Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
The current global healthcare crisis underpins the importance of point-of-care technologies to provide a cost-effective solution to address the unmet healthcare needs [39,40,41]. With a focus on providing rapid detection, POC devices are pivotal in containing disease, particularly those that are highly infectious. Additionally, the availability of POC devices will save time, cost, and travel for patients, particularly those who require frequent testing. This will also increase access to medical care for the underserved population [41,42]. Overall, POC devices promise to alleviate the tradeoff between high cost and poor accessibility of testing, which makes them a robust solution.
- optical sensor
- fluorescence sensor
- biosensor
1. Detection of Bacterial Infection
Bacterial infection is one of the leading causes of global mortality, owing to widespread use of antibiotics, giving rise to antimicrobial resistant strain, which has now become an emerging threat to the global healthcare system [1][2][3]. More than 2.8 million antimicrobial-resistant infections occur in US each year [3]. Some common methods of detection include bacterial culture, polymerase chain reaction, and immunoassays [4][5][6]. These methods do provide precise results but require sophisticated equipment, dedicated laboratory space, and take a long time to generate results. Many fluorescence-based portable devices have been explored in an effort to overcome the limitations of conventional lab-based techniques [7][8][9][10][11]. For example, Sheini et al. developed an ultrafast detection system for the rapid sensing of Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa, which are responsible for causing sepsis in children [12]. The authors developed an opto-electronic tongue (opto-E tongue) with a paper-based substrate for the effective detection of multiple bacteria species in the serum sample. The E-tongue consists of several selective fluorescent sensors, each of which has a unique response to different bacteria. The response of these sensor molecules against each bacterium species is generated in the form of a specific pattern (Figure 1), therefore making it possible to monitor and differentiate pathogenic bacterial species in a complex mixture. The sensors were based on copper (CuNCs) and gold nanoclusters (AuNCs) stabilized with proteins—pepsin, trypsin, ovalbumin, and glutathione. The detection assay, composed of six rectangular detection zones made of paper substrate (1.5 cm × 1.0 cm), was impregnated with Cu and Au NCs, which were separated by hydrophobic barrier created using an ink printing method (Figure 1). A serum sample with or without bacterial contamination was taken on a clean glass substrate and pressed on the paper sensor for interaction. The response of the sensors to each specified bacteria was monitored at an optimum pH close to serum, using a smartphone-based portable fluorometer. In the fluorometer, the paper assay device (PAD) was placed in a sample holder and irradiated with a UV light at 365 nm, as shown in Figure 1. The cabinet was equipped with a smartphone to record the fluorescence intensity change. Analyses of “before” and “after” images of each detection zone were performed using ImageJ software. The CuNCs under UV light showed a blue emission, and AuNCs showed a red fluorescence emission. After interaction with the bacterial contaminated sample, the NCs underwent fluorescence quenching. This was due to the aggregation of the NCs caused by the functional groups present on the protein shell of the NCs and the active sites of the peptidoglycan in the cell wall of the bacteria, which subsequently caused dynamic or static quenching of the NCs. Each of the CuNCs and AuNCs have a different affinity towards different functional moiety, further distinguishing between “Gram positive” (thick peptidoglycan cell wall, less lipopolysaccharide layer) and “Gram negative” bacteria (thin peptidoglycan layer, thick lipopolysaccharide layer). The limit of detection using this fluorometric PAD device was <50 Cfu/mL for S. aureus, S. pyogenes, E. coli, and P. aeruginosa (RSD < 5%). They further tested a spiked serum sample and reported a recovery percentage of 93–107%.

Figure 1. (A) Schematic diagram showing NCs interaction with bacteria in a sample, followed by fluorescence quenching. (B) Stepwise illustration of a paper-based assay system for sensing bacteria using a smartphone as a readout device for point-of-care application [12].
In another study, Xie et al., designed a point-of-care test for Mycobacterium tuberculosis (Mtb), using a naturally secreted enzyme by tubercle bacilli, BlaC, as a marker and BlaC-specific fluorogenic substrate, a chemically modified cephalosporin-based fluorescence molecule, as probe [13]. The detection mechanism was based on the enzymatic hydrolysis of the fluorogenic substrate by the BlaC enzyme releasing the fluorophore, thus enhancing the fluorescence signal intensity. A green-fluorescent probe—Tokyo green—was modified and coupled with cephalosporin to develop the highly stable CDG-OMe, fluorogenic substrate. In the presence of BlaC, the enzyme reacted selectively with the substrate, and there was a significant increase, more than ~200-fold, in the fluorescence intensity at 520 nm. For the practical point-of-care application, they further fabricated a simple device integrated with an LED light source, excitation and emission filters, and a smartphone. Images captured with a smartphone through the box hole made it possible to detect ~10 c.f.u live Mtb in the human sputum in less than 10 min. Therefore, this rapid TB diagnostic tool can be used for the sensitive and selective detection of low quantities of Mycobacterium present in the human sputum and other bodily fluids, without the need for prior sample preparation and additional complicated steps.
2. Detection of Anions and Cations
Metal ions are essential for maintaining a healthy life, as they are responsible for the regulation of cell-to-cell interaction; the proper functioning of nerve cells, the brain, and heart; muscle cells; DNA regulation; transporting oxygen; maintaining osmotic pressure; and many other biological processes [14][15]. Change in their concentrations can cause severe malfunctions in the body, e.g., growth disorders, carcinogenesis, or even death [15]. Therefore, the precise quantification of anions and cations serves as a major tool for understanding any underlying disease condition, and for maintaining a person’s overall well-being. For example, fluoride ion is an essential element that provides strength to bones and teeth, preventing dental cavities, osteofluorosis, etc. [16][17]. Common detection techniques include ion chromatography, spectroscopy, and ion-selective electrodes, all of which require complicated equipment and sample pre-treatment, making the detection process exhaustive [18][19]. Fluorescent probes, on the other hand, provide sensitive simple detection approach [20][21][22][23][24][25][26][27][28][29][30]. For “on-site” detection, Yu et al. fabricated a multicolor ratiometric fluorescent test paper for the point-of-care detection of fluoride ions (F- ions) in water using filter paper [31]. The test paper (3.5 × 1 cm2) was prepared by an ink-printing method using “ink” made by mixing a fluoride-sensitive organic probe (C-TIPS) and F- ion inert red CdTe quantum dots in optimal proportions. The fluorescent sensor showed a fluorescence “turn-on” effect upon binding to fluoride ions. The test paper exhibited a distinguishable fluorescence color change from red to purple to blue under a UV lamp. The probe C-TIPS was fabricated using 7-hydroxycoumarin, and the blue fluorescence of the compounds was chemically quenched by covalently conjugating it with triisopropylsilyl chloride (TIPS). Upon binding to the F- ion, the recovery of the highly bright blue fluorescence suggested the removal of the TIPS moiety from the compound by cleaving the Si-O bond, thus releasing very bright blue, fluorescent coumarin in the system. The overlapping broad absorbance and excitation wavelength of the CdTe QDs, 7-hydroxycoumarin, and C-TIPS allowed the ratiometric analysis using same excitation wavelength at 365 nm. This sensor showed high sensitivity and selectivity toward F- ions. In the presence of F- ions, the emission peak of the sensor, at 455 nm, gradually increased, which was proportional to the concentration of F- ions, while the emission peak 630 nm remained unchanged. The limit of detection was calculated to be ~0.285 μM. The practicality of the sensor was tested using lake water, tap water, well water, and human urine spiked with F- ions, and showed the excellent potential of the sensor to detect F- ions in a real sample, with a recovery percentage of 96.5%. The change in fluorescence color could be distinguished by the naked eye, thus allowing on-site visual assay of F- ions in environmental water and biological fluids without any pre-treatment.
In another study, Zhang et al. established a point-of-care sweat diagnostics technique for the detection of chloride ions. They achieved this by designing a low-cost citrate-derived fluorescence sensor-based chloridometer, operated using a smartphone [32]. Citric acid (CA)-modified cysteine acted as a potential fluorescent chloride sensor to measure the chloride level present in sweat, which is an essential marker for cystic fibrosis diagnosis [32]. Apart from its cost effectiveness and high chloride selectivity, CA-cysteine was also selected as a fluorescent sensor for its exceptional photostability, high quantum yield (81%), and longer lifetime (10 ns) compared to other chloride sensors. An ultraviolet LED at 365 nm was used to excite CA-cysteine, which generated a sharp blue fluorescence signal with an emission maximum of 441 nm. The fluorescent signal was subsequently captured and measured by the smartphone camera, as shown in Figure 2. In the presence of chloride ions, the blue fluorescence signal of the CA-cysteine solution underwent fluorescence quenching, which can be attributed to the non-radiative relaxation of the excited fluorophore due to the presence of chloride ions. The detection limit was reported to be ~0.8 mM. Furthermore, successful clinical validation of the device was performed using sweat from both healthy subjects and cystic fibrosis patients.
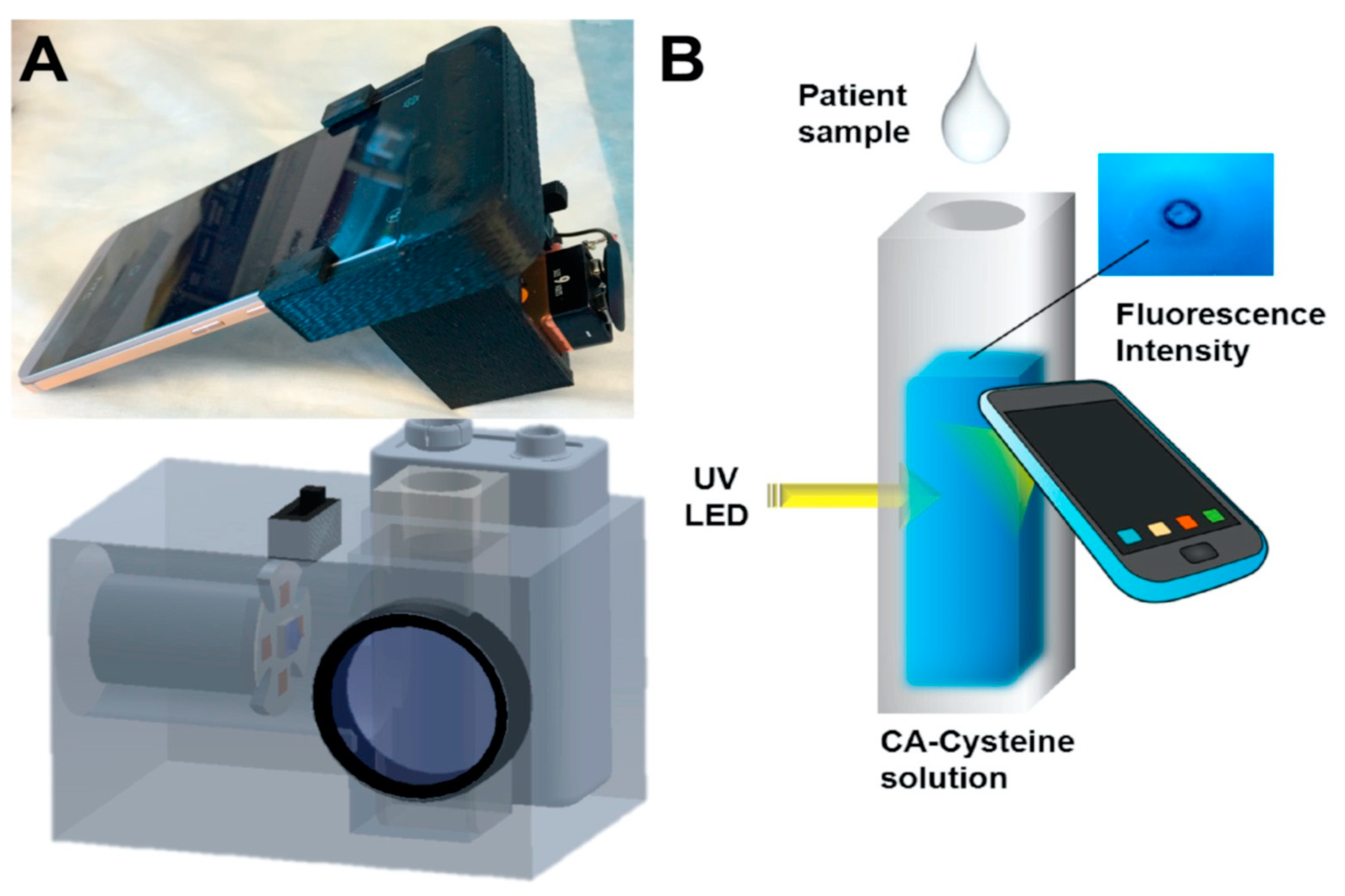
Figure 2. (A) Photo image and schematic diagram of chloridometer system. (B) Schematic illustration of the detection of chloride ions using a smartphone device for point-of-care application [32].
Chromium (Cr3+) is another important cation in the body, and its imbalance (excess or deficiency) has been correlated to diabetes, kidney toxicity, and cardiovascular diseases [33]. Zhang et al., fabricated a hydrogel digital assay based on metal-AIEgen frameworks (MAFs) for the ultrasensitive detection of Cr3+ ions in water, food, and biological fluids by successfully integrating the hydrogel assay with a smartphone readout device [33]. They developed a white emissive MAFs@QDs-PVP hydrogel sensor complex by incorporating blue emissive MAFs (Ex./Em ~310 nm/465 nm) with high quantum yield ~99% and red emissive QDs (Em. 604 nm) into a polyvinylpyrrolidone (PVP) hydrogel matrix, and the combined fluorescent sensor was freeze dried to form a sponge-like hydrogel sensor. The hydrogel swells once the analyte solution comes into contact with it. The analytical performance of the sensor was tested using Cr3+ ions, and blue fluorescence-quenching of the MAFs was observed almost instantaneously. The limit of detection was calculated to be ~0.1 nM. In the presence of Cr3+ ions, the color of the white emissive sensor transitioned to red fluorescence, due to the quenching of blue emissive MAFs. Cr3+ ions selectively bind to the surface of the MAFs, thereby quenching the blue fluorescence of the hydrogel sensor due to the energy transfer between the Cr3+ ions and the MAFs, owing to their overlapping absorption and fluorescence emission wavelength. Furthermore, the sensor was tested with biological fluids such as saliva, urine, and serum, and a recovery percentage of ~103–110% was observed in spiked samples. For the point-of-care application, the images of the hydrogel sensor before and after Cr3+ ion interaction were captured using a smartphone and quantitatively analyzed using RGB color space. Additionally, they also demonstrated a paper-based lateral flow fluorescence immunoassay (LFIA), using MAFs for the effective detection of alpha-fetoprotein (AFP), a serum biomarker for hepatocellular carcinoma. The LFIA comprised of AFP-antibody1 (Ab1)-conjugated MAFs, a secondary Ab (Ab2) for capturing AFP, and an anti-Ab on a paper platform. Once the AFP was captured and deposited on the test line, a bright fluorescent light was observed under UV light. The estimated LOD of 0.6 pg/mL was achieved with this method. The reliability of the LFIA was evaluated using AFP-spiked human serum and with patient urine samples. The sensitivity of this MAFs LFIA was 100–1000 fold higher than traditional QDs-based LFIAs [33].
3. Detection of Biochemical Analytes
Biochemical compounds are the building blocks of life. For example, carbohydrates, lipids, proteins, nucleic acids, and enzymes are responsible for different functions such as energy storage, acting as messengers, helping in the maintenance of cell structure, and carrying out important chemical reactions, amongst others [34][35]. An imbalance of these biomolecules can cause several life-threatening diseases, such as kidney dysfunction, jaundice, sickle cell anemia, cardiovascular diseases, hormonal imbalance, diabetes etc. [36][37]. Therefore, the precise monitoring of biochemical analytes is crucial for disease management and to maintain a healthy life. Guo et al., developed a dual emissive ratiometric fluorescent sensor molecule integrated with a smartphone for the accurate sensing of glucose (Glu) and cholesterol (Chol) in patients with metabolic syndrome [38]. The amount of Glu and Cho was determined by the quantification of H2O2, which is the principal product produced in the presence of glucose oxidase (GOx) and cholesterol oxidase (ChOx). The dual emissive sensor molecule was composed of AgNPs/UiO-66-NH2, where silver nanoparticles (AgNPs) acted as sensing molecules. The AgNPs were absorbed on a metal organic framework (MOF) UiO-66-NH2, with sodium borohydride and OPD (o-phenylenediamine) acting as a chromogenic substrate. In the presence of GOx and ChOx, the analyte glucose and cholesterol are converted to H2O2 which, in turn, etches AgNPs to release Ag+ ions, and further oxidizes OPD to fluorescent 2,3-diaminophenazine (DAP) in situ. Due to the overlapping emission profile of AgNPs/UiO-66-NH2 (Ex. 360 nm, Em. 425 nm) and the absorbance of DAP (emission 555 nm), the fluorescence intensity ratio of F555nm/F425nm showed gradual enhancement, which was proportional to the target analyte, inducing inner-filter effect-based ratiometric sensing. The sensor showed excellent sensitivity and selectivity toward H2O2, with a detection limit of 0.2 μmol/L. As both GOx and ChOx could convert O2 to H2O2, the H2O2 dependent signal was translated to quantify the amount of glucose and cholesterol in the human serum samples. The quantified Glu and Cho were determined to be ~0.5 and ~0.7 μmol/L, respectively. Additionally, the fluorescence color changes from blue to yellow green are easily distinguishable by the naked eye as well. For on-site application, the sensor AgNPs/UiO-66-NH2, along with OPD, was immobilized on a paper substrate, which was integrated with a 3D printed smartphone attachment device, where the test strip was placed in a holder and excited by an in-built UV lamp at 365 nm, as shown in Figure 3. The captured images were processed in the RGB color space with a smartphone app. The recovery rates were found to be ~90% to 101%, using a spiked human serum sample, justifying the potential of these test strips for the quantitative analysis of glucose (Glu) and cholesterol (Chol) present in the human serum sample without any pre-treatment.
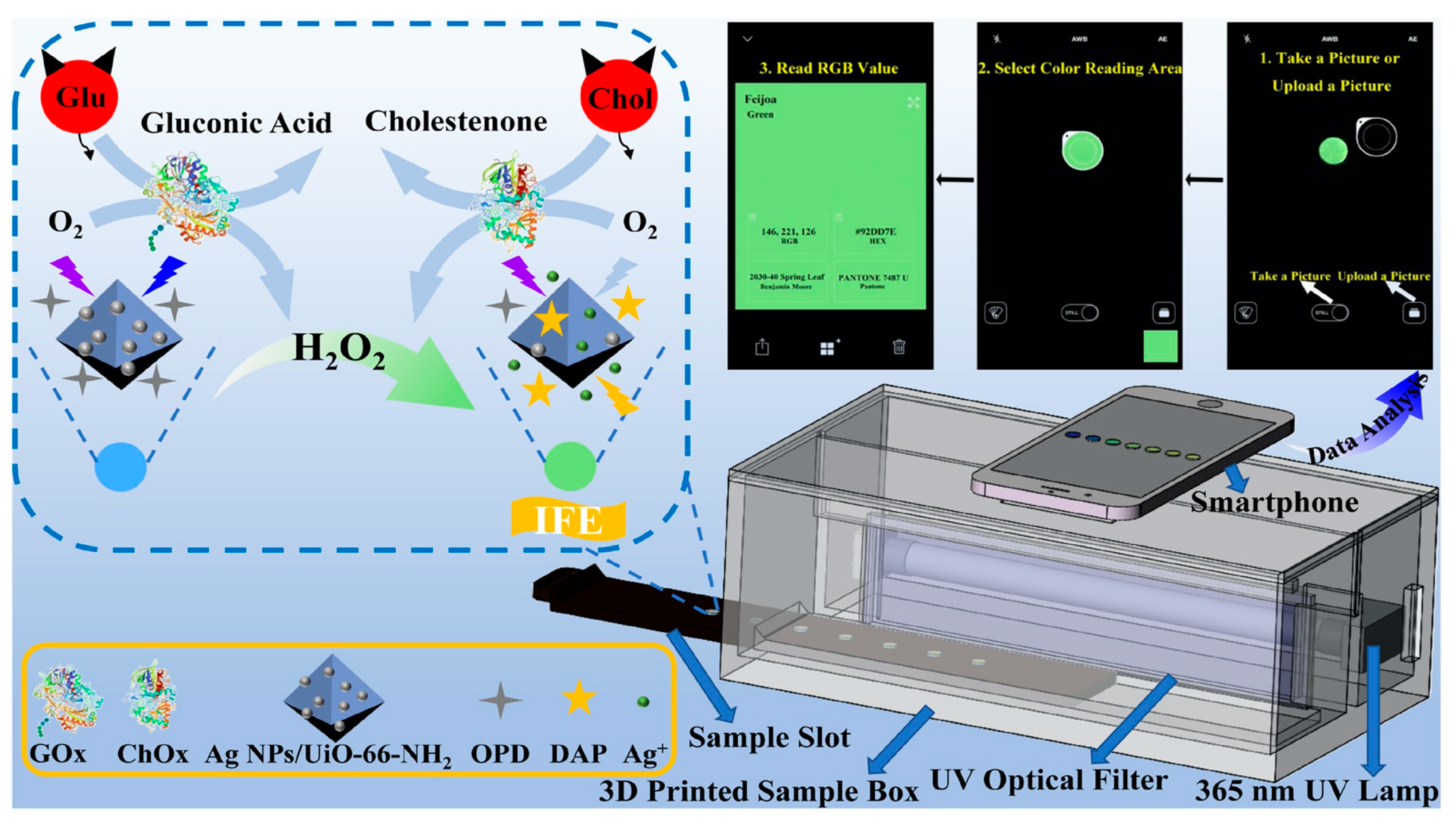
Figure 3. Schematic diagram of the o-Phenylenediamine (OPD) and Ag NPs/UiO-66-NH2 composite film with a smartphone readout device for glucose (Glu) and cholesterol (Chol) testing at the point- of-care [38].
In another study, Wang et al. developed a hybrid ratiometric fluorescence sensor molecule based on an aggregation induced emission (AIE) of hyperbranched polymer nanoaggregate of tetraphenylethylene (HPA-TPE) and Rhodamine B (RhB) for the selective and sensitive testing of free bilirubin in water and urine samples [39]. In patients with liver dysfunction, free bilirubin tends to get accumulated in the body, causing gallbladder disease, anemia, and neurotoxicity [40][41]. The HPA-TPENA/RhB hybrid fluorescence sensor molecule was fabricated by dissolving and mixing HPA-TPE polymer nanoaggregate and Rhodamine B individually in phosphate buffer (pH = 7.4). When the system was irradiated with UV-light at 355 nm, both RhB and HPA-TPENA were excited, thereby producing dual emission peaks at 577 nm and 465 nm [39]. HPA-TPENA/RhB together showed weaker emission intensity at 465 nm, compared to HPA-TPENA alone. This is due to the overlap between emissions of HPA-TPENA (465 nm) and the broad absorption peak of RhB at ~400–550 nm, causing an energy transfer between them. The addition of bilirubin exhibited a linear reduction of the fluorescence intensity ratio I465/I577 with the increment of bilirubin concentration. The blue emission, with a peak at 465 nm, was significantly suppressed due to the Förster resonance energy transfer (FRET) quenching between the bilirubin and polymer nanoaggregate, whereas the Rhodamine B emission peaked at 577 nm and did not show any sensitivity to bilirubin. This eventually shifted the fluorescence peak from blue to orange, which was observable. This mechanism resulted in a detection limit of ~25 nM in the solution phase, with recovery values in the range of ~92.5–103.3%, and relative standard deviations being less than 6.1%. They further built a portable and affordable point-of-care bilirubin detection device by incorporating xanthum gum hydrogel with this hybrid fluorescence sensor, coupled with a handheld UV light and smartphone for capturing images, as shown in Figure 4. The hydrogel showed a blue–white emission, which gradually changed to a yellow color upon increasing the concentration of bilirubin. This device was also successfully tested on human urine for quantifying bilirubin.
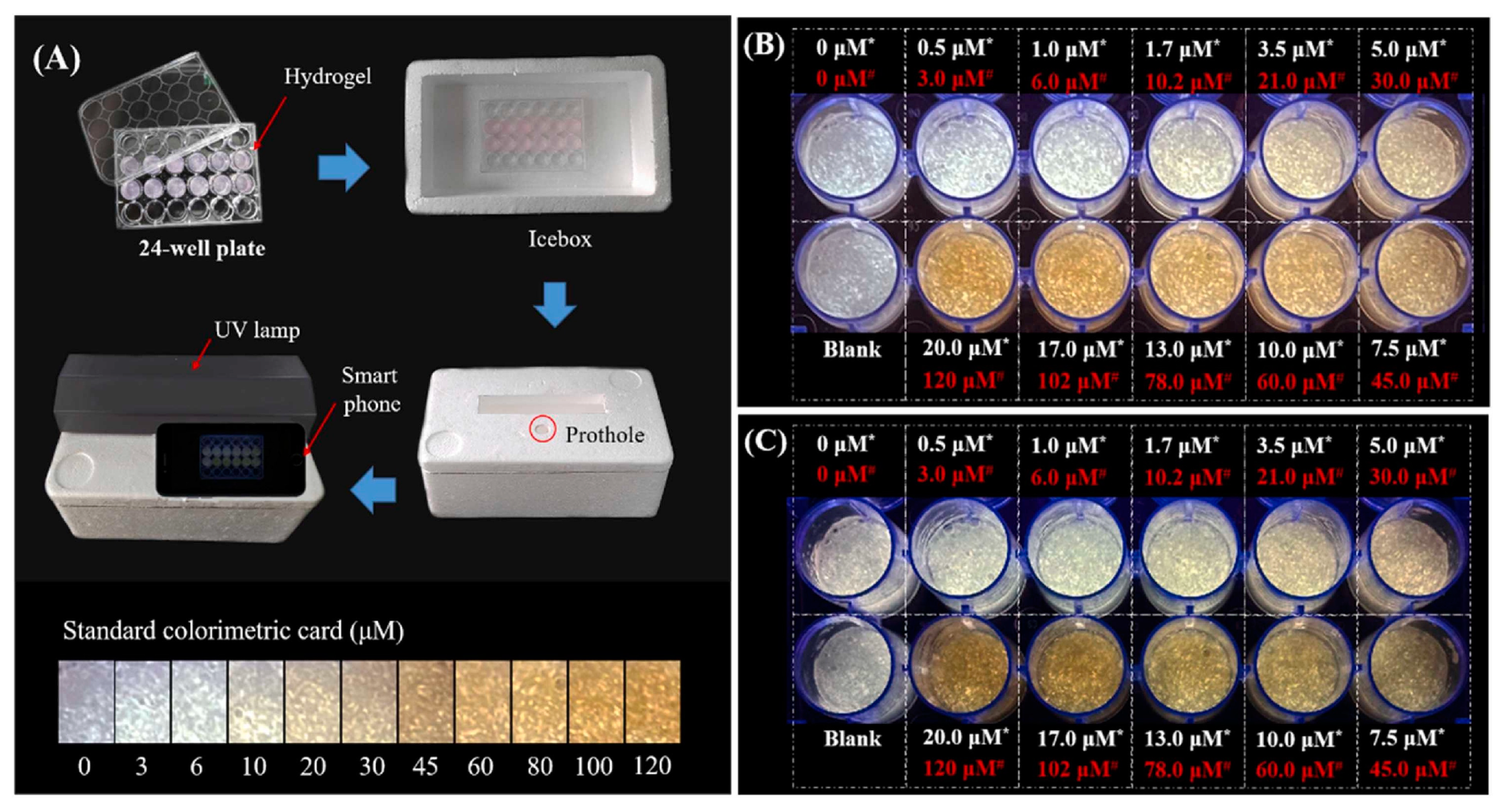
Figure 4. (A) Schematic diagram of the point-of-care testing system set-up; (B,C) the hydrogel fluorescence images after incubation with bilirubin spiked PBS buffer and urine, respectively. (Here, the hydrogel-incorporated hybrid nanosensor exhibited a white emission, while yellow represents the concentration of bilirubin in water or urine samples before mixing) [39].
Enzymes are essential elements for metabolic digestion in humans. They also serve as a specific biomarker, for example, of elevated levels of trypsin or amylase, which are associated with patients suffering from pancreatitis. The normal range of trypsin in human serum is 115–350 ng/mL, an excess of which can lead to pancreatic cancer and cystic fibrosis [42][43][44]. Li et al. developed a fluorescence nano-sensing platform for the detection of trypsin (TRY) by utilizing glutathione-capped gold nanoclusters (AuNCs) [45]. Glutathione (GSH) was used as a template and stabilizer during the AuNC synthesis process. AuNCs typically have an excitation peak at 418 nm, with an emission peak observed at 625 nm. The fluorescence intensity of the AuNC was quenched by conjugating positively charged cytochrome C (Cyt C) through an electron transfer (ET) process. The catalyzing effect of TRY hydrolyzed the Cyt C, causing it to break down into negatively charged heme-peptide particles, thus weakening the ET transfer, which subsequently induced the fluorescence recovery of AuNCs. The sensor reportedly has a detection limit of ~0.08 μg mL−1 in a phosphate buffer solution. The practical implementation of the AuNCs-Cyt C-based platform was successfully demonstrated by analyzing TRY in spiked human serum and urine samples, with a reported recovery range between 95% and 109%, with RSD less than 4.06%. Furthermore, paper-based test strips were developed by immobilizing the AuNCs-Cyt C on absorbent papers for point-of-care detection application. The fluorescent images were captured using a smartphone and split into RGB values using ImageJ software. With the help of an image processing algorithm, a relationship between the RGB values and the logarithmic TRY concentration was established, in order to quantify the concentration.
Recently, a novel ultrasensitive fluorescence-based bioanalytical platform for the detection of multiple different biomarkers was developed [46][47][48]. In this study the authors explored nanostructures comprised of silica nanoparticles immobilized with natural NAD+-dependent or chimeric PQQ-dependent enzymes. The nanoconjugate was spotted on a fiberglass support. PQQ and NAD+-dependent enzymes are important in many cellular processes, and their activity can be used as a biomarker for various diseases. The substrate was designed to produce a fluorescent signal upon reaction with PQQ or NAD+-dependent enzymes via a substrate-stimulated enzymatic reduction of a fluorescence probe phenazine methosulfate or its derivatives (Ex. 365 nm, Em 465 nm). In the presence of the analyte substrate, the immobilized enzymes reacted with it, changing the color of the spot attributed to the reduced form of the dye. Images of the colored spot were captured using a smartphone camera in grayscale and processed via ImageJ software. The sensitivity was enhanced by using the multilayer formation of enzyme-functionalized on the silica nanoparticles, resulting in the amplification of the fluorescence intensity of the reduced dye in the sensor spot. The sensor platform was successfully tested on different biological fluids, such as serum, urine, saliva, and sweat, for the detection of different biomarkers, such as glucose and alcohol (LOD 10 μM), α-amylase (LOD 2 pM), and some immunosuppressive drugs (cyclosporin A and tacrolimus with LOD 2 pM).
Albumin is a protein abundantly present in the human body. Abnormal levels of albumin, especially in urine, was observed in patients with kidney disease, indicating acute kidney failure, polycystic kidney disease, vascular disease, and neoplasia, and it therefore serves as an essential biomarker [49][50]. Most of the fluorescent probes are incapable of detecting urinary albumin with precise accuracy, as urine itself displays strong fluorescence, causing significant interference with high background noise [51][52]. Zhao et al. developed a novel olefin-extended chalcone fluorescent probe DNC, with an enlarged transition dipole moment (Δμ) and an emission wavelength >600 nm, for the point-of-care detection of albuminuria in urine [53]. The red emissive fluorescent probe, DNC, far from the excitation and emission range of the urine, helped in avoiding the spectral interference from urine fluorescence. Upon excitation at 480 nm, the fluorescence from urine was negligible at 610 nm, thereby ensuring a low background signal compared to the DNC fluorescence emission in response to albumin. This eventually provided an improved and amplified signal response against albumin. The fluorescence of DNC was significantly enhanced due to binding with the specific sites (drug sites) of albumin, and it demonstrated a highly sensitive and selective determination of albumin with a detection limit of 23 nM in solution. The sensor was tested using a urine sample spiked with albumin, and it was able to detect albumin concentration of ~61 nM with RSD < 12%. An easy-to-use, smartphone-based POC system was developed for testing clinical samples collected from the patient. The mobile phone was used to capture images of the sensor, DNC, which was then analyzed using ImageJ software. The negative samples exhibited a blue color emission, while the sample spiked with albumin gradually changed from blue to a blue-violet to carmine color, which was easily distinguished by the naked eye. These results showed that the DNC probe can be successfully used to diagnose clinical A2-level and A3-level albuminuria in urine samples without pre-treatment.
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P T 2015, 40, 277–283.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318.
- Kadri, S.S. Key Takeaways From the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945.
- Nath, P.; Kabir, A.; Khoubafarin Doust, S.; Kreais, Z.J.; Ray, A. Detection of Bacterial and Viral Pathogens Using Photonic Point-of-Care Devices. Diagnostics 2020, 10, 841.
- Peri, A.M.; Stewart, A.; Hume, A.; Irwin, A.; Harris, P.N.A. New Microbiological Techniques for the Diagnosis of Bacterial Infections and Sepsis in ICU Including Point of Care. Curr. Infect. Dis. Rep. 2021, 23, 12.
- Tsalik, E.L.; Bonomo, R.A.; Fowler, V.G., Jr. New Molecular Diagnostic Approaches to Bacterial Infections and Antibacterial Resistance. Annu. Rev. Med. 2018, 69, 379–394.
- Kim, J.; Park, J.-Y.; Park, Y.-J.; Park, S.-Y.; Lee, M.-S.; Koo, C. A portable and high-sensitivity optical sensing system for detecting fluorescently labeled enterohaemorrhagic Escherichia coli Shiga toxin 2B-subunit. PLoS ONE 2020, 15, e0236043.
- Zhou, Q.; Pan, J.; Mo, L.; Luo, Z.; Qin, Z.; Dai, Z.; Yi, C. Fluorescent on-site detection of multiple pathogens using smartphone-based portable device with paper-based isothermal amplification chip. Mikrochim. Acta 2022, 189, 333.
- Papadakis, G.; Pantazis, A.K.; Fikas, N.; Chatziioannidou, S.; Tsiakalou, V.; Michaelidou, K.; Pogka, V.; Megariti, M.; Vardaki, M.; Giarentis, K.; et al. Portable real-time colorimetric LAMP-device for rapid quantitative detection of nucleic acids in crude samples. Sci. Rep. 2022, 12, 3775.
- Huang, J.; Zhong, Y.; Li, W.; Wang, W.; Li, C.; Wang, A.; Yan, H.; Wan, Y.; Li, J. Fluorescent and Opt-Electric Recording Bacterial Identification Device for Ultrasensitive and Specific Detection of Microbials. ACS Sens. 2021, 6, 443–449.
- Shin, J.; Yoon, T.; Park, J.; Park, K.S. Sensitive and simultaneous detection of hygiene indicator bacteria using an enhanced CRISPR/Cas system in combination with a portable fluorescence detector. Sens. Actuators B Chem. 2022, 365, 131871.
- Sheini, A. A point-of-care testing sensor based on fluorescent nanoclusters for rapid detection of septicemia in children. Sens. Actuators B Chem. 2021, 328, 129029.
- Xie, H.; Mire, J.; Kong, Y.; Chang, M.; Hassounah, H.A.; Thornton, C.N.; Sacchettini, J.C.; Cirillo, J.D.; Rao, J. Rapid point-of-care detection of the tuberculosis pathogen using a BlaC-specific fluorogenic probe. Nat. Chem. 2012, 4, 802–809.
- Moustakas, M. The Role of Metal Ions in Biology, Biochemistry and Medicine. Materials 2021, 14, 549.
- Lakatos, B.; Szentmihályi, K.; Vinkler, P.; Balla, J.; Balla, G. The role of essential metal ions in the human organism and their oral supplementation to the human body in deficiency states. Orv. Hetil. 2004, 145, 1315–1319.
- Martínez-Mier, E.A. Fluoride: Its Metabolism, Toxicity, and Role in Dental Health. J. Evid. Based. Complement. Altern. Med. 2011, 17, 28–32.
- Duffin, S.; Duffin, M.; Grootveld, M. Revisiting Fluoride in the Twenty-First Century: Safety and Efficacy Considerations. Front. Oral Health 2022, 3, 873157.
- Zheng, X.; Cheng, W.; Ji, C.; Zhang, J.; Yin, M. Detection of metal ions in biological systems: A review. Rev. Anal. Chem. 2020, 39, 231–246.
- Dean, K.M.; Qin, Y.; Palmer, A.E. Visualizing metal ions in cells: An overview of analytical techniques, approaches, and probes. Biochim. Biophys. Acta 2012, 1823, 1406–1415.
- Zhang, L.; Gao, X.; Chen, X.; Zhao, M.; Wu, H.; Liu, Y. A smartphone integrated ratiometric fluorescent sensor for point-of-care testing of fluoride ions. Anal. Bioanal. Chem. 2022, 414, 3999–4009.
- Yang, Q.; Jia, C.; Chen, Q.; Du, W.; Wang, Y.; Zhang, Q. A NIR fluorescent probe for the detection of fluoride ions and its application in in vivo bioimaging. J. Mater. Chem. B 2017, 5, 2002–2009.
- Dey, S.; Kumar, A.; Mondal, P.K.; Chopra, D.; Roy, R.; Jindani, S.; Ganguly, B.; Mayya, C.; Bhatia, D.; Jain, V.K. Ultrasensitive colorimetric detection of fluoride and arsenate in water and mammalian cells using recyclable metal oxacalixarene probe: A lateral flow assay. Sci. Rep. 2022, 12, 17119.
- Zhang, S.; Sun, M.; Yan, Y.; Yu, H.; Yu, T.; Jiang, H.; Zhang, K.; Wang, S. A turn-on fluorescence probe for the selective and sensitive detection of fluoride ions. Anal. Bioanal. Chem. 2017, 409, 2075–2081.
- Liu, X.; Liu, X.; Shen, Y.; Gu, B. A Simple Water-Soluble ESIPT Fluorescent Probe for Fluoride Ion with Large Stokes Shift in Living Cells. ACS Omega 2020, 5, 21684–21688.
- Yan, L.; Li, D.; Le, Y.; Dong, P.; Liu, L. Phenothiazine-based fluorescent probe for fluoride ions and its applications in rapid detection of endemic disease. Dye. Pigment. 2022, 201, 110200.
- Ma, Y.; Mou, Q.; Yan, P.; Yang, Z.; Xiong, Y.; Yan, D.; Zhang, C.; Zhu, X.; Lu, Y. A highly sensitive and selective fluoride sensor based on a riboswitch-regulated transcription coupled with CRISPR-Cas13a tandem reaction. Chem. Sci. 2021, 12, 11740–11747.
- Nakata, E.; Nazumi, Y.; Yukimachi, Y.; Uto, Y.; Hori, H.; Morii, T. Self-Assembled Fluorescent Nanoprobe for the Detection of Fluoride Ions in Aqueous Solutions. Bull. Chem. Soc. Jpn. 2014, 88, 327–329.
- Wu, X.; Wang, H.; Yang, S.; Tian, H.; Liu, Y.; Sun, B. Highly Sensitive Ratiometric Fluorescent Paper Sensors for the Detection of Fluoride Ions. ACS Omega 2019, 4, 4918–4926.
- Ke, B.; Chen, W.; Ni, N.; Cheng, Y.; Dai, C.; Dinh, H.; Wang, B. A fluorescent probe for rapid aqueous fluoride detection and cell imaging. Chem. Commun. (Camb) 2013, 49, 2494–2496.
- Chansaenpak, K.; Kamkaew, A.; Weeranantanapan, O.; Suttisintong, K.; Tumcharern, G. Coumarin Probe for Selective Detection of Fluoride Ions in Aqueous Solution and Its Bioimaging in Live Cells. Sensors 2018, 18, 2042.
- Yu, X.; Yang, L.; Zhao, T.; Zhang, R.; Yang, L.; Jiang, C.; Zhao, J.; Liu, B.; Zhang, Z. Multicolorful ratiometric-fluorescent test paper for determination of fluoride ions in environmental water. RSC Adv. 2017, 7, 53379–53384.
- Zhang, C.; Kim, J.P.; Creer, M.; Yang, J.; Liu, Z. A smartphone-based chloridometer for point-of-care diagnostics of cystic fibrosis. Biosens. Bioelectron. 2017, 97, 164–168.
- Zhang, J.; Li, Y.; Chai, F.; Li, Q.; Wang, D.; Liu, L.; Tang, B.Z.; Jiang, X. Ultrasensitive point-of-care biochemical sensor based on metal-AIEgen frameworks. Sci. Adv. 2022, 8, eabo1874.
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367.
- Shine, B.; Guha, N. The use of biochemical analysis for diagnosis and management. Oxford Textb. Med. 2020, 6577–C29.1.T11.
- Treacy, O.; Brown, N.N.; Dimeski, G. Biochemical evaluation of kidney disease. Transl. Androl. Urol. 2019, 8, S214–S223.
- Zadhoush, F.; Sadeghi, M.; Pourfarzam, M. Biochemical changes in blood of type 2 diabetes with and without metabolic syndrome and their association with metabolic syndrome components. J. Res. Med. Sci. 2015, 20, 763–770.
- Guo, L.; Chen, S.; Yu, Y.-L.; Wang, J.-H. A Smartphone Optical Device for Point-of-Care Testing of Glucose and Cholesterol Using Ag NPs/UiO-66-NH2-Based Ratiometric Fluorescent Probe. Anal. Chem. 2021, 93, 16240–16247.
- Wang, B.; Zhou, X.-Q.; Li, L.; Li, Y.-X.; Yu, L.-P.; Chen, Y. Ratiometric fluorescence sensor for point-of-care testing of bilirubin based on tetraphenylethylene functionalized polymer nanoaggregate and rhodamine B. Sens. Actuators B Chem. 2022, 369, 132392.
- Johnson, S.E.; Sherding, R.G. Diseases of the Liver and Biliary Tract. Saunders Man. Small Anim. Pract. 2006, 747–809.
- VanWagner, L.B.; Green, R.M. Evaluating elevated bilirubin levels in asymptomatic adults. JAMA 2015, 313, 516–517.
- Meher, S.; Mishra, T.S.; Sasmal, P.K.; Rath, S.; Sharma, R.; Rout, B.; Sahu, M.K. Role of Biomarkers in Diagnosis and Prognostic Evaluation of Acute Pancreatitis. J. Biomark. 2015, 2015, 519534.
- Kylänpää-Bäck, M.L.; Kemppainen, E.; Puolakkainen, P. Trypsin-based laboratory methods and carboxypeptidase activation peptide in acute pancreatitis. JOP 2002, 3, 34–48.
- Heinrich, H.C.; Gabbe, E.E.; Icagić, F. Immunoreactive serum trypsin in diseases of the pancreas. Klin. Wochenschr. 1979, 57, 1237–1238.
- Li, H.; Yang, M.; Kong, D.; Jin, R.; Zhao, X.; Liu, F.; Yan, X.; Lin, Y.; Lu, G. Sensitive fluorescence sensor for point-of-care detection of trypsin using glutathione-stabilized gold nanoclusters. Sens. Actuators B Chem. 2019, 282, 366–372.
- Melman, Y.; Wells, P.K.; Katz, E.; Smutok, O. A universal nanostructured bioanalytical platform for NAD+-dependent enzymes based on the fluorescent output reading with a smartphone. Talanta 2022, 243, 123325.
- Wells, P.K.; Smutok, O.; Guo, Z.; Alexandrov, K.; Katz, E. Fluorometric biosensing of α-amylase using an artificial allosteric biosensor immobilized on nanostructured interface. Talanta 2023, 255, 124215.
- Wells, P.K.; Smutok, O.; Guo, Z.; Alexandrov, K.; Katz, E. Nanostructured Interface Loaded with Chimeric Enzymes for Fluorimetric Quantification of Cyclosporine A and FK506. Anal. Chem. 2022, 94, 7303–7310.
- Ninomiya, T.; Perkovic, V.; de Galan, B.E.; Zoungas, S.; Pillai, A.; Jardine, M.; Patel, A.; Cass, A.; Neal, B.; Poulter, N.; et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 2009, 20, 1813–1821.
- Butt, L.; Unnersjö-Jess, D.; Höhne, M.; Edwards, A.; Binz-Lotter, J.; Reilly, D.; Hahnfeldt, R.; Ziegler, V.; Fremter, K.; Rinschen, M.M.; et al. A molecular mechanism explaining albuminuria in kidney disease. Nat. Metab. 2020, 2, 461–474.
- Shibata, K. Urine 3-hydroxykynurenine is higher during the postovulatory phase than in the preovulatory phase indicating a higher vitamin B6 requirement. Biosci. Biotechnol. Biochem. 2014, 78, 1757–1760.
- Birková, A.; Valko-Rokytovská, M.; Hubková, B.; Zábavníková, M.; Mareková, M. Strong Dependence between Tryptophan-Related Fluorescence of Urine and Malignant Melanoma. Int. J. Mol. Sci. 2021, 22, 1884.
- Zhao, X.; Zheng, W.; Qin, T.; Du, X.; Lei, Y.; Lv, T.; Zhou, M.; Xu, Z.; Wang, L.; Liu, B.; et al. An anti-interference fluorescent probe for point-of-care diagnosis of albuminuria. Sens. Actuators B Chem. 2022, 351, 130980.
More
