You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Kenneth Nugent and Version 1 by Busara Songtanin.
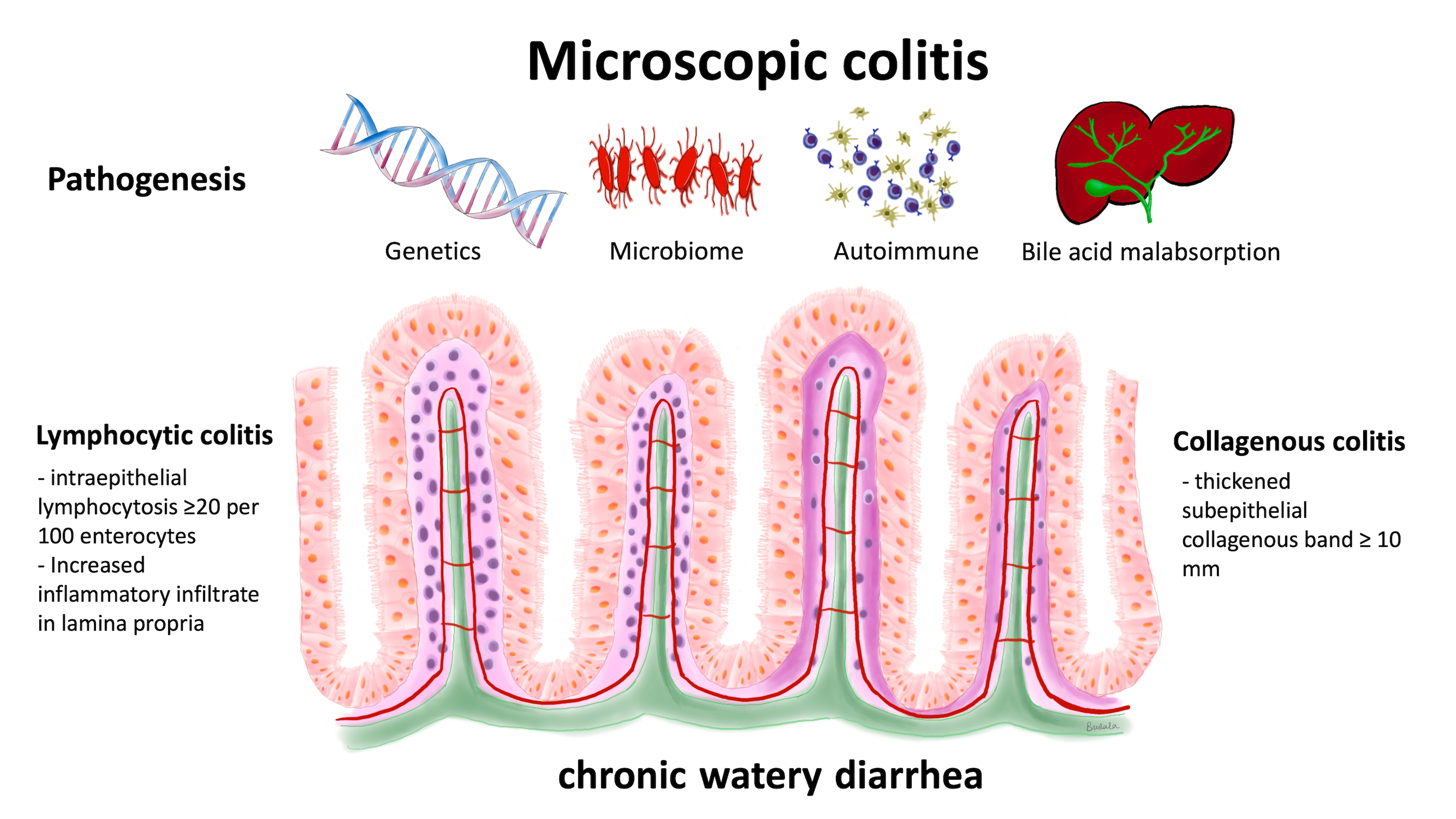
Microscopic colitis is a type of inflammatory bowel disease and is classified as either collagenous colitis or lymphocytic colitis. The typical presentation is chronic watery diarrhea.
- microscopic colitis
- collagenous colitis
- lymphocytic colitis
- chronic diarrhea
1. Microscopic Colitis
Microscopic colitis is an inflammatory bowel disease possibly caused by a chronic immune-mediated process. The incidence rate of microscopic colitis is estimated to be 11.4 per 100,000 person-years [1]. The mean age at diagnosis is 60–65 years with a female-to-male ratio as high as 9:1 [2]. It is a common cause of chronic watery non-bloody diarrhea. The disease is classified into collagenous colitis and lymphocytic colitis based on histologic findings. Collagenous colitis was first described in 1976 [3], and lymphocytic colitis was first described in 1989 [4]. Microscopic colitis is a common cause of chronic watery diarrhea and has a clinicopathological triad of chronic watery non-bloody diarrhea, normal mucosal appearance on colonoscopy, and distinct characteristic histopathology that meet the diagnostic criteria of collagenous colitis or lymphocytic colitis [5]. Microscopic colitis affects all segments of the colon, excluding the rectum; however, the disease process does not affect the colon uniformly [6]. Current literature suggests that the histological findings may be patchy and not continuous throughout the colon and that it is the most severe in the proximal part of the colon [7]. Furthermore, biopsies taken from the rectum have the highest rates of false negative results and are not recommended [8].
2. Pathogenesis of Microscopic Colitis
To date, the pathogenesis of microscopic colitis remains unknown and is likely complex and multifactorial [1,9]. The possible mechanisms include intraluminal factors, such as abnormal microbiota, genetic predisposition, bile acids, and autoimmunity, which could trigger chronic inflammation [9,10,11].2.1. Genetic Predisposition
The role that genetics have in the pathogenesis of microscopic colitis is not completely understood. It has been proposed that microscopic colitis is the result of an abnormal immune response to luminal antigens in genetically predisposed individuals [10]. However, there have been very few genome-wide studies that explored the associations between genes and microscopic colitis. Westerlind et al. used immunochip and targeted single nucleotide polymorphism (SNP) genotype data from 314 collagenous colitis patients, 122 lymphocytic colitis patients, and 4299 controls to analyze possible associations. This study found that human leukocyte antigen (HLA) 8.1 haplotype variants were associated with an increased risk for collagenous colitis [12]. Using the same technique, the authors found no associations for an increased risk for lymphocytic colitis [13]. Genetic similarities were also found between inflammatory bowel disease (IBD) and collagenous colitis [12]. These results have been confirmed by two more recent studies. Green et al. used UK Biobank SNP genotypes of white Europeans, which included 483 patients with microscopic colitis and 450,616 controls. This study confirmed the results of Westerlind et al. and found that the HLA 8.1 haplotype SNP was associated with microscopic colitis [14]. The authors also found that white Europeans with microscopic colitis had an eight times higher risk of celiac disease and a 12 times higher risk of IBD than did controls. Stahl et al. further supported these findings by combining retrospective data and added cases for a total of 804 collagenous colitis DNA samples. These authors reported similar results of an increased risk for collagenous colitis in those with HLA alleles related to the ancestral haplotype 8.1. They also found genetic overlap between collagenous colitis, celiac disease, Crohn’s disease, and ulcerative colitis [15]. Jarnerot reported five families with familial microscopic colitis proven by histological tissue in sisters [16]. This study suggested that there is no association with environmental factors in the pathogenesis of microscopic colitis since the two sisters in one family did not live in the same country. This report included the following relationships: family A: sister 1 collagenous colitis–sister 2 collagenous colitis; family B: sister 1 collagenous colitis–sister 2 microscopic colitis (but another sister without microscopic colitis); family C: sister 1 collagenous colitis–sister 2 collagenous colitis; family D: sister 1 collagenous colitis–sister 2 collagenous colitis; family E: sister 1 collagenous colitis–sister 2 lymphocytic colitis. This study suggests that there may be a genetic basis for some cases with microscopic colitis and makes family history useful information during the evaluation of patients with chronic diarrhea.2.2. Gut Microbiome
The gut microbiome is another factor that may have implications in the pathogenesis of microscopic colitis. Early studies have noted the development of microscopic colitis after infections from Clostridium difficile and Yersinia [17,18]. These findings, combined with reports that diversion of fecal material reduces mucosal inflammation in collagenous colitis [16,19], support the proposal that changes in the microbiota and, thus, the microbiome, affect microscopic colitis development. This idea is supported by a fecal study that compared the microbiota of 10 patients with collagenous colitis with 10 healthy controls, both before and after treatment with budesonide [20]. At baseline, the diversity of the microbiota was less in the patients with collagenous colitis compared to the healthy individuals. Treatment with budesonide led to increased diversity in the collagenous colitis microbiome, which brought it closer to the composition found in the healthy controls. This suggests that the microbiome may contribute to the development of microscopic colitis and that targeting the microbiome could be a potential therapeutic approach. Similar findings were reported by Morgan et al., who collected fecal samples from 20 patients with microscopic colitis during both active and remission phases of this disorder, from 20 patients with functional diarrhea (i.e., non-organic) and from 20 healthy control subjects [21]. There was a significant decrease in variability in the microbiota during the active phase compared to the remission phase. Although not statistically significant, there was also lower diversity in active microscopic colitis than in the healthy controls. In addition, microbial dysbiosis was significantly higher in an active phase microscopic colitis compared to patients in a remission phase, healthy controls, and patients with functional diarrhea. Analysis of the microbiota composition found a higher proportion of Haemophilus parainfluenzae, Veillonella parvula, and Veillonella species in patients with microscopic colitis than in healthy controls, and fewer Alistipes putredinis. This may be a significant finding due to the protective anti-inflammatory implications of Alistipes species [22]. Lower diversity in the microbiota was also reported in studies by Sun et al. and Hertz et al. [23,24]. Sun and colleagues compared microbiota composition from biopsies of the ascending and descending colons from 52 microscopic colitis patients and 153 healthy controls. They found increased Proteobacteria in microscopic colitis and increased Collinsella in healthy controls. These changes occurred in both the ascending and descending colon. Hertz and colleagues reported increased Prevotella enrichment in microscopic colitis gut microbiota from 15 fecal samples compared to 21 healthy control fecal samples [21]. There have been additional smaller-scale studies conducted to examine the microbiota composition in patients with microscopic colitis. Gustafsson et al. studied the colon microbiota of two women with collagenous colitis and found a predominance of Bacteroides and Firmicutes species similar to that of a healthy colon [25]. However, it was found that there was a higher proportion of Bacteroides (47.0% and 31.1% of clones in these two patients) compared to previously published data on healthy guts (3.4–24.4% of clones) [26,27,28]. In addition, Clostridium clostridioforme was found in both patients, which has been historically associated with a variety of infections [29]. Akkermansia mucinciphila, a protective species known to increase the turnover of the mucin layer that helps protect against toxic material [30], was not found in these two women. Fischer supported these results using DNA sequencing to compare the fecal microbiota from 10 female patients with microscopic colitis with the microbiota from seven healthy control women. As in the study by Gustafsson et al., patients with microscopic colitis had a significant reduction in Akkermansia species compared to the healthy control subjects [31]. From colon tissue samples of 20 microscopic colitis patients and 20 controls, Millien et al. found that patients had an increased number of the proinflammatory family Desulfovibrionales and had fewer numbers of the Coriobacteriaceae family that is abundant in a healthy gut [32]. Although these small-scale studies have emphasized the importance of studying microbiota, larger-scale longitudinal studies are needed to better understand the role that the microbiome has on the pathophysiology of Desulfovibrionales and other inflammatory microbes. Studies on the colonic microbiome in patients with microscopic colitis could provide the basis for diagnostic testing if there are consistent changes and if treatment changed the microbiome and improved symptoms.2.3. Autoimmune-Related Colonic Injury
Autoimmunity is a possible pathophysiological mechanism in microscopic colitis. A nationwide case-controlled study performed in Denmark recruited 15,597 microscopic colitis patients and 155,910 controls and reported a significant correlation of autoimmune disease in microscopic colitis with an adjusted odds ratio (OR) of 2.9 (95% CI: 2.0–2.2). The highest ORs were in patients with celiac disease (OR 10.2; 95% CI: 8.2–12.6), Crohn’s disease (OR 2.5; 95% CI; 2.1–2.9), and ulcerative colitis (OR: 6.7; 95% CI; 6.2–7.3) [33]. The same study also demonstrated an increase in the prevalence of autoimmune disease in collagenous colitis compared to lymphocytic colitis (OR 2.4; 95% CI, 2.3–2.5) and (OR 1.9; 95% CI: 1.7–2.0), respectively. However, the positive correlation of IBD and microscopic colitis in this study might have had a misclassification bias due to overlapping histologic and endoscopic findings in these two groups combined with a lack of clinical data. The association was higher in younger patients aged 18–49 compared to older patients aged 50–59. Another retrospective study review of 103 patients with microscopic colitis reported that 40 (39%) of patients had an underlying autoimmune disease with no difference between patients with collagenous colitis and lymphocytic colitis [34]. Hashimoto thyroiditis was the most prevalent autoimmune disease in fourteen patients (35%), followed by rheumatoid arthritis in seven (17.5%), and Sjogren’s syndrome in seven (17.5%). Studies on the immunopathological mechanisms in microscopic colitis are limited to small case series. Göranzon studied the characteristics of lymphocytes in the colonic biopsy tissue samples from 23 patients with microscopic colitis and 17 control patients. In the microscopic colitis group (both collagenous colitis and lymphocytic colitis), the biopsies showed a significant increase in the number of CD8+ lymphocytes in the epithelium and lamina propria and a decreased number of CD4+ lymphocytes in the lamina propria [35]. This study reported an increase in CD3+ lymphocytes in the epithelium in collagenous colitis and lymphocytic colitis compared to the control patients, with a significant increase in CD3+ in the lymphocytic colitis group. This study also found an increase in CD3+ and CD 20 (B lymphocytes) in microscopic colitis patients. It should be noted that the controls in this study were not healthy controls, and some likely had IBS as the study recruited patients who had a normal colonoscopy but had presented with intestinal bleeding and changes in bowel habits. Sandler et al. studied the association between clinical symptoms in patients with microscopic colitis and lymphocyte infiltration in the epithelium and lamina propria in 97 microscopic colitis patients and 165 controls [36]. There were increased numbers of CD8+ lymphocytes in both the epithelium and lamina propria of the patients with microscopic colitis, but there was no associations between diarrhea symptoms and T-cell infiltration in the patients with microscopic colitis. This study indicates that patients with microscopic colitis with more frequent stools do not necessarily have more T-cell infiltration in the colonic mucosa. An autoimmune disorder might explain the development of microscopic colitis in some younger patients and the beneficial effects of treatment with an oral corticosteroid medication. In addition, developing tests for antibodies against colonic antigens could lead to a simple diagnostic test. For example, Kuwada et al. demonstrated that the majority of patients with ulcerative colitis had autoantibodies against integrin αvβ6 and suggested that this antibody might serve as a potential diagnostic marker with high sensitivity and specificity in these patients [37].2.4. Bile Acid Malabsorption
The association between bile acid malabsorption and microscopic colitis remains poorly understood and is complicated by the complex physiology and metabolism of bile acids in the intestinal tract [38,39,40]. In addition, there are several receptors in the intestinal tract that bind bile acids, have important physiologic effects, and complicate any analysis of bile acid physiology in the colon. These acids have both beneficial physiologic effects that help maintain colonic health and support nutrition and can have adverse effects that contribute to the development of diarrhea. Bile acids help maintain intestinal epithelial barrier function and contribute to the formation of tight junctions. They have antibacterial effects and help maintain a “healthy” intestinal microbiome, and they support beneficial immune responses in the colon. There are at least seven receptors in colonic tissue that bind bile acids and have physiologic effects [38]. For example, the Farnesoid X-receptor (FXR) decreases the synthesis of bile acids in the liver, and it also regulates innate and adaptive immunity. The binding of bile acids to this receptor decreases the secretion of tumor necrosis factor alpha, and this limits or controls the development of inflammation. The density of this receptor is increased by luminal bile acids and decreased by inflammation; therefore, the number of receptors will depend on a complex set of conditions and may not be predictable. The adverse effects of bile acids include increased secretion of chloride, decreased absorption of fluid, and increased colonic motility which can contribute to chronic diarrhea. The development of diarrhea associated with bile acid malabsorption should reflect either increased synthesis of bile acids or decreased absorption in the terminal ileum. In addition, the metabolism of primary bile acids into secondary bile acids depends on the bacteria present in the colon, and alterations in the bacterial flora could influence the composition of bile acids in the colon. In some patients, bile acids could have a primary effect and cause microscopic colitis. Alternatively, these acids could have a secondary effect and increase diarrhea in patients with established microscopic colitis. The number of bile acid receptors and the complexity of the metabolism of bile acids in the intestinal tract make the analysis of any effect on bile acids on colonic disease quite difficult. The pathogenesis of bile acid malabsorption in patients with microscopic colitis likely has several mechanisms. There is evidence of villous atrophy and inflammation in the ileum in some patients with microscopic colitis, and this could lead to bile acid malabsorption and increased concentration of bile acids in the colon [41,42,43]. Padmanabhan et al. studied the histological features of the terminal ileum in thirty-two patients with lymphocytic and collagenous colitis [44]. The mean number of lymphocytes per 100 epithelial cells was significantly higher in patients than in controls. In lymphocytic colitis the mean number was 22.3 ± 10.2, in collagenous colitis the mean number was 16.7 ± 6.1, and in control biopsies the mean number was 8.2 ± 2.9. Fourteen patients out of eighteen with lymphocytic colitis had a mean number of intraepithelial lymphocytes greater than two standard deviations above the control mean. Seven out of fourteen patients with collagenous colitis had a mean number greater than two standard deviations above the control. The lymphocytes in these patients were T cells. In some patients the surface of the ileum was abnormal; four had villous atrophy, five had epithelial damage with loss of mucin, and three had an increase in subepithelial collagen. This study demonstrates that patients with microscopic colitis can have abnormal mucosa in the terminal ileum. Involvement of the ileum in these patients may reflect “spread” of the disease from the colon back into the ileum. Alternatively, some of these patients may have undiagnosed celiac disease, which involves the ileum. These patients could have decreased bile absorption of the terminal ileum and therefore increased bile acids in the colon, which could influence the development and persistence of diarrhea. A few studies have used 75SeHCAT, a highly sensitive and specific test for bile acid malabsorption, to determine the prevalence of bile acid malabsorption in patients with microscopic colitis. However, the hypothesis that bile acid malabsorption is associated with microscopic colitis is complicated by several other studies that show that microscopic colitis patients with normal 75SeHCAT results also responded to cholestyramine [45,46,47]. This result might suggest that bile acids can have an important effect in patients with a “vulnerable” mucosa even at low levels. The following studies provide more information on the association of microscopic colitis with bile acid malabsorption and/or treatment of patients with microscopic colitis with bile acid sequestrants. Ung et al. comprehensively evaluated twenty-seven patients with collagenous colitis. Twelve of these patients had bile acid malabsorption based on the 75SeCHCAT test [45]. Stool frequency was higher in patients with colitis and bile acid malabsorption than in patients without bile acid malabsorption. Eleven of these patients had at least one autoimmune disease. All patients received a bile acid binder. Rapid improvement occurred in twenty-one of twenty-seven patients. Eleven of the twelve patients with bile acid malabsorption had a rapid response; ten of the fifteen patients without bile acid malabsorption had a rapid response. These authors note that the etiology of the bile acid malabsorption of these patients is unclear, and that the effect of bile acids on colonic function and the production of diarrhea is also unclear. Presumably, the bile acids could cause a low-grade injury to the mucosa and/or alter electrolyte absorption. Ung and coinvestigators did a similar study on patients with lymphocytic colitis [46]. Only two out of twenty-three patients had a low retention of 75SeCHAT. Six patients out of thirteen treated with a bile acid binder responded to treatment. During follow-up, two patients developed collagenous colitis. Both studies indicate that patients with bile acid malabsorption associated with microscopic colitis can have a good clinical response to bile acid sequestrants. In addition, some patients without biochemical evidence of bile acid malabsorption also can respond to bile sequestrants. Fernandez et al. reported that 43.1% of their patients with microscopic colitis also had bile acid malabsorption [7]. In their study, malabsorption was more frequent in lymphocytic colitis patients than collagenous colitis patients, affecting 60% compared to 27%, respectively. Northcutt analyzed the effect of bile acid sequestrant therapy in seventy-nine patients with microscopic colitis following a registry in United States [48]. Forty-six of these seventy-nine patients (58.2%) had a response to this therapy. In forty-six budesonide-dependent patients, twenty-three patients had a response to treatment. Several patients in this registry also had an autoimmune disease; five patients had celiac disease, eleven patients had hypothyroidism, and six patients had one of the following disorders, including diabetes, psoriasis, ulcerative colitis, lichen planus, and systemic lupus. These authors noted that bile acid malabsorption is present in patients with microscopic colitis in 24% to 44% based on 75SeHCAT retention. However, they noted that some patients without bile acid malabsorption based on this test also responded to bile acid sequestrant therapy. Possible mechanisms include the fact that bile acid sequestrant therapy binds deoxycholic acid, which increases colonic secretion of water and electrolytes. Bile acid sequestrants also bind chenodeoxycholic acid, which has a prokinetic effect through increased colonic contractions. Budesonide may have its clinical effect explained in part by the fact that there is a reduced bile acid load in the colon in patients on budesonide. Gurbuz et al. reported a 44-year-old man who developed lymphocytic colitis diagnosed by biopsy after a cholecystectomy [49]. The patient’s diarrhea symptoms were controlled with cholestyramine but relapsed after initially stopping the medication. His symptoms eventually resolved after continuing the cholestyramine again. This case indicates that surgical history is important in some patients with microscopic colitis. However, other studies showed that microscopic colitis is not usually associated with cholecystectomies or appendectomies [50]. The European guidelines for microscopic colitis recommend testing for bile acid malabsorption as part of a routine diagnostic work-up in patients with microscopic colitis who do not respond to budesonide treatment, but the guideline did not recommend testing for bile acid diarrhea as part of a routine diagnostic workup in microscopic colitis, even though MC and bile acid malabsorption can present with similar symptoms. Some patients with MC with both bile acid malabsorption and without bile acid malabsorption respond to bile acid sequestrants [1]. In summary, these studies indicate that bile acids may have a primary or secondary effect in microscopic colitis. Multiple factors, including the bile acid load in the colonic lumen, bile acid metabolism, and complex physiologic effects mediated by several receptors, make this association very difficult to study. The simplest approach for clinicians managing these patients would involve the measurement of bile acid malabsorption and the use of bile acid sequestrants in empiric trials with these patients.