Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Jeffrey Schlom.
Identifying effective immunotherapies for solid tumors remains challenging despite the significant clinical responses observed in subsets of patients treated with immune checkpoint inhibitors. Interleukin-15 (IL-15) is a promising cytokine for the treatment of cancer as it stimulates NK and CD8+ lymphocytes. However, unfavorable pharmacokinetics and safety concerns render recombinant IL-15 (rIL-15) a less attractive modality. These shortcomings were addressed by the clinical development of heterodimeric IL-15 agonists, including N803.
- IL-15
- N803
- Anktiva®
- cancer immunotherapy
- cytokine
1. Introduction
The success of immune checkpoint inhibition (ICI) in a small subset of cancer patients highlights the promise and challenges of immune therapy for cancer. There is an unmet clinical need for novel therapeutic interventions beyond ICI able to rescue lymphocyte homing and dysfunction in the tumor microenvironment (TME) and to overcome the mechanisms of ICI resistance such as the dysregulation of interferon gamma (IFNγ) signaling, poor major histocompatibility complex I (MHC-I) expression, or defects in antigen processing and presentation (APM). The use of novel immunocytokine delivery strategies and combination therapy approaches may allow for targeting these barriers and increasing the clinical benefit of the treatment of solid malignancies.
Interleukin-15 (IL-15) is a promising immunocytokine for cancer therapy. It is a member of the common receptor gamma chain family together with IL-2, IL-4, IL-7, IL-9, and IL-21. This family of cytokines elicits a broad spectrum of activity in both innate and adaptive immunity, with important clinical implications, which has been reviewed elsewhere [1,2][1][2]. Biologically, the IL-15 cytokine is produced as a stable heterodimer encompassing the IL-15 polypeptide single-chain bound to co-expressed IL-15 receptor α (IL-15Rα) which can be cleaved from the cell surface [3,4][3][4]. IL-15Rα displays a high affinity for IL-15, functioning not as a receptor but as the specific binding protein to the IL-15 single-chain, allowing the heterodimeric complex to form in the endoplasmic reticulum and enabling its transport to the cell surface as a membrane-bound cytokine [5,6][5][6]. IL-15Rα is critical for the bioactivity of this complex as it stabilizes and anchors IL-15, allowing the producing cells to “trans-present” IL-15 to neighboring cells expressing its receptor [4]. A variety of cell types, including blood endothelial cells, lymph node and bone marrow stromal cells, monocytes, macrophages, and dendritic cells, constitutively express IL-15 and IL-15Rα mRNA in a coordinated manner [7,8,9][7][8][9]. In vivo studies using IL-15 reporter mice have demonstrated monocytes, macrophages, and dendritic cells as the main IL-15 producers [10,11][10][11].
IL-15 signals through a receptor complex comprising the IL-2/IL-15 receptor β (CD122) and the shared common γ chain subunit (CD132) on immune cells, thereby initiating multiple signaling pathways that support cell expansion and maintenance, with important immune consequences (Figure 1) [12].
The shared IL-2β chain of the IL-15 signaling complex renders some overlapping functions with IL-2, including the induction of T-cell proliferation and cytotoxicity, and natural killer (NK) cell differentiation/activation [13,14][13][14]. However, IL-15 is required for NK development, expansion, and functional maturation as shown by the marked reduction in NK cells in IL-15-deficient but not IL-2-deficient mice [15]. While IL-2 can promote T-cell activation-induced cell death (AICD), IL-15 serves as an anti-apoptotic factor for T cells, thereby preventing AICD [16,17,18,19,20][16][17][18][19][20]. In addition, IL-15 stimulates the proliferation of memory CD8+ CD44hi T cells expressing high levels of the IL-2β chain [13,16,18,20,21,22][13][16][18][20][21][22]. In contrast to IL-2, IL-15 does not support the proliferation, function, and differentiation of regulatory T cells (Tregs) [13,16,18][13][16][18]. An important clinical distinction between these cytokines is the induction of severe capillary leak syndrome by IL-2 therapy, a toxicity not shared by IL-15 [18]. These functional distinctions render IL-15 more favorable than IL-2 as a cancer immunotherapy given its ability to activate immune cells with tumor-suppressive ability and the potential for lower toxicity.
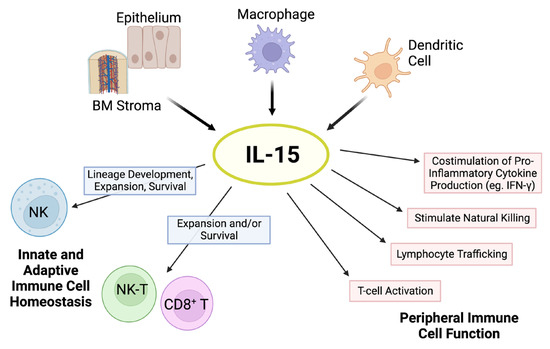
Figure 1. Pleiotropic effects of IL-15. The cytokine IL-15 is produced by multiple cell types, such as activated dendritic cells, monocytes/macrophages, epithelial cells, and bone marrow (BM) stromal cells. IL-15 is critical for natural killer (NK) cell lineage development, survival, and proliferation. This cytokine holds important modulatory effects in the expansion and survival of CD8+ T cells, including memory formation, and lymphocyte cytotoxic function. In addition, IL-15 has pleiotropic effects on peripheral innate and adaptive immunity. Schematic adapted from ([23]; Copyright © 2001 American Society of Hematology) and created with BioRender.com.
2. N803
N803 is a novel mutated (N72D) human IL-15 (IL-15N72D) bound to a dimeric IL-15Rα Sushi domain and an IgG1 Fc fusion protein forming a stable heterodimeric complex (Figure 2A). This complex was designed to increase IL-15′s stability and half-life while mimicking its endogenous biology. Heterodimerization of the IL-15 chain with IL-15Rα has been shown to preserve IL-15 chain stability [35][24]. Moreover, the IL-15 asparagine to aspartic acid substitution lends N803 a 4–5-fold increase in biological activity compared to single-chain wild-type IL-15 via increased binding to IL2Rβ and agonism of the IL2Rβ/IL2Rγ receptor [36,37][25][26]. In vitro studies in the presence of soluble human IL-15Rα-Fc reported the N72D IL-15 mutation to confer ~3.4-fold increase in biological activity relative to rhIL-15, suggesting increased biological activity of the hIL-15N72D heterodimer versus native heterodimeric IL-15 [37][26]. It is possible that heterodimerization and the N72D mutation may add to N803 stability through decreased IL-15 deamidation [38][27]. N803 is a potent inducer of the activation, proliferation, and cytolytic function of NK cells and CD8+ T cells, eliciting pleiotropic immune effects supportive of tumor suppression as a monotherapy and in combination with other agents (Figure 2).
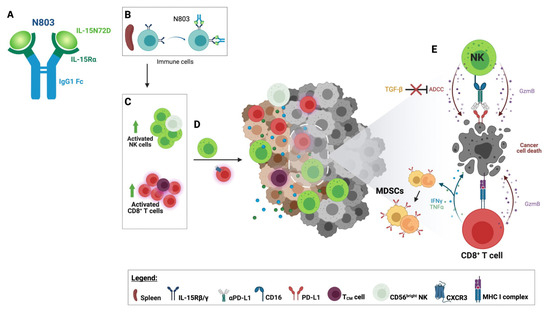
Figure 2. Immune effects of N803. (A) N803 comprises mutated (N72D) human IL-15 bound to the IL15Rα fused with a human IgG1 Fc. (B) N803 binds to circulating immune cells through the IL-15 receptor. (C) The binding of N803 to lymphocytes leads to the activation and expansion of natural killer (NK) cells and CD8+ T-cell populations, resulting in the expansion of high effector CD56dim and CD56bright NK cells and central memory T cells (TCM). (D) The upregulation of CXCR3 on the surface of splenic CD8+ T cells increases the potential for homing into the tumor microenvironment (TME). Activated NK cells also migrate to the tumor. (E) Activated tumor-infiltrating CD8+ T lymphocytes (TILs) recognize cancer cells through recognition of the tumor-associated antigen epitopes presented by the MHC class I complex. Activated CD8+ T cells and NK cells display enhanced cytotoxicity leading to cancer cell death. CD8+ T cells release Th1 cytokines such as IFNγ and TNF⍺, promoting an inflamed TME and upregulating PD-L1 on granulocytic and monocytic myeloid-derived suppressor cells (MDSCs). N803 renders NK TILs resistant to the effects of TGF-β and capable of enhanced antibody-dependent cellular cytotoxicity (ADCC). Figure created with BioRender.com.
2.1. N803 as Single Agent
The safety, pharmacokinetics, and immune modulatory effects of N803 were first studied in murine hosts and cynomolgus monkeys [36,39,40][25][28][29]. In mice, N803 exhibited a 35-fold higher half-life relative to rhIL-15, allowing for a longer stimulation of NK and CD8+ T cells [37,41][26][30]. In cynomolgus monkeys, N803 displayed a half-life of ~8 h, inducing a wide dose-dependent expansion of peripheral blood lymphocytes after intravenous administration without increased IL-2, IFNγ, TNFα, IL-4, IL-5, or IL-6 plasma levels. No toxicity was observed [41][30].
In murine tumor models, N803 has demonstrated significant tumor suppression and/or anti-metastatic effects against multiple transplanted murine solid tumors such as the 4T1 breast, B16F10 melanoma, CT26 colon carcinoma, and GL261-luc glioblastoma models [39,41,42][28][30][31]. Antitumor effects have also been reported in carcinogen-induced orthotopic non-muscle invasive bladder tumors [43][32]. The ability of N803 to induce antitumor effects stems from its ability to induce significant expansion and activation of NK cells and CD8+ central memory T cells in the periphery, as well as IFNγ production and infiltration of CD8 T cells into the tumor microenvironment [41,42][30][31].
The regulation of NK and T-cell populations by N803 has been associated with the preferential stimulation of Th1 versus Th2 cytokines. In vitro, N803 has been shown to induce IFN-γ production by human peripheral blood mononuclear cells (PBMCs) without modulating anti-inflammatory and immune suppressive cytokines, such as IL-4, IL-10, or IL-17A [41][30]. In mice, N803 induced an 11-fold increase in IFN-γ 1 day post-dosing, followed by ≤5-fold increases in TNF-α, IL-5, IL-6, and IL-10 [39][28]. The proliferative effects of N803 appear more pronounced in NK cell populations and innate memory CD8+ T cells expressing NKG2D [39][28]. N803 has been shown to increase the lytic ability of the human herpes virus 16 (HPV16) E7-specific cytotoxic CD8+ T cells in vitro against HPV16+ carcinoma targets, including after target exposure to sublethal radiation [44][33].
N803 exposure modulates murine and human NK cells in multiple and comparable ways. N803 promotes the development of highly cytolytic murine CD11b+ CD27high NK cells, with increased cytokine production and migratory capacity [39][28]. The induction of this high-effector NK cell population was associated with anti-metastatic activity in the lung elicited by N803 in a 4T1 orthotopic breast cancer model [39][28]. Similarly, N803 has been demonstrated to increase the lytic ability of human NK cells to lyse a spectrum of triple-negative breast (TNB) cancer cell lines in vitro, further amplified upon tumor target exposure to the estrogen receptor antagonist fulvestrant. These effects translated to a significant suppression of 4T1 tumors in immune-competent mice [45][34]. In addition, N803 has been shown to protect human NK cells from the immunosuppressive effects of transforming growth factor beta (TGFβ), rescuing their cytolytic function through the inhibition of TGFβ1 signaling and Smad2/3-induced gene transcription [46][35]. Additional evidence of N803 affecting the development of NK cells can be seen from the priming of human CD56bright NK cells, conventionally considered immature and associated with immunomodulatory effects. However, upon in vitro exposure to N803, CD56bright NK cells displayed increased cytokine production, cytotoxicity, and degranulation, indicating an enhancement of tumor-suppressing ability [47][36]. These findings were demonstrated in vivo where NSG mice inoculated with N803-primed human CD56bright NK cells experienced better control of the K562 leukemia tumor burden [47][36]. N803 also demonstrated similar effects on the expansion and lytic ability of human NK cells against leukemia and GD2+ pediatric solid tumor xenografts [48,49][37][38]. Recent studies have demonstrated the potential of N803 as a therapeutic for the treatment of small cell lung cancer (SCLC). In vitro studies demonstrated that exposure to N803 enabled NK cells to efficiently lyse a spectrum of human SCLC cell lines irrespective of their molecular subtype and MHC I expression. These effects translated into effective tumor control in a human SCLC xenograft model [50][39].
2.2. N803 in Combination Therapy
N803 has been shown to increase the cell-surface expression of programmed cell death ligand 1 (PD-L1) in immune cells infiltrating murine TNB 4T1 tumors, particularly granulocytic and monocytic myeloid-derived suppressor cells (MDSCs) via IFNγ induction [41,51][30][40]. Combination therapy with N803 and αPD-L1 monoclonal antibody (mAb) has been evaluated in both “warm” (MC38-CEA colon) and “cold” (4T1 breast) murine tumor models [51,52][40][41]. In contrast to either monotherapy, N803 plus αPD-L1 reduced 4T1 lung metastasis and MC38-CEA tumor burden, resulting in survival benefit [51][40]. These effects were associated with increased activation, proliferation, and cytotoxicity of CD8+ T cells and NK cells, both determinants of tumor suppression [51][40]. Consistent with these findings, N803 has been shown to augment NK cell-mediated ADCC of human carcinoma in the presence of mAbs targeting PD-L1 [48,53][37][42]. Notably, these cytotoxic effects elicited by N803 resulted in efficient lysis of cancer-stem cell-rich chordoma cell lines in vitro via mAbs targeting PD-L1 and the epidermal growth factor receptor (EGFR) [54][43]. The stimulatory effects mediated by N803 have also been observed in human xenograft models of oral cavity squamous carcinomas, where the addition of N803 to the combination of a PD-1-targeting mAb plus adoptive transfer of an irradiated human NK cell line harboring a PD-L1-specific chimeric antigen receptor (CAR) elicited synergistic tumor control [55][44].
Preclinical studies in MC38 expressing CEA colon and 4T1 TNB cancer models examined the effects of N803 in combination with the class I histone deacetylase (HDAC) inhibitor entinostat plus adenovirus-based vaccines targeting the pan-cancer carcinoembryonic antigen (Ad-CEA) or Twist1 (Ad-Twist1), a metastasis-associated transcription factor [56][45]. Whereas N803 in combination with vaccine promoted circulating activated CD8+ T cells with augmented expression of the trafficking chemokine receptor CXCR3, CD8+ T cells showed poor tumor infiltration and granzyme B expression in the TME, resulting in modest antitumor efficacy. In contrast, triple combination therapy promoted significant tumor control versus monotherapies or doublet combinations in both tumor models, and a significant decrease in the number of 4T1 lung metastases. These effects were primarily dependent on CD8+ T cells, whose activity was significantly enhanced by N803 and the vaccine. In the periphery, the N803 component drove CD8+ T cells’ expansion, activation, and memory formation. Further, N803 plus vaccine expanded CD8+ T cells, producing IFNγ and/or TNFα in the tumor microenvironment and the periphery. At the tumor site, N803 in combination with the vaccine alone or plus entinostat induced a significant Treg reduction and increased the activation of CD8+ T lymphocytes (TILs), including those expressing granzyme B. The synergistic effects of triple combination resulted in significant T-cell responses to vaccine and cascade antigens, including neoepitopes, the maximal infiltration of CD8+ T cells, as well as the enhanced transcription of genes associated with tumor inflammation and T-cell chemotaxis, including the CXCR3 ligands CXCL9, CXCL10, and CXCL11.
N803 has been evaluated preclinically in multiple-agent combinations [52,57][41][46]. N803 in combination with the Ad-(CEA or Twist1) vaccine was included in a hexatherapy regimen comprising docetaxel and mAbs targeting OX40, 4-1BB, and PD-L1 [52][41]. Hexatherapy synergized to provide superior CD8+ T-cell-dependent suppression of 4T1 and MC38-CEA tumors and 4T1 lung metastasis, compared to monotherapies or other agent combinations. These effects were associated with the increased activation and tumor infiltration of CD8+ T cells, decreased MDSC populations, and induction of antigen-specific T cells and antigen cascade responses. Within these dynamic effects of hexatherapy, N803 contributed to the increased proliferation and decreased exhaustion of CD8+ T cells in the TME, as well as the enhanced transcription of tumor IFNγ and T-cell-attracting chemokines.
Preclinical studies targeting MC38-CEA, 4T1, and LLC (lung) tumors explored the ability of N803 to activate tumor-specific T cells in combination with Ad-(CEA or Twist1), OX40, and GITR agonists, and the indoleamine 2,3-dioxygenase (IDO) inhibitor epacadostat [57][46]. Tumor therapy with N803 and the vaccine elicited immune-mediated tumor control associated with the enhanced expression of co-stimulatory molecules on immune populations. However, pentatherapy induced the highest tumor suppression via effector T-cell activation and decreased tumor immunosuppression, consistent with the observed protective immunological memory. In a murine model of bladder cancer, the intravesical administration of N803 in combination with BCG treatment induced tumor suppression associated with augmented tumor infiltration of activated NK cells and CD8+ tumor-infiltrating lymphocytes [58][47].
References
- Dwyer, C.J.; Knochelmann, H.M.; Smith, A.S.; Wyatt, M.M.; Rivera, G.O.R.; Arhontoulis, D.C.; Bartee, E.; Li, Z.; Rubinstein, M.P.; Paulos, C.M. Fueling Cancer Immunotherapy with Common Gamma Chain Cytokines. Front. Immunol. 2019, 10, 263.
- Shourian, M.; Beltra, J.-C.; Bourdin, B.; Decaluwe, H. Common gamma chain cytokines and CD8 T cells in cancer. Semin. Immunol. 2019, 42, 101307.
- Bergamaschi, C.; Bear, J.; Rosati, M.; Beach, R.K.; Alicea, C.; Sowder, R.; Pavlakis, G.N. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood 2012, 120, e1–e8.
- Dubois, S.; Mariner, J.; Waldmann, T.A.; Tagaya, Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 2002, 17, 537–547.
- Robinson, T.O.; Schluns, K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017, 190, 159–168.
- Giri, J.G.; Kumaki, S.; Ahdieh, M.; Friend, D.J.; Loomis, A.; Shanebeck, K.; DuBose, R.; Cosman, D.; Park, L.S.; Anderson, D.M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995, 14, 3654–3663.
- Jonuleit, H.; Wiedemann, K.; Müller, G.; Degwert, J.; Hoppe, U.; Knop, J.; Enk, A.H. Induction of IL-15 messenger RNA and protein in human blood-derived dendritic cells: A role for IL-15 in attraction of T cells. J. Immunol. 1997, 158, 2610–2615.
- Carson, W.E.; Ross, M.E.; Baiocchi, R.A.; Marien, M.J.; Boiani, N.; Grabstein, K.; Caligiuri, M.A. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Investig. 1995, 96, 2578–2582.
- Doherty, T.M.; Seder, R.A.; Sher, A. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 1996, 156, 735–741.
- Colpitts, S.L.; Stonier, S.W.; Stoklasek, T.A.; Root, S.H.; Aguila, H.L.; Schluns, K.S.; Lefrançois, L. Transcriptional regulation of IL-15 expression during hematopoiesis. J. Immunol. 2013, 191, 3017–3024.
- Cui, G.; Hara, T.; Simmons, S.; Wagatsuma, K.; Abe, A.; Miyachi, H.; Kitano, S.; Ishii, M.; Tani-Ichi, S.; Ikuta, K. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 1915–1920.
- Mishra, A.; Sullivan, L.; Caligiuri, M.A. Molecular pathways: Interleukin-15 signaling in health and in cancer. Clin. Cancer Res. 2014, 20, 2044–2050.
- Steel, J.C.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012, 33, 35–41.
- Waldmann, T.A.; Tagaya, Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 1999, 17, 19–49.
- Kennedy, M.K.; Glaccum, M.; Brown, S.N.; Butz, E.A.; Viney, J.L.; Embers, M.; Matsuki, N.; Charrier, K.; Sedger, L.; Willis, C.R.; et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000, 191, 771–780.
- Vella, A.T.; Dow, S.; Potter, T.A.; Kappler, J.; Marrack, P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 3810–3815.
- Marks-Konczalik, J.; Dubois, S.; Losi, J.M.; Sabzevari, H.; Yamada, N.; Feigenbaum, L.; Waldmann, T.A.; Tagaya, Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11445–11450.
- Waldmann, T.A. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: Implications for cancer therapy. Cancer Immunol. Res. 2015, 3, 219–227.
- Fontenot, J.D.; Rasmussen, J.P.; Gavin, M.A.; Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005, 6, 1142–1151.
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601.
- Zhang, X.; Sun, S.; Hwang, I.; Tough, D.F.; Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 1998, 8, 591–599.
- Schluns, K.S.; Klonowski, K.D.; Lefrancois, L. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood 2004, 103, 988–994.
- Fehniger, T.A.; Caligiuri, M.A. Interleukin 15: Biology and relevance to human disease. Blood 2001, 97, 14–32.
- Bergamaschi, C.; Rosati, M.; Jalah, R.; Valentin, A.; Kulkarni, V.; Alicea, C.; Zhang, G.-M.; Patel, V.; Felber, B.K.; Pavlakis, G.N. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J. Biol. Chem. 2008, 283, 4189–4199.
- Han, K.P.; Zhu, X.; Liu, B.; Jeng, E.; Kong, L.; Yovandich, J.L.; Vyas, V.V.; Marcus, W.D.; Chavaillaz, P.-A.; Romero, C.A.; et al. IL-15:IL-15 receptor alpha superagonist complex: High-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 2011, 56, 804–810.
- Zhu, X.; Marcus, W.D.; Xu, W.; Lee, H.-I.; Han, K.; Egan, J.O.; Yovandich, J.L.; Rhode, P.R.; Wong, H.C. Novel human interleukin-15 agonists. J. Immunol. 2009, 183, 3598–3607.
- Thaysen-Andersen, M.; Chertova, E.N.; Bergamaschi, C.; Moh, E.; Chertov, O.Y.; Roser, J.D.; Sowder, R.C.; Bear, J.S.; Lifson, J.D.; Packer, N.; et al. Recombinant human heterodimeric IL-15 complex displays extensive and reproducible N- and O-linked glycosylation. Glycoconj. J. 2016, 33, 417–433.
- Kim, P.S.; Kwilas, A.R.; Xu, W.; Alter, S.; Jeng, E.K.; Wong, H.C.; Hodge, J.W. IL-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15SA/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 2016, 7, 16130–16145.
- Liu, B.; Jones, M.; Kong, L.; Noel, T.; Jeng, E.K.; Shi, S.; England, C.G.; Alter, S.; Miller, J.S.; Cai, W.; et al. Evaluation of the biological activities of the IL-15 superagonist complex, ALT-803, following intravenous versus subcutaneous administration in murine models. Cytokine 2018, 107, 105–112.
- Rhode, P.R.; Egan, J.O.; Xu, W.; Hong, H.; Webb, G.M.; Chen, X.; Liu, B.; Zhu, X.; Wen, J.; You, L.; et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol. Res. 2016, 4, 49–60.
- Mathios, D.; Park, C.K.; Marcus, W.D.; Alter, S.; Rhode, P.R.; Jeng, E.K.; Lim, M. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int. J. Cancer 2016, 138, 187–194.
- Chen, W.; Liu, N.; Yuan, Y.; Zhu, M.; Hu, X.; Hu, W.; Wang, S.; Wang, C.; Huang, B.; Xing, D. ALT-803 in the treatment of non-muscle-invasive bladder cancer: Preclinical and clinical evidence and translational potential. Front. Immunol. 2022, 13, 1040669.
- Kowalczyk, J.T.; Fabian, K.P.; Padget, M.R.; Lopez, D.C.; Hoke, A.T.; Allen, C.T.; Hermsen, M.; London, J.N.R.; Hodge, J.W. Exploiting the immunogenic potential of standard of care radiation or cisplatin therapy in preclinical models of HPV-associated malignancies. J. Immunother. Cancer 2022, 10, e005752.
- Wolfson, B.; Padget, M.R.; Schlom, J.; Hodge, J.W. Exploiting off-target effects of estrogen deprivation to sensitize estrogen receptor negative breast cancer to immune killing. J. Immunother. Cancer 2021, 9, e002258.
- Fujii, R.; Jochems, C.; Tritsch, S.R.; Wong, H.C.; Schlom, J.; Hodge, J.W. An IL-15 superagonist/IL-15Ralpha fusion complex protects and rescues NK cell-cytotoxic function from TGF-beta1-mediated immunosuppression. Cancer Immunol. Immunother. 2018, 67, 675–689.
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Investig. 2017, 127, 4042–4058.
- Van der Meer, J.M.R.; Maas, R.J.A.; Guldevall, K.; Klarenaar, K.; de Jonge, P.K.J.D.; Evert, J.H.V.; Dolstra, H. IL-15 superagonist N-803 improves IFNgamma production and killing of leukemia and ovarian cancer cells by CD34(+) progenitor-derived NK cells. Cancer Immunol. Immunother. 2021, 70, 1305–1321.
- Chu, Y.; Nayyar, G.; Jiang, S.; Rosenblum, J.M.; Soon-Shiong, P.; Safrit, J.T.; Lee, D.A.; Cairo, M.S. Combinatorial immunotherapy of N-803 (IL-15 superagonist) and dinutuximab with ex vivo expanded natural killer cells significantly enhances in vitro cytotoxicity against GD2(+) pediatric solid tumors and in vivo survival of xenografted immunodeficient NSG mice. J. Immunother. Cancer 2021, 9, e002267.
- Fousek, K.; Horn, L.A.; Qin, H.; Dahut, M.; Iida, M.; Yacubovich, D.; Hamilton, D.H.; Thomas, A.; Schlom, J.; Palena, C. An Interleukin-15 Superagonist Enables Antitumor Efficacy of Natural Killer Cells Against All Molecular Variants of SCLC. J. Thorac. Oncol. 2023, 18, 350–368.
- Knudson, K.; Hicks, K.; Alter, S.; Schlom, J.; Gameiro, S.R. Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. J. Immunother. Cancer 2019, 7, 82.
- Fabian, K.P.; Padget, M.R.; Fujii, R.; Schlom, J.; Hodge, J.W. Differential combination immunotherapy requirements for inflamed (warm) tumors versus T cell excluded (cool) tumors: Engage, expand, enable, and evolve. J. ImmunoTherapy Cancer 2021, 9, e001691.
- Felices, M.; Wesley, E.; Bendzick, L.E.; Kodal, B.; Hopps, R.; Grzywacz, B.; Hinderlie, P.; Miller, J.S.; Geller, M.A. Reverse translation identifies the synergistic role of immune checkpoint blockade and IL-15 to enhance immunotherapy of ovarian cancer. Cancer Immunol. Res. 2023, 11, 674–686.
- Hoke, A.T.; Padget, M.R.; Fabian, K.P.; Nandal, A.; Gallia, G.L.; Bilusic, M.; Soon-Shiong, P.; Hodge, J.W.; London, J.N.R. Combinatorial Natural Killer Cell-based Immunotherapy Approaches Selectively Target Chordoma Cancer Stem Cells. Cancer Res. Commun. 2021, 1, 127–139.
- Fabian, K.P.; Padget, M.R.; Donahue, R.N.; Solocinski, K.; Robbins, Y.; Allen, C.T.; Hodge, J.W. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J. Immunother. Cancer 2020, 8, e000450.
- Hicks, K.C.; Knudson, K.M.; Lee, K.L.; Hamilton, D.H.; Hodge, J.W.; Figg, W.D.; Ordentlich, P.; Jones, F.R.; Rabizadeh, S.; Soon-Shiong, P.; et al. Cooperative Immune-Mediated Mechanisms of the HDAC Inhibitor Entinostat, an IL15 Superagonist, and a Cancer Vaccine Effectively Synergize as a Novel Cancer Therapy. Clin. Cancer Res. 2020, 26, 704–716.
- Fabian, K.P.; Malamas, A.S.; Padget, M.R.; Solocinski, K.; Wolfson, B.; Fujii, R.; Sater, H.A.; Schlom, J.; Hodge, J.W. Therapy of Established Tumors with Rationally Designed Multiple Agents Targeting Diverse Immune-Tumor Interactions: Engage, Expand, Enable. Cancer Immunol. Res. 2021, 9, 239–252.
- Gomes-Giacoia, E.; Miyake, M.; Goodison, S.; Sriharan, A.; Zhang, G.; You, L.; Egan, J.O.; Rhode, P.R.; Parker, A.S.; Chai, K.X.; et al. Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PLoS ONE 2014, 9, e96705.
More
