Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Dung Tuan Hoang.
The UN Climate Change Conference in Glasgow (COP26) has stressed that stakeholders need to work together to achieve a NetZero target. Technologies involving absorbents for the capture of CO2 from a gas mixture are energy-intensive. Carbon adsorption and conversion (CAC) approaches have been gaining attention since these technologies can mitigate CO2 emissions.
- carbon-based materials
- CO2 adsorption
- CO2 conversion
- fossil fuel
- climate change
- global issues
1. Carbon-Based Materials for CO2 Conversion
The global climate is changing unprecedentedly, and the primary reason behind this catastrophic change is the significant rise in global CO2 emissions. It is critically important to lower the CO2 concentration in the atmosphere, and this can be accomplished by CO2 conversion technology where carbon-based materials are used. CO2 conversion refers to the process of converting CO2 into useful chemicals and fuels, and some common conversion methods are illustrated in Figure 1:
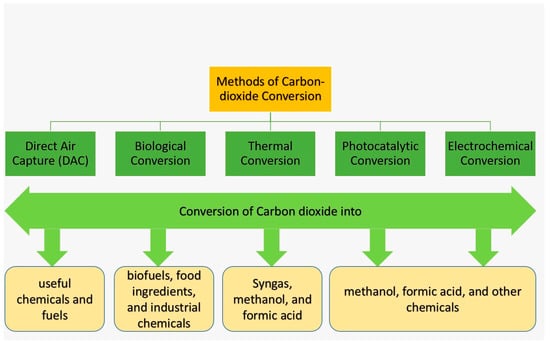
Figure 1.
CO
2
conversion methods.
-
Direct Air Capture (DAC): This is a potential technology that directly captures CO2 from the air, and then converts it into useful chemicals and fuels.
Different types of materials are potentially used for CO2 conversions into useful chemicals and fuels, including carbon-based materials, namely activated carbon, graphene, and carbon nanotubes. These materials possess a high surface area and can act as catalysts, making them promising for use in CO2 reduction reactions. However, more research is needed to optimize their performance and make the process more efficient and cost-effective.
1.1. Graphene
Graphene has been studied for its potential in converting CO2 into useful chemicals and fuels. One way for CO2 conversion using graphene is through graphene-based catalyst usage, which can facilitate chemical reactions that convert CO2 into CH3OH or formic acid. Another application is membranes synthesized from graphene, which can selectively separate CO2 from other gasses. Graphene has several qualities that make it a promising material for CO2 conversion, and some of them are as follows [77][5]:
-
High surface area: This characteristic makes graphene useful for catalytic reactions;
-
High conductivity: Graphene is an excellent conductor of electricity and heat, which makes it useful in electrochemical reactions;
-
Chemical stability: Graphene is chemically stable, and can be used in harsh environments and high-temperature reactions;
-
Selectivity: Graphene membranes can be made to be highly selective, which makes graphene useful for CO2 separation;
-
Low cost: Graphene is made of carbon, which is abundant and inexpensive;
-
Durability: Graphene is a strong and durable materials that can be used in long-term applications. These qualities make graphene a highly promising material for CO2 conversion.
1.2. Carbon Aerogels (CAs)
These materials are highly porous, lightweight, have a high surface area, are usually made from carbon nanostructures, and have been shown to have the potential for CO2 conversion [49,77][5][6]. Their high surface areas allow these materials to adsorb large amounts of CO2, and they, therefore, can be used in a variety of chemical reactions to convert CO2 into other compounds [77][5].
One of the main ways ACs have been used for CO2 conversion is in the form of adsorbents. CO2 adsorption on carbon aerogels can be done through physisorption or chemisorption processes, which are then followed by the release of CO2 by heating or by changing the pressure.
High surface area: CAs possess a very high surface area, typically from 300 to 2000 square meters per gram. This property allows them to adsorb large amounts of gases, liquids, and other substances [78][7];
-
Low density: CAs are extremely lightweight, with densities as low as 0.003 g per cubic centimeter;
-
High thermal conductivity: CAs have high thermal conductivity, which makes them useful for thermal insulation and heat dissipation;
-
High electrical conductivity: CAs can be made to be highly conductive, which makes them useful in applications such as super-capacitors and batteries;
-
Porous structure: CAs have a highly porous structure and large pore volumes, which allows for the easy diffusion of gases and liquids;
-
Low cost: CAs can be made from inexpensive, abundant materials, and their production process is relatively simple, which makes them a cost-effective material. However, these properties can vary depending on the specific type of carbon aerogel and how it was manufactured;
1.3. Activated Carbons (ACs)
Activated carbons, usually known as activated charcoal, are carbon-based materials with a highly porous surface area which makes them useful for CO2 conversion. ACs can also be used as a catalyst to convert CO2 into other chemical compounds. For example, they have been used to catalyze the conversion of CO2 into formic acid and methanol, which are useful chemicals for other industrial processes [81][10].
ACs can be used as a CO2 adsorbent, which involves both the physical and chemical adsorption processes. The CO2 can then be released by heating or by changing the pressure, making it possible to capture and store CO2 in this way. ACs are particularly effective at adsorbing CO2 at high pressures and temperatures, making them a potential material for capturing CO2 from industrial processes [82][11].
Though those materials are promising in CO2 conversion at research and lab scales, the CO2 conversion performance of those materials at industrial scales still need to be tested and verified.
Yellow tuff, a natural tuff, and low-cost adsorption material, has been reported to be conveniently employed in a vacuum swing for CO2 adsorption processes [83][12]. MOFs are proving to be effective adsorbents for CO2 capture due to their microporous structure and chemical and thermal stabilities [84][13]. MOFs are capable of providing both physical and chemical interactions with CO2 [83][12], while some other chemical adsorbents, such as amine-functionalized adsorbents, are capable of interacting strongly with the acidic CO2 molecules [83][12]. Zeolites have been shown to have significant potential for CO2 adsorption due to their high porosity, ultra-small pores, structural diversity, high stability, excellent recyclability, and chemical reactivity [35][14].
2. Carbon Capture Technologies for Climate Change Mitigation
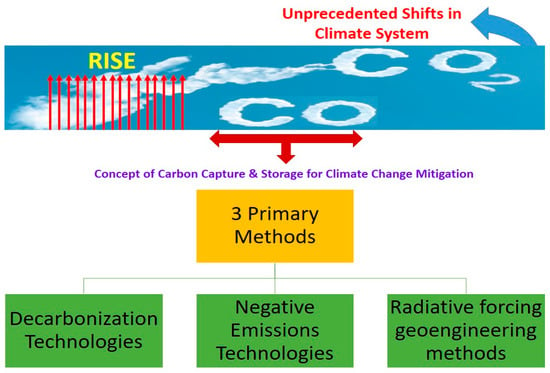
Figure 2.
CO
2
conversion methods for climate change mitigation.
Direct Air Carbon Capture Technology
One of the emerging technologies for artificially removing CO2 from the atmosphere is direct air carbon capture and storage (DACCS). This technology uses molecular bonding to extract CO2 from the atmosphere, and it is then either kept in geological reservoirs or put to some other use, such as in the production of compounds or mineral carbonates adsorption. The stability of CO2 storage is a major issue for this technology as well, just as it is for carbon capture and storage and bioenergy carbon capture and storage. Capturing CO2 from atmospheric air is much more challenging than capturing CO2 from highly concentrated combustion gas streams since direct air carbon capture and storage requires more energy and materials. If unforeseen issues with large-scale implementation can be resolved, the global capacity for CO2 removal will be between 0.5 and 5 GtCO2 per year by 2050 and could increase to 40 GtCO2 per year by the end of the century. The cost of CO2 removal is estimated to run from USD 600 to 1000 per metric ton of moved (tCO2) at the beginning but to drop to USD 100 to 300 per metric ton of CO2 removed in the future [94][24]. Several companies have developed and implemented direct air capture technology at an industrial scale. These companies include Carbon Engineering, Climeworks, Global Thermostat, Hydro Cell, Sky Tree, and Infinitree. Carbon Engineering has constructed a DAC plant located in the Permian Basin in Texas, USA, with a capture capacity of 1 MtonCO2/year [95][25]. There are no legislative instruments in place to aid this technology, as is the case with many of the other negative emissions options that have been investigated. The direct air capture of CO2 by physisorbent materials, namely TEPA-SBA-15 (amine-modified mesoporous silica, chemisorbent, and Zeolite 13X (inorganic), HKUST-1, Mg-MOF-74/Mg-dobdc, and SIFSIX-3-Ni (a hybrid ultra-microporous material including four physisorbents), were found to be able to capture carbon from CO2-rich gas mixtures [96][26]. The greenhouse gas removal efficiency was 79–91%, while in the best case, the removal efficacy can be as high as 97% [97][27]. However, competition and reaction with atmospheric moisture significantly reduced the direct air capture (DAC) performance [96][26], and the drawback of DAC lies in the significant amount of energy required for capturing CO2 from the atmosphere [98][28]. Amine-based cellulose adsorbents or silica gel are normally used in the DAC system [99,100][29][30]. An estimate of US$610–780/tC CO2 was reported for the facility using sodium hydroxide, which is a strong base [95][25]. The thermal energy required for a DAC adsorption system is between 1400 and 2000 kWhth/ton CO2 [100][30].3. Carbon Recycling through CO2 Conversion
References
- Muraza, A.G.O.J.R.; Galadima, A. Reviews, Catalytic thermal conversion of CO2 into fuels: Perspective and challenge. Renew. Sustain. Energy Rev. 2019, 115, 109333–109353.
- Bhattacharya, A.; Selvaraj, A. Photocatalytic conversion of CO2 into beneficial fuels and chemicals—A new horizon in atmospheric CO2 mitigation. Process Saf. Environ. Prot. 2021, 156, 256–287.
- Malkhandi, S.; Yeo, B.S. Electrochemical conversion of carbon dioxide to high value chemicals using gas-diffusion electrodes. Curr. Opin. Chem. Eng. 2019, 26, 112–121.
- Chakraborty, S.; Ponrasu, T.; Chandel, S.; Dixit, M.; Muthuvijayan, V. Reduced graphene oxide-loaded nanocomposite scaffolds for enhancing angiogenesis in tissue engineering applications. R. Soc. Open Sci. 2018, 5, 172017.
- Milow, B. Carbon Aerogels Synthesis, Properties and Applications. In Proceedings of the Department Chemistry and Physics of Materials at the Paris-Lodron University Salzburg, Salzburg, Austria, 12 September 2019.
- Kwiatkowski, M.; Broniek, E. An analysis of the porous structure of activated carbons obtained from hazelnut shells by various physical and chemical methods of activation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 443–453.
- Hsieh, T.-H.; Huang, Y.-S.; Shen, M.-Y. Mechanical properties and toughness of carbon aerogel/epoxy polymer composites. J. Mater. Sci. 2015, 50, 3258–3266.
- Pinelli, F.; Piras, C.; Rossi, F. A perspective on graphene based aerogels and their environmental applications. Flatchem 2022, 36, 100449.
- Ramezanipour Penchah, H.; Ghaemi, A.; Jafari, F. Piperazine-modified activated carbon as a novel adsorbent for CO2 capture: Modeling and characterization. Environ. Sci. Pollut. Res. 2021, 29, 5134–5143.
- Pellerano, M.; Pré, P.; Kacem, M.; Delebarre, A. CO2 capture by adsorption on activated carbons using pressure modulation. Energy Procedia 2009, 1, 647–653.
- Ammendola, P.; Raganati, F.; Chirone, R.; Miccio, F. Fixed bed adsorption as affected by thermodynamics and kinetics: Yellow tuff for CO2 capture. Powder Technol. 2020, 373, 446–458.
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849.
- Hoang, T.-D.; Nghiem, N. Recent developments and current status of commercial production of fuel ethanol. Fermentation 2021, 7, 314.
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251.
- Bustreo, C.; Giuliani, U.; Maggio, D.; Zollino, G. How fusion power can contribute to a fully decarbonized European power mix after 2050. Fusion Eng. Des. 2019, 146, 2189–2193.
- Palmer, C. Mitigating Climate Change Will Depend on Negative Emissions Technologies; Elsevier: Amsterdam, The Netherlands, 2019.
- Yan, J.; Obersteiner, M.; Möllersten, K.; Moreira, J.R. Negative Emission Technologies–NETs; Elsevier: Amsterdam, The Netherlands, 2019; p. 113749.
- Goglio, P.; Williams, A.; Balta-Ozkan, N.; Harris, N.; Williamson, P.; Huisingh, D.; Zhang, Z.; Tavoni, M. Advances and challenges of life cycle assessment (LCA) of greenhouse gas removal technologies to fight climate changes. J. Clean. Prod. 2020, 244, 118896.
- Lin, A.C. Carbon dioxide removal after Paris. Ecol. Law Q. 2019, 45, 533–582.
- Pires, J. Negative emissions technologies: A complementary solution for climate change mitigation. Sci. Total Environ. 2019, 672, 502–514.
- Shinnar, R.; Citro, F. Decarbonization: Achieving near-total energy independence and near-total elimination of greenhouse emissions with available technologies. Technol. Soc. 2008, 30, 1–16.
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97.
- Ma, X.; Zhang, X.; Tian, D.J. Farmland degradation caused by radial diffusion of CO2 leakage from carbon capture and storage. J. Clean. Prod. 2020, 255, 120059.
- Tcvetkov, P.; Cherepovitsyn, A.; Fedoseev, S. Public perception of carbon capture and storage: A state-of-the-art overview. Heliyon 2019, 5, e02845.
- Arning, K.; Heek, J.O.-V.; Linzenich, A.; Kaetelhoen, A.; Sternberg, A.; Bardow, A.; Ziefle, M. Same or different? Insights on public perception and acceptance of carbon capture and storage or utilization in Germany. Energy Policy 2019, 125, 235–249.
- Aresta, M.; Dibenedetto, A.; Pastore, C. Biotechnology to develop innovative syntheses using CO2. Environ. Chem. Lett. 2005, 3, 113–117.
- Leonzio, G.; Fennell, P.S.; Shah, N. Analysis of technologies for carbon dioxide capture from the air. Appl. Sci. 2022, 12, 8321.
- Gambhir, A.; Tavoni, M. Direct air carbon capture and sequestration: How it works and how it could contribute to climate-change mitigation. One Earth 2019, 1, 405–409.
- Kumar, A.; Madden, D.G.; Lusi, M.; Chen, K.-J.; Daniels, E.A.; Curtin, T.; Perry, J.J., IV; Zaworotko, M.J. Direct air capture of CO2 by physisorbent materials. Angew. Chem. Int. Ed. 2015, 54, 14372–14377.
- Terlouw, T.; Treyer, K.; Bauer, C.; Mazzotti, M. Life cycle assessment of direct air carbon capture and storage with low-carbon energy sources. Environ. Sci. Technol. 2021, 55, 11397–11411.
- Terlouw, T.; Bauer, C.; Rosa, L.; Mazzotti, M. Life cycle assessment of carbon dioxide removal technologies: A critical review. Energy Environ. Sci. 2021, 14, 1701–1721.
- Elfving, J.; Bajamundi, C.; Kauppinen, J. Characterization and performance of direct air capture sorbent. Energy Procedia 2017, 114, 6087–6101.
- Leonzio, G.; Mwabonje, O.; Fennell, P.S.; Shah, N. Environmental performance of different sorbents used for direct air capture. Sustain. Prod. Consum. 2022, 32, 101–111.
- Aresta, M.; Dibenedetto, A. Carbon Recycling Through CO2-Conversion for Stepping Toward a Cyclic-C Economy. A Perspective. Front. Energy Res. 2022, 8, 159.
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208.
More
