Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Liliana Villao and Version 3 by Conner Chen.
One of the strategies to overcome diseases or abiotic stress in crops is the use of improved varieties. Genetic improvement could be accomplished through different methods, including conventional breeding, induced mutation, genetic transformation, or gene editing. The gene function and regulated expression through promoters are necessary for transgenic crops to improve specific traits. The variety of promoter sequences has increased in the generation of genetically modified crops because they could lead to the expression of the gene responsible for the improved trait in a specific manner.
- transcription
- gene expression
- genetic engineering
1. Promoters and Their Importance in Genetically Modified Crops
Sustainable maintenance in agriculture with a high productivity level has also been thanks to advances in plant biotechnology, especially through the implementation of genetically modified crops. Therefore, the search and availability of new promoters have been a high priority among researchers since they provide spatial and temporal control in transgene expression. Over the years, various promoters have been identified in different organisms for use in genetically modified plants because they are a potent tool in regulating gene expression leading to improved traits [1]. The success of gene expression depends to a great extent on the use and efficient selection of promoters, even being able to drive multiple transgenes by the same promoter in a single plant [2][3][2,3].
Promoters are located in the 5′ region of a gene and are composed of a specific nucleotide sequence controlling the expression of DNA in a physical, adjacent, and functional manner. In this way, gene regulation mainly depends on these essential regulatory elements for transcription initiation [1]. A DNA sequence located at the 5′ end of the coding region of a gene, which includes different binding regions for transcription factors, is known as a promoter [4]. Promoters are divided into a central core and several regulatory regions, usually in the 5′ region. The promoter core may also have a TATA box (consensus DNA sequence rich in adenine and thymine) and an initiator element that binds to transcription factors. It is already known that promoters can regulate the expression level of various transgenes and, in turn, these can be obtained from different sources; thus, their classification is divided into pol II (constitutive, inducible, and tissue-specific) and pol III (U3, U6); both are activated due to recognition by RNA polymerases [5].
On the other hand, the initiator elements can signal the start of the transcription in specific promoters that lack a TATA box [6]. The recognition of plant promoters would generally involve identifying and characterizing genes expressed in tissues or under conditions of some physiological stress [7] to identify promoters activated in these circumstances. The structurally characterized promoters could be fused with a coding sequence for later use in the genetic transformation of plants [8]; thus, the promoters are necessary to determine the expression of transgenes in genetically modified crops (Figure 1).
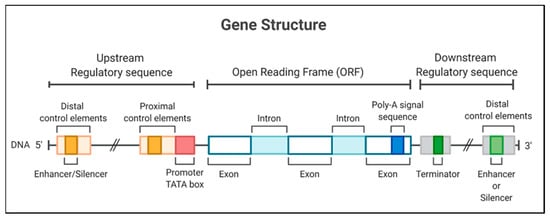
Figure 1. Gene structure, including the promoter region for a eukaryotic gene, encodes a protein. The gene diagrammed here contains a TATA box and different regulatory elements in 5′ and 3′ regions.
The study of promoters is necessary to understand the regulation of gene expression in plants [9]. The isolation of genes could be used as a starting point for identifying the promoters which drive the expression of the isolated gene. If the genome of the organism is already sequenced, basic local alignment tool (blast) analysis could be performed using the query of the sequence of the isolated gene; then, the location of the promoters will be at the 5′ region of the coding sequence of the gene. In the case that the genome of an organism is not sequenced, first, genes could be isolated through the creation of libraries, and once the gene sequence is known, the promoters of corresponding genes could be searched using genome walking strategies, such as PCR-based techniques [10][11][10,11]. The importance of isolating and identifying promoters lies in knowing which regions of interest are located on the 5′ side of a known genomic sequence.
Whole-genome sequencing of many plants has facilitated the isolation of promoters from genomes, in which primers can be designed in the 5′ region of the open reading frame [12]. Once the promoter is isolated and sequenced, in silico analysis can be performed using bioinformatics tools such as PlantCARE [13], PlantProm DB [14], iProm-Zea [15], and TSSPlant [16]. Recent studies have shown that promoters play an essential role in processes such as gene editing with clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9), increasing the effectiveness and specificity in cutting double-stranded DNA. For example, in soybean, the function of the gRNA and Cas9 expressed under the control of the double 35S Cauliflower Mosaic Virus (CaMV) promoter was tested in hairy soybean roots where a high rate of mutations was obtained; which were even visualized in the next generation; opposite when using egg cell-specific promoters [17][18][17,18].
2. Types of Promoters
Promoters can be classified as constitutive, tissue-specific, inducible, and synthetic. The classification will depend on the function, type, and gene expression level [13][14][13,14].3. Constitutive Promoters
Constitutive promoters are primarily used in plant transformation, promoting the expression of transgenes with different purposes, whether resistant to diseases, biotic stress, or insects [19]. One of the characteristics of the constitutive promoters is that they maintain control of the genes during most of the plant development; the expression levels will depend clearly on the type of cell with which they work. The 35S promoter derived from the CaMV is genetically modified crops’ most popular constitutive promoter (Table 1).Table 1.
Constitutive promoters are used in the generation of genetically modified plants.
| Promoter | Source | Expression | References |
|---|---|---|---|
| Act1 | Actin gene, rice | The whole plant, preferably monocots | [20][21][20,21] |
| Adh1 | Alcohol dehydrogenase gene, maize | Roots, meristematic tissue, endosperm, and pollen (anaerobic regulation) preference in monocots | [22][23][22,23] |
| HSP18.2 | Arabidopsis thaliana | Leaves, vascular system | [24] |
| ScBV | Bacilliform virus, sugarcane | Leaves, vascular system, monocots, and dicots | [25][26][25,26] |
| Ubi-1 | Ubiquitin gene, maize | Protoplast, monocots. | [27] |
| RUBQ1/RUBQ2 | Ubiquitin gene, rice | Genes expression, monocots. | [28] |
| Gmubi | Soybean | The whole plant | [29] |
| CaMV35S | Cauliflower mosaic virus | Expression throughout the plant, monocots, and dicots | [30] |
| nos | Nopaline synthase gene, Agrobacterium tumefaciens | Expression throughout the plant, monocots, and dicots | [31][32][31,32] |
| CmYLCV | Cestrum Yellow Leaf Curling Virus (CmYLCV) | Growth and development | [33] |
| KST1 | Solanum tuberosum (potato) | Guard cell promoter | [34] |
| Cula11/Cula08 | Cunninghamia lanceolate (Chinese fir) | Protoplast, monocots, and dicots | [35] |
| P OsCon1 | Rice | The whole plant, monocots, and dicots | [36] |
| TCTP | Oil palm | Immature embryo, embryogenic callus, embryoid, a young leaflet from a mature palm, green leaf, mesocarp, and stem | [37] |
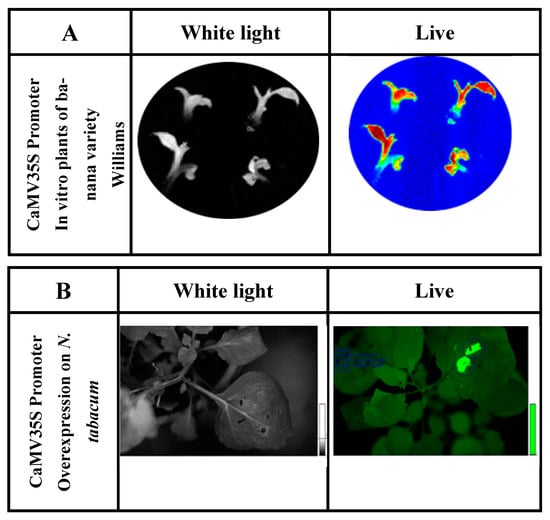
Figure 2. (A) Determination of luciferase reporter gene activity of in vitro ‘Williams’ banana plants transformed with the construct containing P35S::luc2. Exposure time was 3 min in total darkness; the photo was captured using the Stella 3200 equipment camera (Raytest, Straubenhardt, Germany). Luciferin dissolved in water (500 µm) was applied to in vitro plants. ‘Live’ indicates luciferase enzyme activity detected under conditions of total darkness. (B) Luciferase activity in N. tabacum leaves agroinfiltrated for the luciferase reporter gene expression fused to the CaMV 35S promoter (P35S::luc2).
