You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Sergio Arthuro Mota-Rolim.
Sleep is not only a whole-brain process but also a complex local phenomenon controlled by specific neurotransmitters that act in different neural networks, which is called “local sleep”. Moreover, the basic states of human consciousness - wakefulness, sleep onset (N1), light sleep (N2), deep sleep (N3), and rapid eye movement (REM) sleep - can concurrently appear, which may result in different sleep-related dissociative states.
- dissociative states
- sleep dissociation
- daydreaming
- lucid dreaming
- false awakenings
- sleep paralysis
- sleepwalking
- REM sleep behavior disorder
- hypnosis
- anesthesia
- psychedelics
1. Introduction
Contemporary works on sleep regulation proposed the term “local sleep” to define physiological characteristics that can be found in specific regions of the brain during the basic states of consciousness, i.e., wakefulness, rapid eye movement (REM) sleep, and non-REM sleep (sleep onset - N1, light sleep - N2, deep sleep - N3) [1,2,3,4][1][2][3][4]. These physiological states of consciousness tend to cycle in a stable manner; however, the transitions among these states are gradual and inconsistent, with the concomitant presence of multiple state-determining biomarkers. Accordingly, this may result in mixed states in which sleep and wakefulness occur asynchronously in different regions of the brain, resulting in the so-called sleep-related dissociative states [5].
2. Physiological States of Consciousness
2.1. Daydreaming
Singer and McCraven [6] defined daydreaming (or mind wandering) as a partial detachment from the reality around, directing consciousness towards the own imagination with personal content. Although this state of consciousness is part of human nature and an everyday experience, there is still little systematic knowledge about its frequency, duration, spontaneity of appearance, variations, and patterns. Researchers have hypothesized about the advantages and importance of daydreaming, considering that it occupies more than half of the time we are awake [7]. According to these authors, daydreaming may serve for (1) Adaptive function: allowing for planning future activities, creativity, attentional cycling, and resting [8]; (2) Event simulation: there is a correlation between self-reflection, autobiographical memories, and task performance, suggesting that daydreams (as well as night dreaming) may simulate future events [9]; (3) Interpersonal relationships: allowing the development of interpersonal skills, such as compassion, moral reasoning, empathy, etc. [10]; (4) Volitional processes: one can choose to detach from the reality around to focus on one’s own thoughts [11]; (5) Personal goals: self-awareness, personal planning, goal-directed thinking, simulating another person’s perspective, and compassion [7]; (6) Mental health: daydreaming components such as imagination and fantasy support mental health, allowing the mind to varying its attentional content [7]. Singer [12] divided daydreams into three types: positive-constructive daydreaming, guilty-dysphoric daydreaming, and poor attentional control. positive-constructive daydreaming consists of carefree, positive desires and plans, such as imagining inviting a friend to dinner or being in a park with your pet. Guilty-dysphoric daydreaming is about worries and undesirable scenes, such as revenge, and undesirable experiences, such as deaths and separations. Poor attention control is not necessarily related to the emotional charge but rather an obstacle to the individual’s attention. These daydreaming experiences were studied in different ages, ethnicities, and genders [13,14][13][14].2.1.1. Self-Generated Thoughts, Daydreaming, and Depression
Self-generated thoughts are intrusive imaginations, ideas, and desires that are not directly related to environmental stimuli and may arise from daydreaming [16][15]. Self-generated thoughts are associated with loss of reading comprehension [17][16], sustained attention capacity [18][17], and working memory [19][18]. Self-generated thoughts characterized by predominantly negative thoughts correlate with mental diseases, such as depression, anxiety, schizophrenia, and other pathological states of consciousness [20][19]. The tendency to fall into negative daydreamings, such as ruminating thoughts, correlates with depression; daydreaming can not only be related to depression but also self-generated thoughts can be a symptom of depression [21,22][20][21]. It was shown that this relationship between daydreaming and depression was more due to the type of daydreaming, that is, always ruminating on the same thoughts [18,23][17][22]. In this sense, it was found that the greater the focus and attention to the own thoughts, the greater the rumination and, consequently, the more depressed mood [23][22].2.1.2. Daydreaming and the Default Mode Network
The default mode network (DMN) is a brain circuit that is activated when a person does not direct attention to environmental stimuli, during REM sleep, or during daydreaming [26,27][23][24] (Figure 1). In other words, the regions of the brain that constitute the DMN are more active in the absence of external stimuli and sustained attention [28][25], which means that dreaming and imagination processes share neurophysiological similarities [9].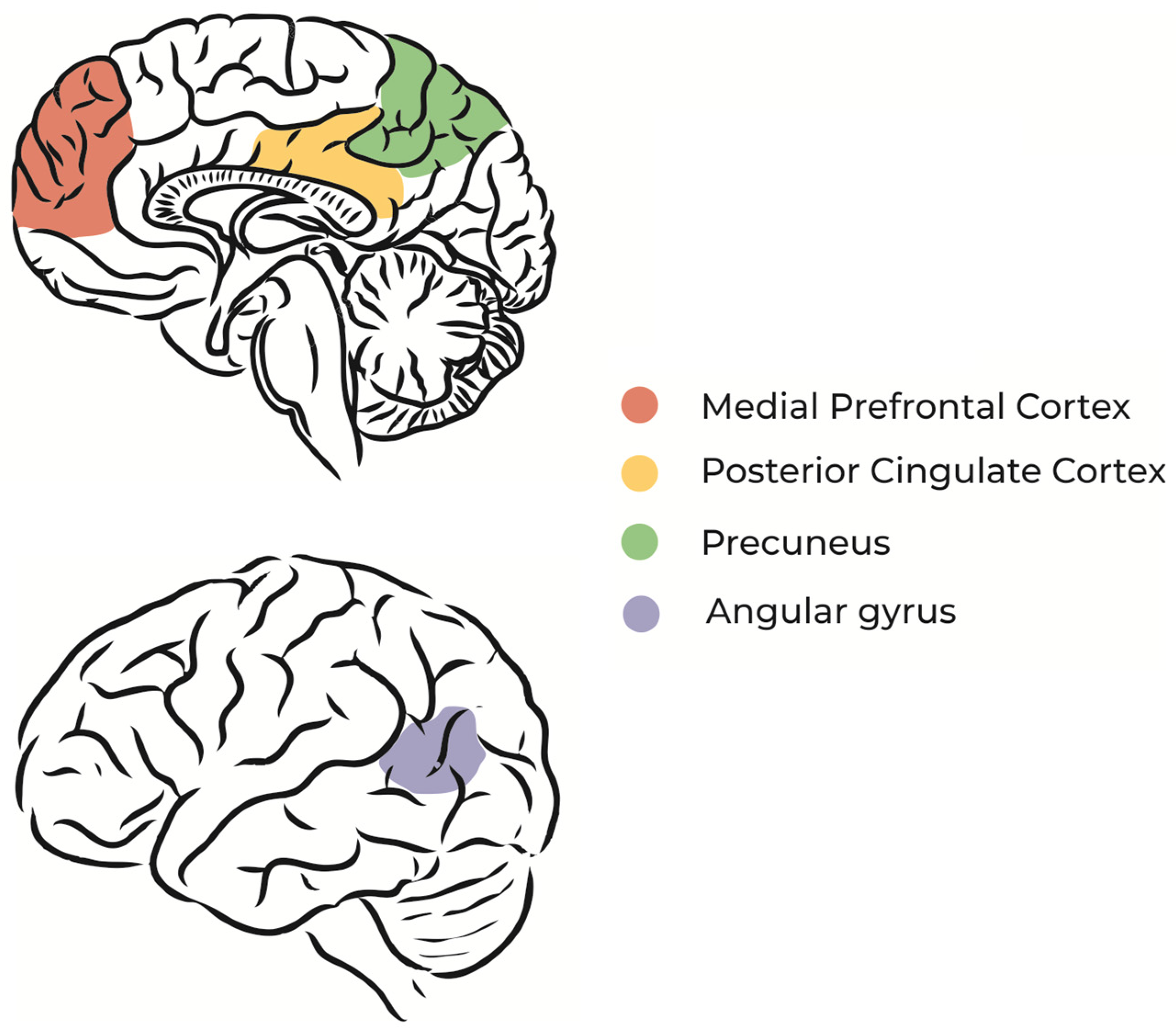
Figure 1.
Schematic illustration of DMN main regions.
Specific regions of the DMN, such as the dorsomedial prefrontal cortex and medial temporal lobe, are associated with thoughts about oneself (self-referential thoughts) and the construction of future scenarios based on memories [29][26]. Studies using functional magnetic resonance imaging during unconscious states, such as sleep and coma, found that the spatial organization of neural networks is similar to that of the resting state [30,31][27][28].
2.1.3. Daydreaming and Brain Rhythms
Even in tasks that require focus, shifts in the attentional focus to internal issues often occur; therefore, changes in the rhythms of the neural networks can be expected. Brabosczc and Delorme [34][29] analyzed the electroencephalographic signals of 12 volunteers who performed a breath-counting test and were supposed to press a button whenever they realized they had another thought occupying their minds. In these moments of concentration break, there was an increase in the frequency power of the slower theta (4–7 Hz) and delta (2–3.5 Hz) waves, whereas the frequency of the faster alpha (9–11 Hz) and beta (15–30 Hz) waves decreased. Therefore, daydreaming is associated with less vigilance and alertness, similar to sleep [35][30].

2.2. Lucid Dreaming
Lucid dreaming (LD) is a specific type of dreaming characterized by the awareness that one is dreaming and the capacity to control the oneiric content [39][31]. LD has been mentioned in several religions and by many philosophers for thousands of years [40,41][32][33]. However, more recently, some authors were skeptical about this dream phenomenon, arguing that individuals were actually awake or had some kind of misperception [42][34], which might be due to the lacking evidence of LDs as genuine subjective experiences at that time. This changed with the pioneering work of Stephen Laberge in 1980. This was based on prior research showing that: (1) eye muscles are exempt from the muscular atonia that accompanies REM sleep [43,44][35][36] and (2) direction shifts of the gaze within a dream can be accompanied by real movements of the sleeper’s eyeballs [45,46][37][38]. Laberge and colleagues thus asked lucid dreamers to move their eyes in a pre-agreed sequence (left–right movements, for example) as soon as they became lucid in the dream. This technique succeeded and laid the foundation for the scientific studies of LD [47][39].2.2.1. Lucid Dreaming, REM Sleep, and the Brain Rhythms
LD occurs predominantly during REM sleep [48,49][40][41]. LD has also been reported during the N1 (sleep onset) and N2 (light sleep) stages but not during the N3 stage (deep sleep) [50,51][42][43]. As for the ordinary non-LD, the LD during non-REM sleep stages 1 and 2 tends to be shorter, less vivid, less story-telling-like, and more similar to a thought or a waking memory [50][42] It is not clear, however, why LD is not common in the general population [52][44], although the majority of people show REM sleep every night. A possible explanation for this inconsistency is that LD is probably associated with specific brain rhythms during REM sleep, with different spectral characteristics from non-lucid REM sleep.2.2.2. Neuroimaging of Non-Lucid and Lucid REM Sleep
During non-lucid REM sleep, the limbic system is activated (hippocampus, amygdala, medial prefrontal cortex, and anterior cingulate cortex), which might be related to the intense emotions that are usually experienced during dreams [61,62][45][46]. On the other hand, the activity in the prefrontal cortex decreases, which may be related to the lack of self-consciousness and meta-cognition during non-lucid dreams [63,64][47][48]. Dresler and collaborators [65][49] observed that during LD frontal regions are activated, including the anterior prefrontal cortex, which may be associated with the insight and self-reflection abilities that characterize LD. More recently, Baird et al. [66][50] observed that frequent lucid dreamers showed increased resting-state functional connectivity between the anterior prefrontal cortex and other frontal, parietal, and temporal regions.2.3. False Awakenings
False awakening (FA) is a type of dream in which people believe they have woken up since the dream content is similar to their daily life, especially regarding their activities when waking up, such as rising from bed, going to the bathroom, having breakfast, brushing the teeth, etc. Only when the subjects actually wake up do they realize they were dreaming [71][51]. In a study with 974 participants, 45% of them had experienced at least one episode of FA in their lifetime, 28% have it sometimes, 8% have experienced a single episode, and 7% have it often. Participants above 50 years have fewer experiences of FA (4%) than the younger ones, especially those between 21–30 years (9%) [72][52]. During an FA episode, the dream scenario is likely to be similar to the usual environment of the dreamer. Often, the trigger for the FAs are external factors, such as a ringing cell phone; however, sometimes even the trigger may be dreamed [71][51]. On the other hand, some people refer to FA as “a dream within a dream”.2.3.1. False Awakenings and REM Sleep
FA happens mostly during REM sleep, which is similar to LD, sleep paralysis (SP; see next section), and out-of-body experiences. Since they all occur in the same sleep stage (REM), these phenomena may be linked to each other. Raduga, Kuyava, and Sevcenko [72][52] found that people who have already experienced one of these REM sleep-dissociated states are likely to have experienced another. SP and FA share the feeling of being in a familiar environment (usually their bed), the perception of being awake, and feelings of anxiety. One main difference is the inability versus freedom to move during SP and FA episodes, respectively.2.3.2. False Awakenings and the Brain Rhythms
Using polysomnography during FA, an increase in alpha frequency alternating with states of REM sleep, slow eye movement, and low muscle tone was found [73][53]. Thise study also found that, although FA and SP are experiences similar to each other and mimic a waking state, in both of them the individual is dreaming and in the REM sleep stage (associated with the predominance of theta waves in the electroencephalogram). Thus, commonalities between SP and FA regarding the characteristics of REM sleep and wakefulness can be explained by the fact that different stages of sleep or wakefulness, especially their transitions, show mixed features that characterize a sleep-related dissociated state [5].3. Pathological States of Consciousness
3.1. Sleep Paralysis
Sleep paralysis (SP) is a dissociative state of consciousness that is characterized by motor, sensory, and cognitive abnormalities that usually happen in the transition from REM sleep to the waking state. It comprises the inability to move, visual hallucinations, tightness in the chest, fear of death, and perception of threatening creatures. The SP experience can induce intense frightening and anxiety due to the loss of voluntary motor activity, which can cause the inability to scream, open the eyes, cry, and even breathe [74,75][54][55]. Most people have experienced a few episodes of SP in their lifetime, but when SP occurs frequently or involves intense negative emotional components, impairing well-being, and social functioning, it can be considered a sleeping disorder (parasomnia). Only sparsely studied by science, many cultures have interpreted SP from a supernatural perspective until today [76][56]. In Japan, the “kanashibari”, which can be translated as “the state of being totally bound, as if constrained by metal chains”, is caused by a vengeful spirit who suffocates his enemies [77][57]. In Thailand, the “phi am” is a ghost that appears when subjects are half asleep and unable to move [78][58]. Egyptians believe that SP is caused by the “jinn”, which are malevolent spirit-like creatures [75][55]. Ethiopians consider the “dukak” an evil spirit that haunts sleep [79][59]. In the USA, the report of “alien abductions”—experienced as the inability to move during awakening with visual hallucinations of aliens—is considered a manifestation of SP [80][60]. The emotional components of SP have been explained by cognitive distortions such as hallucinations, which can be grouped into “Intruder”, “Unusual Bodily Experiences”, and “Incubus”. The first is characterized by the feeling of fear or the sensation of a presence in space accompanied by visual and auditory hallucinations. The second is related to out-of-body experiences. The third refers to the feeling of tightness in the chest and difficulty to breath [81][61], as depicted by the painter Henry Fuseli (Figure 2).
Figure 2.
The Nightmare
(1871), by Henry Fuseli.
The different societies share a common frightening component due to the person’s inability to move and visual hallucinations according to the individual’s culture [82][62]. However, some authors interpret SP in a strictly pathological way. In the past, SP was underdiagnosed and understood as a symptom of narcolepsy. This disorder is characterized by abnormalities of sleep regulation, in which the individual falls asleep abruptly associated with cataplexy, when muscle tone is lost, usually after a very emotional episode. However, despite its similarities with narcolepsy, SP can occur separately from narcolepsy and, overall, shares few relationships with neuropsychiatric disorders. However, SP must be seen from a pathological point of view when the episodes are very frequent or intense, thus implicating physical, mental, and/or social suffering.
3.2. Sleepwalking
Sleepwalking (or somnambulism) is a condition that has been documented by humanity for a long time. In the third century AD, the Stoic philosopher Diogenes Laertius was said to read and write his works while he slept. In the second century, Galen, the “father” of anatomy, reported in his work “De motu musculorum” that he had spent a whole night walking in his sleep and only woke up after stumbling over a stone. These lines are very reminiscent of sleepwalking but they can also have alternative interpretations, especially from a poetic point of view. In the medieval period, sleepwalking and other sleep disorders were intrinsically related to religious beliefs and interpreted as divine designations or diabolical acts. The sixteenth-century Spanish writer Antonio de Torquemada wrote that the devil makes us “dream lascivious dreams” and provokes the sleepers “to commit follies for which we can lose body and mind once”. Supernatural explanations such as these still exist in many cultures [89][63].
Sleepwalking is a type of arousal parasomnia disorder that occurs during NREM sleep but in which the usual distinctions between wakefulness, REM, and NREM sleep are blurred, which is the precise definition of a sleep-related dissociative state. Sleepwalking is closely related to other NREM parasomnias (confusional arousals, sleep terrors, sleep-related sexual behavior, and sleep-related violence) since these sleep disturbs are mostly classified by the displayed behaviors [90][64]. The term disorders of arousal encompass sleepwalking, night terrors, and confusional arousals and also a broader spectrum of specific forms of NREM parasomnias, such as sexsomnia, sleep-related eating, and sleep-related asphyxia syndrome. The underlying factors associated with confusional arousal disorders are mostly unknown [91][65].
Sleepwalking episodes draw a lot of attention because the person is sleeping and performing complex activities, such as gesturing, pointing, walking, or even dressing, cooking, and driving. These activities can last from seconds to more than 30 min. Its main characteristics are speech in response to external stimuli, as the person is sleeping, mental confusion, variable retrograde amnesia, perceived threat, and misperception [92][66]. Moreover, descriptive reports show that, in children, complete amnesia is more common, as well as sleepwalking behavior characterized by automatic movements and higher arousal thresholds. Unlike children, many adults remember at least parts of the events upon waking [93][67]. The condition is most common in children, whereas only 2–3% of adults show sleepwalking and only 0.4% of adults sleepwalk every night. About 80% of adult sleepwalkers showed childhood sleepwalking, perhaps due to an immaturity of the sleep state [94][68]. Therefore, this phenomenon might be understood as a complex condition that involves high motor control levels, which we detail next.
3.3. REM Sleep Behavior Disorder
Rapid eye movement (REM) sleep behavior disorder (RBD) is a behavioral and experiential parasomnia that consists of abnormal behavioral release during REM sleep with loss of generalized skeletal muscle paralysis or “REM-atonia” [107][69]. RBD was first identified in humans in a series of five patients [108][70] and resembled findings from an experimental model in cats with pontine tegmental lesions [85][71]. Mahowald and Schenck [109][72] also described the dissociation and admixture of different states of consciousness that underscores the neurophysiological phenomenon in which REM-atonia is compromised and wakeful muscle tone and twitching intrudes, resulting in clinically relevant behavioral release during REM sleep. During an RBD episode, the person moves with eyes closed while attending to the inner dream action and being unaware of the actual bedside surroundings [107][69]. Behavioral release during REM sleep often involves acting out of dreams that are confrontational, aggressive, and violent. The enacted dreams usually involve hostile people and animals and the dreamer is often defending him/herself or loved ones against the perpetrator. The reported dream imagery closely matches the observed physical behaviors during video-polysomnography evaluation. RBD is a dream disorder almost as much as a sleep behavioral disorder. The first textbook on RBD describes various considerations in detail [110][73].4. Altered States of Consciousness
4.1. Hypnosis
The term “hypnosis” derives from the Greek Hypnos, meaning “to sleep”. It was popularized by James Braid in the middle of the 19th century to designate a sleep-like, highly suggestible state based on induction and relaxation techniques [123][74]. In the middle of the 20th century, Milton Erickson revolutionized hypnosis application with his individualized, non-authoritarian approaches, which laid the foundation for the development, use, and acceptance of modern clinical hypnosis [124][75]. Today, hypnosis is often embedded in a broader therapeutic approach and recognized as complemental treatment in psychotherapy, medicine, sports, and forensics [125][76]. In clinical application, it is mostly known as an efficient tool for pain reduction but might also be useful in phobias, depression, eating disorders, posttraumatic stress disorders, and substance use disorders, among many others [126][77]. Over two centuries of research have always strived to better describe the phenomenology, efficacy, and challenges of hypnosis; however, the technique is far from being thoroughly explored. Hypnosis can be understood as an altered state of consciousness produced by suggestions from a hypnotist to a hypnotized person to induce changes in perception, cognition, or behavior [125][76]. In general, the suggestions consist of three phases of: 1—induction, including general suggestions to guide the person into a hypnotic trance; 2—application, including directed suggestions around the specific topic; and 3—termination, including general suggestions to guide the person out of the trance.4.2. Anesthesia
“You will fall asleep now” is the most common sentence anesthesiologists use before administering hypnotics [145][78]. Trying to understand the biological basis of how the human mind shifts from an alert (or responsive) state to (natural) sleep or (artificial) anesthesia is a recent and important object of study in neuroscience. First, it is necessary to differentiate two confusing concepts: wakefulness and an “unconscious” state. In the context of anesthesia, despite the state of consciousness being defined by the behavior of the individual, the lack of response does not indicate unawareness or lack of experiences that were generated only internally, so it does not mean unconsciousness [146][79]. During wakefulness—the “connected” state—the contents that reach consciousness are modulated through a sensory filter, which allows the conscious perception of stimuli. However, during anesthesia—the “disconnected (or dissociated)” state—the contents of consciousness revolve around internally generated experiences, such as the individual’s imagination [147][80].4.3. Psychedelics
Classic serotonergic psychedelics, such as lysergic acid diethylamide (LSD), psilocybin, and ayahuasca, are gaining growing scientific attention due to their therapeutic potential in diverse psychiatric conditions, especially mood and substance use disorders [154,155,156][81][82][83]. After a research hiatus following political motivations by the end of the 1960s, their profound effects on the human mind are being increasingly studied [157][84]. Findings from the last century and modern studies highlight the fundamental effects of psychedelics on perception, cognition, and emotion, including altered perceptions of the senses, self, body, time, and space, reduced cognitive control, and heightened emotionality [158,159][85][86].References
- Krueger, J.M.; Nguyen, J.T.; Dykstra-Aiello, C.J.; Taishi, P. Local Sleep. Sleep Med. Rev. 2019, 43, 14–21.
- Scarpelli, S.; Alfonsi, V.; Gorgoni, M. Parasomnias and Disruptive Sleep-Related Disorders: Insights from Local Sleep Findings. J. Clin. Med. 2022, 11, 4435.
- Huber, R.; Felice Ghilardi, M.; Massimini, M.; Tononi, G. Local Sleep and Learning. Nature 2004, 430, 78–81.
- Vyazovskiy, V.V.; Olcese, U.; Hanlon, E.C.; Nir, Y.; Cirelli, C.; Tononi, G. Local Sleep in Awake Rats. Nature 2011, 472, 443–447.
- Mahowald, M.W.; Cramer Bornemann, M.A.; Schenck, C.H. State Dissociation, Human Behavior, and Consciousness. Curr. Top. Med. Chem. 2011, 11, 2392–2402.
- Singer, J.L.; McCraven, V.G. Some Characteristics of Adult Daydreaming. J. Psychol. 1961, 51, 151–164.
- McMillan, R.L.; Kaufman, S.B.; Singer, J.L. Ode to Positive Constructive Daydreaming. Front. Psychol. 2013, 4, 626.
- Schooler, J.W.; Smallwood, J.; Christoff, K.; Handy, T.C.; Reichle, E.D.; Sayette, M.A. Meta-Awareness, Perceptual Decoupling and the Wandering Mind. Trends Cogn. Sci. 2011, 15, 319–326.
- Ribeiro, S. O Oráculo Da Noite: A História e a Ciência Do Sonho; Editora Companhia das Letras: Rio de Janeiro, Brazil, 2019.
- Immordino-Yang, M.H.; Christodoulou, J.A.; Singh, V. Rest Is Not Idleness: Implications of the Brain’s Default Mode for Human Development and Education. Perspect. Psychol. Sci. 2012, 7, 352–364.
- Smallwood, J.; Fitzgerald, A.; Miles, L.K.; Phillips, L.H. Shifting Moods, Wandering Minds: Negative Moods Lead the Mind to Wander. Emotion 2009, 9, 271–276.
- Singer, J.L. The Inner World of Daydreaming, 1st ed.; Harper & Row: New York, NY, USA, 1975; ISBN 978-0-06-013907-0.
- Giambra, L.M. Daydreaming across the Life Span: Late Adolescent to Senior Citizen. Int. J. Aging Hum. Dev. 1974, 5, 115–140.
- Giambra, L.M. A Laboratory Method for Investigating Influences on Switching Attention to Task-Unrelated Imagery and Thought. Conscious. Cogn. 1995, 4, 1–21.
- Klinger, E. Goal Commitments and the Content of Thoughts and Dreams: Basic Principles. Front. Psychol. 2013, 4, 415.
- Franklin, M.S.; Smallwood, J.; Schooler, J.W. Catching the Mind in Flight: Using Behavioral Indices to Detect Mindless Reading in Real Time. Psychon. Bull. Rev. 2011, 18, 992–997.
- Smallwood, J.; O’Connor, R.C.; Heim, D. Rumination, Dysphoria, and Subjective Experience. Imagin. Cogn. Personal. 2005, 24, 355–367.
- McVay, J.C.; Kane, M.J. Why Does Working Memory Capacity Predict Variation in Reading Comprehension? On the Influence of Mind Wandering and Executive Attention. J. Exp. Psychol. Gen. 2012, 141, 302–320.
- Whitfield-Gabrieli, S.; Ford, J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76.
- Giambra, L.M.; Traynor, T.D. Depression and Daydreaming; an Analysis Based on Self-Ratings. J. Clin. Psychol. 1978, 34, 14–25.
- Meyer, T.D.; Finucane, L.; Jordan, G. Is Risk for Mania Associated with Increased Daydreaming as a Form of Mental Imagery? J. Affect. Disord. 2011, 135, 380–383.
- Marchetti, I.; Koster, E.H.W.; De Raedt, R. Mindwandering Heightens the Accessibility of Negative Relative to Positive Thought. Conscious. Cogn. 2012, 21, 1517–1525.
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682.
- Domhoff, G.W.; Fox, K.C.R. Dreaming and the Default Network: A Review, Synthesis, and Counterintuitive Research Proposal. Conscious. Cogn. 2015, 33, 342–353.
- Raichle, M.E.; Snyder, A.Z. A Default Mode of Brain Function: A Brief History of an Evolving Idea. NeuroImage 2007, 37, 1083–1090; discussion 1097–1099.
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 2010, 65, 550–562.
- Horovitz, S.G.; Fukunaga, M.; de Zwart, J.A.; van Gelderen, P.; Fulton, S.C.; Balkin, T.J.; Duyn, J.H. Low Frequency BOLD Fluctuations during Resting Wakefulness and Light Sleep: A Simultaneous EEG-FMRI Study. Hum. Brain Mapp. 2008, 29, 671–682.
- Vincent, J.L.; Patel, G.H.; Fox, M.D.; Snyder, A.Z.; Baker, J.T.; Van Essen, D.C.; Zempel, J.M.; Snyder, L.H.; Corbetta, M.; Raichle, M.E. Intrinsic Functional Architecture in the Anaesthetized Monkey Brain. Nature 2007, 447, 83–86.
- Braboszcz, C.; Delorme, A. Lost in Thoughts: Neural Markers of Low Alertness during Mind Wandering. NeuroImage 2011, 54, 3040–3047.
- Oken, B.S.; Salinsky, M.C.; Elsas, S.M. Vigilance, Alertness, or Sustained Attention: Physiological Basis and Measurement. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2006, 117, 1885–1901.
- La Berge, S.P. Lucid Dreaming as a Learnable Skill: A Case Study. Percept. Mot. Ski. 1980, 51, 1039–1042.
- Mota-Rolim, S.A.; Bulkeley, K.; Campanelli, S.; Lobão-Soares, B.; de Araujo, D.B.; Ribeiro, S. The Dream of God: How Do Religion and Science See Lucid Dreaming and Other Conscious States During Sleep? Front. Psychol. 2020, 11, 555731.
- Ferreira, G.H.; Prata, T.d.A.; Fontenele-Araujo, J.; de Carvalho, F.T.; Mota-Rolim, S.A. I Dream Therefore I Am: A Review on Lucid Dreaming in Western Philosophy. Dreaming 2021, 31, 69–87.
- Dennett, D.C. Are Dreams Experiences? Philos. Rev. 1976, 85, 151.
- Aserinsky, E.; Kleitman, N. Regularly Occurring Periods of Eye Motility, and Concomitant Phenomena, During Sleep. Science 1953, 118, 273–274.
- Jouvet, M. Research on the neural structures and responsible mechanisms in different phases of physiological sleep. Arch. Ital. Biol. 1962, 100, 125–206.
- Dement, W.; Wolpert, E.A. The Relation of Eye Movements, Body Motility, and External Stimuli to Dream Content. J. Exp. Psychol. 1958, 55, 543–553.
- Mota-Rolim, S.A. On Moving the Eyes to Flag Lucid Dreaming. Front. Neurosci. 2020, 14, 361.
- La Berge, S.P.; Nagel, L.E.; Dement, W.C.; Zarcone, V.P. Lucid Dreaming Verified by Volitional Communication during REM Sleep. Percept. Mot. Ski. 1981, 52, 727–732.
- LaBerge, S.; Levitan, L.; Dement, W.C. Lucid Dreaming: Physiological Correlates of Consciousness during REM Sleep. J. Mind Behav. 1986, 7, 251–258.
- Brylowski, A.; Levitan, L.; LaBerge, S. H-Reflex Suppression and Autonomic Activation during Lucid REM Sleep: A Case Study. Sleep 1989, 12, 374–378.
- Stumbrys, T.; Erlacher, D. Lucid Dreaming during NREM Sleep: Two Case Reports. Int. J. Dream Res. 2012, 5, 151–155.
- Mota Rolim, S.A.; Brandão, D.S.; Andrade, K.C.; de Queiroz, C.M.T.; Araujo, J.F.; de Araujo, D.B.; Ribeiro, S. Neurophysiological Features of Lucid Dreaming During N1 And N2 Sleep Stages: Two Case Reports. Sleep Sci. 2015, 8, 215.
- Mota-Rolim, S.; Targino, Z.; Souza, B.; Blanco, W.; Araujo, J.; Ribeiro, S. Dream Characteristics in a Brazilian Sample: An Online Survey Focusing on Lucid Dreaming. Front. Hum. Neurosci. 2013, 7, 836.
- Braun, A. Regional Cerebral Blood Flow throughout the Sleep-Wake Cycle. An H2(15)O PET Study. Brain 1997, 120, 1173–1197.
- Maquet, P.; Péters, J.; Aerts, J.; Delfiore, G.; Degueldre, C.; Luxen, A.; Franck, G. Functional Neuroanatomy of Human Rapid-Eye-Movement Sleep and Dreaming. Nature 1996, 383, 163–166.
- Hobson, J.A. REM Sleep and Dreaming: Towards a Theory of Protoconsciousness. Nat. Rev. Neurosci. 2009, 10, 803–813.
- Nir, Y.; Tononi, G. Dreaming and the Brain: From Phenomenology to Neurophysiology. Trends Cogn. Sci. 2010, 14, 88–100.
- Dresler, M.; Wehrle, R.; Spoormaker, V.I.; Koch, S.P.; Holsboer, F.; Steiger, A.; Obrig, H.; Sämann, P.G.; Czisch, M. Neural Correlates of Dream Lucidity Obtained from Contrasting Lucid versus Non-Lucid REM Sleep: A Combined EEG/FMRI Case Study. Sleep 2012, 35, 1017–1020.
- Baird, B.; Castelnovo, A.; Gosseries, O.; Tononi, G. Frequent Lucid Dreaming Associated with Increased Functional Connectivity between Frontopolar Cortex and Temporoparietal Association Areas. Sci. Rep. 2018, 8, 17798.
- Buzzi, G. False Awakenings in Light of the Dream Protoconsciousness Theory: A Study in Lucid Dreamers. Int. J. Dream Res. 2011, 4, 110–116.
- Raduga, M.; Kuyava, O.; Sevcenko, N. Is There a Relation among REM Sleep Dissociated Phenomena, like Lucid Dreaming, Sleep Paralysis, out-of-Body Experiences, and False Awakening? Med. Hypotheses 2020, 144, 110169.
- Mainieri, G.; Maranci, J.-B.; Champetier, P.; Leu-Semenescu, S.; Gales, A.; Dodet, P.; Arnulf, I. Are Sleep Paralysis and False Awakenings Different from REM Sleep and from Lucid REM Sleep? A Spectral EEG Analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2021, 17, 719–727.
- Sharpless, B.A.; McCarthy, K.S.; Chambless, D.L.; Milrod, B.L.; Khalsa, S.-R.; Barber, J.P. Isolated Sleep Paralysis and Fearful Isolated Sleep Paralysis in Outpatients with Panic Attacksb. J. Clin. Psychol. 2010, 66, 1292–1306.
- Jalal, B.; Hinton, D.E. Rates and Characteristics of Sleep Paralysis in the General Population of Denmark and Egypt. Cult. Med. Psychiatry 2013, 37, 534–548.
- de Sá, J.F.R.; Mota-Rolim, S.A. Sleep Paralysis in Brazilian Folklore and Other Cultures: A Brief Review. Front. Psychol. 2016, 7, 1294.
- Fukuda, K.; Miyasita, A.; Inugami, M.; Ishihara, K. High Prevalence of Isolated Sleep Paralysis: Kanashibari Phenomenon in Japan. Sleep 1987, 10, 279–286.
- Cassaniti, J.L.; Luhrmann, T.M. The Cultural Kindling of Spiritual Experiences. Curr. Anthropol. 2014, 55, S333–S343.
- Sharpless, B.A.; Doghramji, K. Sleep Paralysis: Historical, Psychological, and Medical Perspectives; Oxford University Press: New York, NY, USA, 2015; pp. xiii, 287. ISBN 978-0-19-931380-8.
- McNally, R.J.; Clancy, S.A. Sleep Paralysis, Sexual Abuse, and Space Alien Abduction. Transcult. Psychiatry 2005, 42, 113–122. Available online: https://journals.sagepub.com/doi/10.1177/1363461505050715 (accessed on 18 March 2023).
- Cheyne, J.A.; Rueffer, S.D.; Newby-Clark, I.R. Hypnagogic and Hypnopompic Hallucinations during Sleep Paralysis: Neurological and Cultural Construction of the Night-Mare. Conscious. Cogn. 1999, 8, 319–337.
- Hinton, D.E.; Hufford, D.J.; Kirmayer, L.J. Culture and Sleep Paralysis. Transcult. Psychiatry 2005, 42, 5–10.
- Umanath, S.; Sarezky, D.; Finger, S. Sleepwalking through History: Medicine, Arts, and Courts of Law. J. Hist. Neurosci. 2011, 20, 253–276.
- Harris, M.; Grunstein, R.R. Treatments for Somnambulism in Adults: Assessing the Evidence. Sleep Med. Rev. 2009, 13, 295–297.
- Idir, Y.; Oudiette, D.; Arnulf, I. Sleepwalking, Sleep Terrors, Sexsomnia and Other Disorders of Arousal: The Old and the New. J. Sleep Res. 2022, 31, e13596.
- Zadra, A.; Desautels, A.; Petit, D.; Montplaisir, J. Somnambulism: Clinical Aspects and Pathophysiological Hypotheses. Lancet Neurol. 2013, 12, 285–294.
- Busby, K.A.; Mercier, L.; Pivik, R.T. Ontogenetic Variations in Auditory Arousal Threshold during Sleep. Psychophysiology 1994, 31, 182–188.
- Plazzi, G.; Vetrugno, R.; Provini, F.; Montagna, P. Sleepwalking and Other Ambulatory Behaviours during Sleep. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2005, 26 (Suppl. S3), s193–s198.
- Schenck, C.H.; Mahowald, M.W. REM Sleep Behavior Disorder: Clinical, Developmental, and Neuroscience Perspectives 16 Years After Its Formal Identification in SLEEP. Sleep 2002, 25, 120–138.
- Schenck, C.H.; Bundlie, S.R.; Ettinger, M.G.; Mahowald, M.W. Chronic Behavioral Disorders of Human REM Sleep: A New Category of Parasomnia. Sleep 1986, 9, 293–308.
- Jouvet, M. Locus Coeruleus et Sommeil Paradoxal. Comptes Rendus Séances Société Biol. Ses Fil. 1965, 159, 895–899.
- Mahowald, M.W.; Schenck, C.H. Dissociated States of Wakefulness and Sleep. Neurology 1992, 42, 44–51; discussion 52.
- Schenck, C.; Högl, B.; Videnovic, A. (Eds.) Rapid-Eye-Movement Sleep Behavior Disorder; Springer: Cham, Switzerland, 2019; ISBN 978-3-319-90152-7.
- Hammond, D.C. A Review of the History of Hypnosis through the Late 19th Century. Am. J. Clin. Hypn. 2013, 56, 174–191.
- Matthews, W.J.; Lankton, S.; Lankton, C. An Ericksonian Model of Hypnotherapy. In Handbook of Clinical Hypnosis; American Psychological Association: Washington, DC, USA, 1993; pp. 187–214. ISBN 978-1-55798-440-1.
- Kirsch, I.; Lynn, S.J.; Rhue, J.W. Introduction to Clinical Hypnosis. In Handbook of Clinical Hypnosis; American Psychological Association: Washington, DC, USA, 1993; pp. 3–22. ISBN 978-1-55798-440-1.
- Montgomery, G.H.; DuHamel, K.N.; Redd, W.H. A Meta-Analysis of Hypnotically Induced Analgesia: How Effective Is Hypnosis? Int. J. Clin. Exp. Hypn. 2000, 48, 138–153.
- Lendner, J.; Helfrich, R. How Can I Run Sleep and Anesthesia Studies with Intracranial EEG? PsyArXiv 2022.
- Huang, Z.; Tarnal, V.; Vlisides, P.E.; Janke, E.L.; McKinney, A.M.; Picton, P.; Mashour, G.A.; Hudetz, A.G. Asymmetric Neural Dynamics Characterize Loss and Recovery of Consciousness. NeuroImage 2021, 236, 118042.
- Bonhomme, V.; Staquet, C.; Montupil, J.; Defresne, A.; Kirsch, M.; Martial, C.; Vanhaudenhuyse, A.; Chatelle, C.; Larroque, S.K.; Raimondo, F.; et al. General Anesthesia: A Probe to Explore Consciousness. Front. Syst. Neurosci. 2019, 13, 36.
- Domínguez-Clavé, E.; Soler, J.; Elices, M.; Pascual, J.C.; Álvarez, E.; de la Fuente Revenga, M.; Friedlander, P.; Feilding, A.; Riba, J. Ayahuasca: Pharmacology, Neuroscience and Therapeutic Potential. Brain Res. Bull. 2016, 126, 89–101.
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M.; The Work Group on Biomarkers and Novel Treatments; A Division of the American Psychiatric Association Council of Research. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410.
- Palhano-Fontes, F.; Mota-Rolim, S.; Lobão-Soares, B.; Galvão-Coelho, N.; Maia-Oliveira, J.P.; Araújo, D.B. Recent Evidence on the Antidepressant Effects of Ayahuasca. In Ayahuasca Healing and Science; Labate, B.C., Cavnar, C., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 21–41. ISBN 978-3-030-55687-7.
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355.
- Vollenweider, F.X.; Preller, K.H. Psychedelic Drugs: Neurobiology and Potential for Treatment of Psychiatric Disorders. Nat. Rev. Neurosci. 2020, 21, 611–624.
- Wießner, I.; Falchi, M.; Palhano-Fontes, F.; Feilding, A.; Ribeiro, S.; Tófoli, L.F. LSD, Madness and Healing: Mystical Experiences as Possible Link between Psychosis Model and Therapy Model. Psychol. Med. 2023, 53, 1151–1165.
More
