Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 4 by Jessie Wu.
Hydrothermal liquefaction (HTL) of biomass is establishing itself as one of the leading technologies for the conversion of virtually any type of biomass feedstock into drop-in biofuels and renewable materials.
- HTL
- catalyst
1. Catalysis during Hydrothermal Liquefaction
The role of a catalyst for biomass processing in hydrothermal liquefaction (HTL) to enhance biocrude yield and quality is intensely dependent upon several factors such as temperature, residence time, reactor system, etc. [1][2][3]. Among them, temperature is the most dominant operating parameter for HTL. Many studies have proven that temperature strongly affects the biocrude yield and elemental composition [4][5][6][7][8]. The overall temperature range for HTL varies from 270 to 400 °C [9]. Regardless of feedstock type, many studies show that biocrude yield increases with the rise in temperature (from 280 to 350 °C); however, a further increase in temperature generally decreases the biocrude yield [4][10][11][12]. In the majority of cases, the catalysts were applied in a subcritical temperature range (270−350 °C) [13][14][15][16][17][18], except in a few studies, where the catalyst effects in supercritical temperature (>373.94 °C) were explored [12][19][20][21][22][23][24][25]. All these authors stated different conclusions depending on the nature and type of catalyst employed.
Moderate temperatures (300−350 °C) facilitate the hydrolysis of biomass, condensation, and repolymerization of reactive substances to form biocrude [14][26]. However, temperatures above the critical point (373.94 °C) improve the degree of deoxygenation and offer higher HHVs [20]. Retention time (RT) is another important parameter, longer residence time, higher than 10 min, mostly increases the biocrude yield, however, above the threshold level, biocrude yield decreases on account of higher organic loss in the form of water-soluble organics to the aqueous phase or gases by cracking reactions [27]. Xu et al. stated the possible reasons behind the leveling off or decreasing the biocrude yield at prolonging retention times, which include cracking of biocrude components to gases, repolymerization to form char, and condensation to aqueous products [28]. Malins et al. used a catalyst (FeSO4) for sewage sludge at 300 °C in an autoclave under reaction times of 10 to 100 mins and reported a maximum biocrude yield of 48% at 40 min. On the other hand, prolonged RTs enhance the gaseous products, and the biocrude quality is improved through intermingling tar substances with biocrude that could positively affect the HHV [10]. Seehar et al. derived a different conclusion by conducting a catalytic (K2CO3) reaction time study on eucalyptus at 350 °C from 10 min to 25 mins and reported that 15 min is the best reaction time for the eucalyptus conversion [11]. Another study indicated that 10 min is the optimum RT for the HTL of lignocellulosic biomass (Cunninghamia lanceolata) at 320 °C [29].
The reactor system also influences the overall energy recovery of the HTL system, typically, longer RTs are selected for autoclave-based reactors that give slightly lower yields due to lower heating rates [6][7][10]. Alternatively, improved biocrude productivity has been observed by many studies adopting micro-batch reactors. Even so, shorter RTs in the range of 10 to 20 min are declared as ideal for biomass liquefaction in all micro-batch reactor-oriented systems [11][12][27][30].
2. Catalysis for Hydrothermal Liquefaction Biocrude Upgrading
HTL biocrude is a diverse pool of unsaturated organics containing significant amounts of contaminants such as oxygen, nitrogen, and inorganics in higher amounts and sulfur in lower amounts. Inevitably, the presence of these organic contaminants makes HTL biocrude an intermediate product with high TAN, high viscosity/density, low H/C, and poor thermal stability. Therefore, a downstream refining step is essential before HTL biocrudes can be utilized for the production of drop-in fuels. To date, the removal of organic contaminants via catalytic hydrotreatment has been a widely explored research area. During catalytic hydrotreatment, the removal of inorganics, O, N, and S takes place with reactions involving hydrodemetallization (HDM), hydrodeoxygenation (HDO), decarboxylation, decarbonylation, hydrodenitrogenation (HDN), hydrodesulfurization (HDS), and hydrogenation (HYD) [31][32][33]. In the literature, researchers have largely utilized both non-sulfided and sulfided catalysts. Most of these studies have been carried out in batch units. The main purpose of these efforts was to demonstrate the practicability of hydroprocessing for the treatment of HTL biocrudes from different feedstocks (such as lignocellulosic residues, algae, sewage sludge etc.) toward the production of drop-in fuels. Moreover, these batch hydrotreating studies also documented the effect of different sulfided/non-sulfided catalysts and operating conditions. Likewise, both families of catalysts have also been tested to some extent in continuous units. Hereafter, this section will comprehensively discuss and compare the effect of both non-sulfided and sulfided catalysts on the properties of hydrotreated oils (i.e., H/C, O/C, and N/C atomic ratios). Details of batch hydrotreating studies that utilized different non-sulfided and sulfided catalysts are listed in Table 1 and Table 2, respectively. The documented results of these batch hydrotreating studies are discussed and compared based on the fuel properties (such as H/C, O/C, and N/C atomic ratios) in this section.Table 1. Batch hydrotreatment of different HTL biocrudes in the presence of non-sulfided catalysts.
| HTL Biocrude | Non-Sulfided Catalysts | T | PH2 | Time | Hydrotreated Oils | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| (°C) | (MPa) | (h) | H/C | N/C | O/C | |||
| Chlorella pyrenoidosa | Pt/C | 400 | 6 | 4 | 1.76 | 0.026 | 0.029 | [34] |
| Chlorella pyrenoidosa | Ru/C | 400 | 6 | 4 | 1.68 | 0.026 | 0.010 | [34] |
| Chlorella pyrenoidosa | Pd/C | 400 | 6 | 4 | 1.64 | 0.027 | 0.043 | [34] |
| Chlorella pyrenoidosa | Activated carbon | 400 | 6 | 4 | 1.63 | 0.031 | 0.054 | [34] |
Table 2. Batch hydrotreatment of different HTL biocrudes in the presence of sulfided catalysts.
| HTL Biocrude | Sulfided Catalysts | T | PH2 | Time | Hydrotreated Oils | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| (°C) | (MPa) | (h) | H/C | N/C | O/C | |||
| Chlorella pyrenoidosa | MoS2 | 400 | 6 | 4 | 1.68 | 0.031 | 0.044 | [34] |
| Chlorella pyrenoidosa | CoMo/γ-Al2O3 | 400 | 6 | 4 | 1.74 | 0.028 | 0.045 | [34] |
| Chlorella | NiMo/γ-Al2O3 | 405 | 6.6 | 2 | 1.64 | 0.024 | 0.013 | [47] |
| Chlorella | CoMo/γ-Al2O3 | 405 | 6.6 | 2 | 1.68 | 0.027 | 0.009 | [47] |
| Chlorella pyrenoidosa | Raney-Ni | 400 | 6 | 4 | 1.77 | 0.017 | 0.031 | [34 |
| Hard wood | NiMo/γ-Al2O3 | ] | ||||||
| 350 | 14.7 | max | 2 | 1.67 | 0.004 | 0.003 | [ | 48] |
Table 3. Continuous hydrotreatment of different HTL biocrudes in the presence of sulfided catalysts.
| HTL Biocrude | Process Parameters | Hydrotreated Oils | Ref. | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sulfided Catalysts | T | PH2 | WHSV/LHSV i | |||||||||||||||||
| (°C) | (MPa) | (h−1) | H/C | N/C | O/C | |||||||||||||||
| Nannochloropsis–Solix LEA | CoMo/fluorinated-Al2O3 | 405 | 13.6 | 0.14 LHSV | 1.99 | 0.001 | 0.007 | [61] | ||||||||||||
| Nannochloropsis–NB238 | CoMo/fluorinated-Al2O3 | 405 | 13.6 | 0.20 LHSV | 1.86 | 0.002 | 0.011 | [61] | ||||||||||||
| Nannochloropsis–Cellana LL | CoMo/fluorinated-Al2O3 | 405 | 13.6 | 0.20 LHSV | 1.91 | 0.003 | 0.016 | [61] | ||||||||||||
| Nannochloropsis–Celana HL | CoMo/fluorinated-Al2O3 | 405 | 13.6 | 0.20 LHSV | 1.98 | 0.001 | 0.015 | [61] | ||||||||||||
| Chlorella–Standard Lipid | CoMo guard bed with CoMo/Al2O3 | 400 | 10.3 | 0.20 LHSV | 1.97 | 0.001 | 0.015 | [62] | ||||||||||||
| Chlorella pyrenoidosa | Ru/C + Raney-Ni | 400 | 6 | Nannochloropsis oceanica | ||||||||||||||||
| Chlorella–High Lipid | NiMo/γ-Al | 4 | 2 | 1.74 | 0.021 | O | 0.018 | 3 + NiMo/γ-Al2O3[34] | ||||||||||||
| 350 + 350 | 4 + 4 | 2 + 2 | 1.85 | 0.021 | 0.007 | [ | 49 | ] | ||||||||||||
| CoMo guard bed with CoMo/Al | 2O3 | 400 | 10.3 | 0.20 LHSV | 2.02 | 0.0005 | 0.015 | [62] | Chlorella pyrenoidosa | Ru/C:Pd/C | 400 | 6 | 4 | 2.06 | 0.020 | 0.030 | [35] | |||
| Nannochloropsis oceanica | NiMo guard catalyst with NiMo/γ-Al2O3 | 350 + 350 | 6 + 6 | 1 + 1 | ||||||||||||||||
| Primary sludge | CoMo guard bed with CoMo/Al2O3 | 400 | 10.6 | 1.98 | 0.001 | 0.005 | 0.16 LHSV | [50] | ||||||||||||
| 2.00 | 0.0003 | 0.010 | [ | 63 | ] | Chlorella pyrenoidosa | Ru/C:Pt/C | 400 | Aspen wood | NiMo/γ-Al2O6 | 4 | 3 | 350 | 101.83 | 0.026 | 4 | 1.25 | 0.0050.008 | 0.006[35] | |
| [ | 43 | ] | Chlorella pyrenoidosa | Ru/C:Pt/γ-Al2O3 | 400 | 6 | 4 | 1.73 | 0.019 | |||||||||||
| Spirulina | NiMo/γ-Al2O | 0.040 | 3 | [ | 35 | ] | ||||||||||||||
| 375 | 7 | 3 | 1.79 | 0.031 | 0.000 | [ | 51 | ] | Chlorella pyrenoidosa | Ru/C:Rh/γ-Al2O3 | 400 | 6 | 4 | 1.80 | 0.025 | 0.028 | [35] | |||
| Spirulina | NiMo/γ-Al2O3 | 400 | 8 | 4 | 1.76 | 0.042 | 0.000 | [ | Chlorella pyrenoidosa | Ru/C:Mo2C | 400 | 6 | 4 | 1.85 | 0.032 | 0.001 | [35] | |||
| 52 | ] | |||||||||||||||||||
| Sewage sludge | NiMo/γ-Al2O3 | 400 | 8 | Chlorella pyrenoidosa | Ru/C:Raney-Ni | 400 | 6 | 4 | 1.73 | 0.020 | 0.017 | [35] | ||||||||
| Digested solids | CoMo guard bed with CoMo/Al2O3 | 400 | 10.6 | 0.16 LHSV | 1.93 | 0.0006 | 0.008 | [63] | ||||||||||||
| Corn stover | CoMo guard bed with CoMo/Al2O3 | 400 | 10.3 | 0.21 LHSV | 2.00 | 0.002 | 0.010 | [64] | ||||||||||||
| Forestry residues | CoMo/γ-Al2O3 | 350 | 9.5 | 0.3 WHSV | 1.52 | - | 0.056 | [60] | 4 | 1.95 | 0.009 | |||||||||
| Forestry residues | CoMo/γ-Al2O3 + NiMo/γ-Al2O | 0.000 | 3 | [ | 52 | ] | ||||||||||||||
| 350 + 350 | 9.5 + 9.5 | 0.3 + 0.3 WHSV | 1.62 | - | 0.032 | [ | 60 | ] | Miscanthus | NiMo/γ-Al2O3 | 400 | 8 | 4 | 1.45 | ||||||
| Pine wood | NiMo/γ-Al | 0.015 | 2 | 0.007 | [ | 52 | ] | Chlorella pyrenoidosa | Ru/C:Activated carbon | 400 | ||||||||||
| O | 3 | 400 | 12.4 | 0.10 LHSV | 1.60 | 0.0005 | 0.004 | [65] | 6 | 4 | 1.96 | 0.024 | 0.033 | Chlorella vulgaris[ | NiMo/γ-Al2O335] | |||||
| 400 | 12 | 9.9 | 1.91 | 0.003 | 0.000 | |||||||||||||||
| Sludge/Fog–GLWA | CoMo guard bed with NiMo/Al2O3 | [ | 53 | ] | 400 | [ | 10.3 | 54] | ||||||||||||
| 0.39 WHSV | 2.03 | 0.001 | 0.009 | [ | 66 | ] | Chlorella pyrenoidosa | Ru/C:Alumina | Chlorococcum sp.400 | NiMo/Al-SBA-156 | 4 | |||||||||
| Sludge–CCCSD | CoMo guard bed with NiMo/Al | 425 | 2O3 | 1.87 | 3 | 4000.15 | 1.620.026 | 0.004 | [ | 0.03135 | 0.032] | |||||||||
| [ | 55 | ] | ||||||||||||||||||
| 10.3 | 0.39 WHSV | 2.00 | 0.007 | 0.004 | [ | 66 | ] | Chlorella pyrenoidosa | SpirulinaPt/γ-Al2O | NiMo/γ-Al23 | O3 + NiMo/γ-Al400 | 2O6 | 3 | 350 + 4001 | 1.48 | 8 + 8 | 4 + 4 | 2.070.051 | 0.053 | [36] |
| 0.006 | 0.000 | [ | 56 | ] | Scenedesmus almeriensis | Pt/γ-Al2O3 | 400 | 8 | 4 | 1.59 | 0.044 | 0.017 | [37] | |||||||
| Sewage sludge | NiMo/γ-Al2O3 + NiMo/γ-Al | Nannochloropsis gaditana | Pt/γ-Al2O3 | 400 | 8 | 4 | 1.67 | 0.024 | 0.014 | [37] | ||||||||||
| Nannochloropsis sp. | Ni/C | 350 | 6.9 | 10 | 1.59 | 0.018 | 0.003 | [38] | ||||||||||||
| Nannochloropsis sp. | Ru/C | 350 | 6.9 | 10 | 1.72 | 0.014 | 0.009 | [38] | ||||||||||||
| Nannochloropsis sp. | Pt/C | 350 | 6.9 | 10 | 1.59 | 0.013 | 0.005 | [38] | ||||||||||||
| Nannochloropsis sp. | Ru/γ-Al2O3 | 400 | 5 | 1 | 1.66 | 0.033 | 0.04 | [39] | ||||||||||||
| Nannochloropsis sp. | Pt/γ-Al2O3 | 400 | 5 | 1 | 1.77 | 0.030 | 0.014 | [39] | ||||||||||||
| Nannochloropsis sp. | Pd/γ-Al2O3 | 400 | 5 | 1 | 1.72 | 0.035 | 0.025 | [39] | ||||||||||||
| Nannochloropsis sp. | Pt/C | 400 | 5 | 1 | 1.76 | 0.034 | 0.018 | [39] | ||||||||||||
| Nannochloropsis sp. | Ru/C | 400 | 5 | 1 | 1.76 | 0.037 | 0.034 | [39] | ||||||||||||
| Nannochloropsis sp. | Pd/C | 400 | 5 | 1 | 1.75 | 0.034 | 0.058 | [39] | ||||||||||||
| Nannochloropsis sp. | Ni-Ru/CeO2 | 450 | 2 | 1 | 1.42 | 0.044 | 0.045 | [40] | ||||||||||||
| Nannochloropsis sp. | Ni/CeO2 | 450 | 2 | 1 | 1.33 | 0.049 | 0.055 | [40] | ||||||||||||
| Chlorella | NiMoW/Al2O3 | 400 | 3.4 | 4 | 1.38 | 0.059 | 0.067 | [41] | ||||||||||||
| Chlorella | CoMoW/Al2O3 | 400 | 3.4 | 4 | 1.31 | 0.063 | 0.072 | [41] | ||||||||||||
| Chlorella | CoNiMoW/Al2O3 | 400 | 3.4 | 4 | 1.43 | 0.056 | 0.065 | [41] | ||||||||||||
| Chlorella vulgaris | NiW/Al2O3 | 400 | 13.9 | 4 | 1.62 | 0.042 | 0.013 | [42] | ||||||||||||
| Nannochloropsis gaditana | NiW/Al2O3 | 400 | 13.9 | 4 | 1.75 | 0.047 | 0.024 | [42] | ||||||||||||
| Aspen wood | NiW/Al2O3 | 350 | 7.5 | 2 | 1.20 | 0.006 | 0.062 | [43] | ||||||||||||
| Sweet sorghum bagasse | Ru/C | 350 | 3.5 | 4 | 1.24 | 0.006 | 0.02 | [44] | ||||||||||||
| Duckweed (Lemna minor) | Ru/C | 400 | 6 | 1 | 1.51 | 0.013 | 0.018 | [45] | ||||||||||||
2.1. Non-Sulfided Catalysts in Batch Hydrotreating
Bai et al. [34] carried out an extensive catalytic screening study by employing a wide range of different non-sulfided catalysts (such as Pt/C, Ru/C, Pd/C, activated carbon, Raney-Ni, and Ru/C + Raney-Ni) to HTL biocrude from Chlorella pyrenoidosa algae under hydrotreating conditions. Their results showed that Pt/C has the highest HYD activity (resulting in an increase in H/C in the biocrude), Ru/C has the highest HDO, and Raney-Ni has the highest HDN. However, Ru/C + Raney-Ni (two-component catalyst) exhibited optimal HYD, HDO, and HDN [34]. Based on these results, Xu et al. [35] further investigated two-component catalysts (Ru/C + others metals) in 1:1 mass fraction. Ru/C was employed in all experiments because of its proven ability of achieving higher HDO. In comparison to a single-component catalyst (i.e., Ru/C), the two-component catalysts (Ru/C:Pd/C, Ru/C:Pt/C, Ru/C:Pt/γ-Al2O3, Ru/C:Rh/γ-Al2O3, Ru/C:Mo2C, Ru/C:Raney-Ni, Ru/C:Activated carbon, and Ru/C:Alumina) showed reduced coke yield, reduced gas formation, and increased HYD. Duan et al. [36] studied the influence of catalyst loading on the properties of hydrotreated oils. They found out that with 40% catalyst loading, high HDN (low N/C) and high HDO (low O/C) were achieved [36]. Moreover, Barreiro et al. [37] reported the hydrotreatment of two different microalgae HTL biocrudes with Pt/γ-Al2O3 catalyst. They noticed a reduction in the heteroatom content and an increase in volatility of both microalgae HTL biocrudes [37]. Shakya et al. [38] also reported the hydrotreatment of Nannochloropsis sp. algae with several non-sulfided catalysts (Ni/C, Ru/C and Pt/C). Ru/C and Pt/C resulted in a better oil quality in terms of HHV, HDN, and TAN. However, Ni/C showed the highest upgraded oil yields. They also observed a significant decrease in the pore volume and surface area of the catalysts (Ni/C, Ru/C, and Pt/C), primarily because of coke formation [38]. Patel et al. [39] carried out the hydrotreatment of algae biocrude in the presence of noble metal catalysts (Pt, Pd, and Ru) with both carbon and γ-Al2O3 supports. They documented an improvement in HDO when the γ-Al2O3 support was added to Pt and Ru [39]. Xu et al. [40] investigated the hydrotreatment of algae biocrude with the Ni-Ru/CeO2 and Ni/CeO2 catalysts. They recorded higher HDS for Ni-Ru/CeO2 and considered it as an optimal catalyst for the hydrotreatment of algal biocrude [40]. Xu et al. [41] explored the applicability of multi-metallic catalysts (NiMoW/γ-Al2O3, CoMoW/γ-Al2O3, and CoNiMoW/γ-Al2O3) during the hydrotreatment of Chlorella microalgae HTL biocrude. They noted that both CoMoW/γ-Al2O3 and CoNiMoW/γ-Al2O3 effectively reduced both the molecular weight distribution and boiling point distribution. Guo et al. [42] investigated the hydrotreatment of Chlorella vulgaris and Nannochloropsis gaditana HTL biocrudes in the NiW/γ-Al2O3 catalyst and reported higher HDS activity in comparison to the conventional hydrotreating catalyst. Yu et al. [43] also explored the NiW/γ-Al2O3 catalyst during the hydrotreatment of aspen wood HTL biocrude and recorded an increase in H/C, HHV, and HDO activity. Yue et al. [44] presented the hydrotreatment of sweet sorghum bagasse by utilizing Ru/C as a HDO catalyst under mild operating conditions (350 °C and 3.5 MPa). Furthermore, Duan et al. [45] utilized Ru on activated carbon (Ru/C) and successfully upgraded the duckweed HTL biocrude by reducing the heteroatom content and increasing the overall H/C and HHV.2.2. Sulfided Catalysts in Batch Hydrotreating
Sulfided catalysts represent the state-of-the-art in hydrotreating and have been widely employed in fossil oil refineries for the desulfurization of oil fractions [46]. Sulfided catalysts are often represented by supported CoMo and NiMo. Although sulfur removal is generally not the main issue in biocrude hydrotreating, sulfided catalysts have also proven to be effective for the removal of other heteroatoms such as O and N as well as for hydrogenation. Bai et al. [34] investigated sulfided CoMo/γ-Al2O3 and MoS2 catalysts during the hydrotreatment of Chlorella pyrenoidosa algae HTL biocrude. Both sulfided catalysts reduced the heteroatom content and increased the H/C and HHV of hydrotreated oils. During their investigation, they found that CoMo/γ-Al2O3 tends to reduce coke formation compared to other non-sulfided catalysts [34]. Biller et al. [47] reported the hydrotreatment of HTL biocrude from Chlorella microalgae with conventional sulfided catalysts (CoMo/γ-Al2O3 and NiMo/γ-Al2O3). They achieved higher HDN activity with sulfided NiMo and higher HDO activity with sulfided CoMo at given hydrotreating conditions (405 °C and 6.6 MPa) [47]. Jensen et al. [48] carried-out the hydrotreatment of hardwood biocrude with a commercial NiMo/γ-Al2O3 catalyst. They found that the operating temperature and hydrogen to oil ratio had a positive influence on overall HYD and HDO. However, operating pressure mostly affects the HYD and HDO of low reactivity oxygenates [48| 2 |
| O |
| 3 |
| 350 + 400 |
| 8 + 8 |
| 4 + 4 |
| 2.16 |
| 0.003 |
| 0.000 |
| [ |
| 15 |
Figure 1 illustrates the effect of non-sulfided and sulfided catalysts on the properties of the hydrotreated HTL biocrudes by means of van Krevelen-like plots. Sulfided catalysts perform better compared to non-sulfided ones under given hydrotreated conditions. HTL biocrudes treated with sulfided catalysts are on the extreme left side of both diagrams, meaning that the upgraded oil possesses a higher degree of HYD (highest H/C atomic ratio) along with the highest HDO (lowest O/C atomic ratio) and HDN (lowest N/C atomic ratio) activity. In contrast, non-sulfided noble metal catalysts were comparatively not conducive to enhanced HYD, HDO, and HDN activity. Thereby, non-sulfided noble metal catalysts retain a lower drop-in fuel properties. The lower efficiency of non-sulfided noble metal catalysts is probably due to the rapid catalyst deactivation due to sulfur molecules [57]. Similar concerns regarding sulfur poisoning of precious noble metal catalysts (Pt, Pd, Rh, Ru, etc.) are also suggested during the catalytic HDO of pyrolysis bio-oils where sulfur concentrations up to a few hundred ppm are found [58]. However, the short-term nature of batch hydrotreating HTL experiments does not allow for a correct evaluation of the deactivation mechanism by sulfur poisoning.
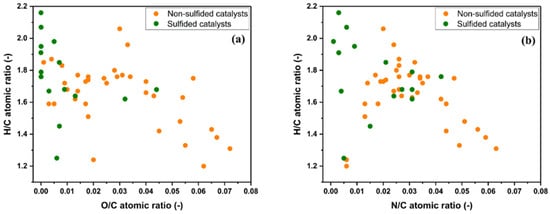

Figure 1. Van Krevelen diagram showing the atomic ratio of H/C as a function of O/C (a) and modified van Krevelen diagram with H/C as a function of N/C (b) of hydrotreated biocrudes with non-sulfided and sulfided catalysts. The atomic ratios are those reported in Table 1 and Table 2.
2.3. Catalysis in Continuous Hydrotreating
Only a handful of continuous hydrotreating studies on HTL biocrudes are present in the open literature. Continuous processing indeed requires more complex facilities than batch units and, normally, also higher volumes of catalysts and biocrude feed. Continuous operations are, however, more significant toward the scale up of the process. Processing in a continuous unit may be substantially different from the batch, and results are often difficult to compare. Indeed, batch units often experience significant equilibrium and mass transfer limitations, which lead to lower performance compared to continuous operations in fixed beds or trickle beds. All of the available continuous studies are carried-out in the presence of sulfided CoMo/γ-Al2O3 and NiMo/γ-Al2O3 hydrotreating catalysts (Table 1). However, only one continuous hydrotreating study [59] based on two-stage non-sulfided noble metal catalysts (NiW/SiO2/Al2O3References
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342.
- Kumar, M.; Olajire Oyedun, A.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770.
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156.
- Zhu, Z.; Toor, S.S.; Rosendahl, L.; Chen, G. Analysis of product distribution and characteristics in hydrothermal liquefaction of barley straw in subcritical and supercritical water. Environ. Prog. Sustain. Energy 2014, 33, 737–743.
- Suzuki, A.; Nakamura, T.; Yokoyama, S.; Ogi, T.; Koguchi, K. Conversion of sewage sludge to heavy oil by direct thermochemical liquefaction. J. Chem. Eng. Jpn. 1988, 21, 288–293.
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal liquefaction of barley straw to bio-crude oil: Effects of reaction temperature and aqueous phase recirculation. Appl. Energy 2015, 137, 183–192.
- Li, R.; Ma, Z.; Yang, T.; Li, B.; Wei, L.; Sun, Y. Sub–supercritical liquefaction of municipal wet sewage sludge to produce bio-oil: Effect of different organic–water mixed solvents. J. Supercrit. Fluids 2018, 138, 115–123.
- Xu, C.; Lancaster, J. Conversion of secondary pulp/paper sludge powder to liquid oil products for energy recovery by direct liquefaction in hot-compressed water. Water Res. 2008, 42, 1571–1582.
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165.
- Malins, K.; Kampars, V.; Brinks, J.; Neibolte, I.; Murnieks, R.; Kampare, R. Bio-oil from thermo-chemical hydro-liquefaction of wet sewage sludge. Bioresour. Technol. 2015, 187, 23–29.
- Seehar, T.H.; Toor, S.S.; Sharma, K.; Nielsen, A.H.; Pedersen, T.H.; Rosendahl, L.A. Influence of process conditions on hydrothermal liquefaction of eucalyptus biomass for biocrude production and investigation of the inorganics distribution. Sustain. Energy Fuels 2021, 5, 1477–1487.
- Shah, A.A.; Toor, S.S.; Conti, F.; Nielsen, A.H.; Rosendahl, L.A. Hydrothermal liquefaction of high ash containing sewage sludge at sub and supercritical conditions. Biomass Bioenergy 2020, 135, 105504.
- Biller, P.; Ross, A.B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225.
- Shakya, R.; Whelen, J.; Adhikari, S.; Mahadevan, R.; Neupane, S. Effect of temperature and Na2CO3 catalyst on hydrothermal liquefaction of algae. Algal Res. 2015, 12, 80–90.
- Jindal, M.K.; Jha, M.K. Catalytic Hydrothermal Liquefaction of Waste Furniture Sawdust to Bio-oil. Indian Chem. Eng. 2016, 58, 157–171.
- Nazari, L.; Yuan, Z.; Souzanchi, S.; Ray, M.B.; Xu, C.C. Hydrothermal liquefaction of woody biomass in hot-compressed water: Catalyst screening and comprehensive characterization of bio-crude oils. Fuel 2015, 162, 74–83.
- Kaur, R.; Biswas, B.; Kumar, J.; Jha, M.K.; Bhaskar, T. Catalytic hydrothermal liquefaction of castor residue to bio-oil: Effect of alkali catalysts and optimization study. Ind. Crops Prod. 2020, 149, 112359.
- Zhang, B.; He, Z.; Chen, H.; Kandasamy, S.; Xu, Z.; Hu, X.; Guo, H. Effect of acidic, neutral and alkaline conditions on product distribution and biocrude oil chemistry from hydrothermal liquefaction of microalgae. Bioresour. Technol. 2018, 270, 129–137.
- Seehar, T.H.; Toor, S.S.; Shah, A.A.; Pedersen, T.H.; Rosendahl, L.A. Biocrude production from wheat straw at sub and supercritical hydrothermal liquefaction. Energies 2020, 13, 3114.
- Conti, F.; Toor, S.S.; Pedersen, T.H.; Seehar, T.H.; Nielsen, A.H.; Rosendahl, L.A. Valorization of animal and human wastes through hydrothermal liquefaction for biocrude production and simultaneous recovery of nutrients. Energy Convers. Manag. 2020, 216, 112925.
- Toor, S.S.; Jasiunas, L.; Xu, C. (Charles); Sintamarean, I.M.; Yu, D.; Nielsen, A.H.; Rosendahl, L.A. Reduction of inorganics from macroalgae Laminaria digitata and spent mushroom compost (SMC) by acid leaching and selective hydrothermal liquefaction. Biomass Convers. Biorefin. 2018, 8, 369–377.
- Jensen, C.U.; Rosendahl, L.A.; Olofsson, G. Impact of nitrogenous alkaline agent on continuous HTL of lignocellulosic biomass and biocrude upgrading. Fuel Process. Technol. 2017, 159, 376–385.
- Jensen, C.U.; Rodriguez Guerrero, J.K.; Karatzos, S.; Olofsson, G.; Iversen, S.B. Fundamentals of HydrofactionTM: Renewable crude oil from woody biomass. Biomass Convers. Biorefin. 2017, 7, 495–509.
- Sintamarean, I.M.; Grigoras, I.F.; Jensen, C.U.; Toor, S.S.S.; Pedersen, T.H.; Rosendahl, L.A. Two-stage alkaline hydrothermal liquefaction of wood to biocrude in a continuous bench-scale system. Biomass Convers. Biorefin. 2017, 7, 425–435.
- Qian, L.; Wang, S.; Savage, P.E. Hydrothermal liquefaction of sewage sludge under isothermal and fast conditions. Bioresour. Technol. 2017, 232, 27–34.
- Ross, A.B.; Biller, P.; Kubacki, M.L.; Li, H.; Lea-Langton, A.; Jones, J.M. Hydrothermal processing of microalgae using alkali and organic acids. Fuel 2010, 89, 2234–2243.
- Dimitriadis, A.; Bezergianni, S. Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: A state of the art review. Renew. Sustain. Energy Rev. 2017, 68, 113–125.
- Xu, C.; Etcheverry, T. Hydro-liquefaction of woody biomass in sub- and super-critical ethanol with iron-based catalysts. Fuel 2008, 87, 335–345.
- Qu, Y.; Wei, X.; Zhong, C. Experimental study on the direct liquefaction of Cunninghamia lanceolata in water. Energy 2003, 28, 597–606.
- Conti, F.; Toor, S.S.; Pedersen, T.H.; Nielsen, A.H.; Rosendahl, L.A. Biocrude production and nutrients recovery through hydrothermal liquefaction of wastewater irrigated willow. Biomass Bioenergy 2018, 118, 24–31.
- Xu, D.; Lin, G.; Guo, S.; Wang, S.; Guo, Y.; Jing, Z. Catalytic hydrothermal liquefaction of algae and upgrading of biocrude: A critical review. Renew. Sustain. Energy Rev. 2018, 97, 103–118.
- Djandja, O.S.; Wang, Z.; Chen, L.; Qin, L.; Wang, F.; Xu, Y.; Duan, P. Progress in Hydrothermal Liquefaction of Algal Biomass and Hydrothermal Upgrading of the Subsequent Crude Bio-Oil: A Mini Review. Energy Fuels 2020, 34, 11723–11751.
- Chiaberge, S.; Siviero, A.; Passerini, C.; Pavoni, S.; Bianchi, D.; Haider, M.S.; Castello, D. Co-processing of Hydrothermal Liquefaction Sewage Sludge Biocrude with a Fossil Crude Oil by Codistillation: A Detailed Characterization Study by FTICR Mass Spectrometry. Energy Fuels 2021, 35, 13830–13839.
- Bai, X.; Duan, P.; Xu, Y.; Zhang, A.; Savage, P.E. Hydrothermal catalytic processing of pretreated algal oil: A catalyst screening study. Fuel 2014, 120, 141–149.
- Xu, Y.; Duan, P.; Wang, B. Catalytic upgrading of pretreated algal oil with a two-component catalyst mixture in supercritical water. Algal Res. 2015, 9, 186–193.
- Duan, P.; Bai, X.; Xu, Y.; Zhang, A.; Wang, F.; Zhang, L.; Miao, J. Catalytic upgrading of crude algal oil using platinum/gamma alumina in supercritical water. Fuel 2013, 109, 225–233.
- López Barreiro, D.; Gómez, B.R.; Ronsse, F.; Hornung, U.; Kruse, A.; Prins, W. Heterogeneous catalytic upgrading of biocrude oil produced by hydrothermal liquefaction of microalgae: State of the art and own experiments. Fuel Process. Technol. 2016, 148, 117–127.
- Shakya, R.; Adhikari, S.; Mahadevan, R.; Hassan, E.B.; Dempster, T.A. Catalytic upgrading of bio-oil produced from hydrothermal liquefaction of Nannochloropsis sp. Bioresour. Technol. 2018, 252, 28–36.
- Patel, B.; Arcelus-Arrillaga, P.; Izadpanah, A.; Hellgardt, K. Catalytic Hydrotreatment of algal biocrude from fast Hydrothermal Liquefaction. Renew. Energy 2017, 101, 1094–1101.
- Xu, D.; Guo, S.; Liu, L.; Hua, H.; Guo, Y.; Wang, S.; Jing, Z. Ni-Ru/CeO2 Catalytic Hydrothermal Upgrading of Water-Insoluble Biocrude from Algae Hydrothermal Liquefaction. Biomed Res. Int. 2018, 2018, 8376127.
- Xu, D.; Liu, L.; He, Z.; Yang, J.; Wu, Z.; Jing, Z. Hydrothermal upgrading of water-insoluble algal biocrude over γ-Al2O3 supported multi-metallic catalysts. J. Anal. Appl. Pyrolysis 2019, 140, 188–194.
- Guo, B.; Walter, V.; Hornung, U.; Dahmen, N. Hydrothermal liquefaction of Chlorella vulgaris and Nannochloropsis gaditana in a continuous stirred tank reactor and hydrotreating of biocrude by nickel catalysts. Fuel Process. Technol. 2019, 191, 168–180.
- Yu, J.; Biller, P.; Mamahkel, A.; Klemmer, M.; Becker, J.; Glasius, M.; Iversen, B.B. Catalytic hydrotreatment of bio-crude produced from the hydrothermal liquefaction of aspen wood: A catalyst screening and parameter optimization study. Sustain. Energy Fuels 2017, 1, 832–841.
- Yue, Y.; Kastner, J.R.; Mani, S. Two-Stage Hydrothermal Liquefaction of Sweet Sorghum Biomass—Part II: Production of Upgraded Biocrude Oil. Energy Fuels 2018, 32, 7620–7629.
- Duan, P.; Zhang, C.; Wang, F.; Fu, J.; Lü, X.; Xu, Y.; Shi, X. Activated carbons for the hydrothermal upgrading of crude duckweed bio-oil. Catal. Today 2016, 274, 73–81.
- Furimsky, E. Hydroprocessing challenges in biofuels production. Catal. Today 2013, 217, 13–56.
- Biller, P.; Sharma, B.K.; Kunwar, B.; Ross, A.B. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel 2015, 159, 197–205.
- Jensen, C.U.; Hoffmann, J.; Rosendahl, L.A. Co-processing potential of HTL bio-crude at petroleum refineries. Part 2: A parametric hydrotreating study. Fuel 2016, 165, 536–543.
- Zhao, B.; Wang, Z.; Liu, Z.; Yang, X. Two-stage upgrading of hydrothermal algae biocrude to kerosene-range biofuel. Green Chem. 2016, 18, 5254–5265.
- Zhao, B.; Shi, Z.; Yang, X. Upgrading Algae Biocrude for a Low-Nitrogen-Containing Biofuel: Compositions, Intermediates, and Reaction Routes. Ind. Eng. Chem. Res. 2017, 56, 6378–6390.
- Haider, M.; Castello, D.; Michalski, K.; Pedersen, T.; Rosendahl, L. Catalytic Hydrotreatment of Microalgae Biocrude from Continuous Hydrothermal Liquefaction: Heteroatom Removal and Their Distribution in Distillation Cuts. Energies 2018, 11, 3360.
- Castello, D.; Haider, M.S.; Rosendahl, L.A. Catalytic upgrading of hydrothermal liquefaction biocrudes: Different challenges for different feedstocks. Renew. Energy 2019, 141, 420–430.
- Rathsack, P.; Wollmerstaedt, H.; Kuchling, T.; Kureti, S. Analysis of hydrogenation products of biocrude obtained from hydrothermally liquefied algal biomass by comprehensive gas chromatography mass spectrometry (GC×GC-MS). Fuel 2019, 248, 178–188.
- Zuber, J.; Wollmerstädt, H.; Kuchling, T.; Kureti, S.; Rathsack, P. Analysis of Hydrogenation Products of Biocrude Obtained from Hydrothermally Liquefied Algal Biomass Using Fourier-Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2020, 34, 3199–3209.
- Subagyono, R.R.D.J.N.; Marshall, M.; Jackson, W.R.; Auxilio, A.R.; Fei, Y.; Chaffee, A.L. Upgrading Microalgal Biocrude Using NiMo/Al-SBA-15 as a Catalyst. Energy Fuels 2020, 34, 4618–4631.
- Haider, M.S.; Castello, D.; Rosendahl, L.A. Two-stage catalytic hydrotreatment of highly nitrogenous biocrude from continuous hydrothermal liquefaction: A rational design of the stabilization stage. Biomass Bioenergy 2020, 139, 105658.
- Duan, P.; Savage, P.E. Hydrothermal Liquefaction of a Microalga with Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2011, 50, 52–61.
- Elliott, D.C. Biofuel from fast pyrolysis and catalytic hydrodeoxygenation. Curr. Opin. Chem. Eng. 2015, 9, 59–65.
- Jensen, C.U. PIUS - Hydrofaction(TM) Platform with Integrated Upgrading Step. Ph.D. Thesis, Aalborg Universitetsforlag, Aalborg, Denmark, 2018.
- Haghighat, P.; Montanez, A.; Aguilera, G.R.; Rodriguez Guerrero, J.K.; Karatzos, S.; Clarke, M.A.; McCaffrey, W. Hydrotreating of HydrofactionTM biocrude in the presence of presulfided commercial catalysts. Sustain. Energy Fuels 2019, 3, 744–759.
- Elliott, D.C.; Hart, T.R.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process development for hydrothermal liquefaction of algae feedstocks in a continuous-flow reactor. Algal Res. 2013, 2, 445–454.
- Albrecht, K.O.; Zhu, Y.; Schmidt, A.J.; Billing, J.M.; Hart, T.R.; Jones, S.B.; Maupin, G.; Hallen, R.; Ahrens, T.; Anderson, D. Impact of heterotrophically stressed algae for biofuel production via hydrothermal liquefaction and catalytic hydrotreating in continuous-flow reactors. Algal Res. 2016, 14, 17–27.
- Marrone, P.A.; Elliott, D.C.; Billing, J.M.; Hallen, R.T.; Hart, T.R.; Kadota, P.; Moeller, J.C.; Randel, M.A.; Schmidt, A.J. Bench-scale evaluation of hydrothermal processing technology for conversion of wastewater solids to fuels. Water Environ. Res. 2018, 90, 329–342.
- Collett, J.R.; Billing, J.M.; Meyer, P.A.; Schmidt, A.J.; Remington, A.B.; Hawley, E.R.; Hofstad, B.A.; Panisko, E.A.; Dai, Z.; Hart, T.R.; et al. Renewable diesel via hydrothermal liquefaction of oleaginous yeast and residual lignin from bioconversion of corn stover. Appl. Energy 2019, 233–234, 840–853.
- Van Dyk, S.; Ebadian, M.; Su, J.; Larock, F.; Zhang, Y.; Monnier, J.; Wang, H.; Santosa, D.M.; Olarte, M.V.; Neuenschwander, G.; et al. ssessment of Likely Technology Maturation Pathways for Biojet Production from Forest Residues. Available online: https://task39.sites.olt.ubc.ca/files/2019/06/Executive-Summary-ATM-Project-26-June-2019.pdf (accessed on 18 July 2021).
- Snowden-Swan, L.; Billing, J.; Thorson, M.; Schmidt, A.; Santosa, M.; Jones, S.; Hallen, R. Wet Waste Hydrothermal Liquefaction and Biocrude Upgrading to Hydrocarbon Fuels: 2019 State of Technology; Report no.: PNNL-29882; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2020.
- Haider, M.S.; Castello, D.; Rosendahl, L.A. The Art of Smooth Continuous Hydroprocessing of Biocrudes Obtained from Hydrothermal Liquefaction: Hydrodemetallization and Propensity for Coke Formation. Energy Fuels 2021, 35, 10611–10622.
More
