine to develop sizing, i.e., the resistance to liquid penetration of the sheet. The commercial papermaking process is more complex than laboratory hand-sheet making, and the chance of covalent bond formation is even lower than the already low laboratory results due to the following reasons:-
One additive (ASA) is usually studied in the laboratory to prevent the influence of other variables while in real papermaking processes many organics, inorganics, additives and even bacteria compete with ASA to dwell with the fiber. In short, there are more uncontrolled variables in field experiments than in lab ones [30][31][39,40].
-
Lastly, in laboratory studies, sometimes the pulp or the substrate has a special treatment not found in the mill. Such treatment can be ethanol or methanol wash of the pulp or the substrate, although it is well known that these solvents esterify with ASA, and their leftovers in the pulp can mislead the interpretation of FTIR results [14][17][24][32][33][23,26,33,41,42].
4. Chemistry Considerations for Better ASA Sizing Application
Sizing performance is a function of size distribution, retention, and development. For an ASA sizing program, though there was debate on the sizing development mechanism, a few researchers agreed that better ASA emulsion stability and higher retention improves sizing results [18][21][28][40][41][42][27,30,37,49,50,51]. Some chemistry considerations for better ASA stability and retention are provided herein.-
Emulsion Stability (better distribution)
-
Emulsion Shelf Life
-
Emulsion Retention
