Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Koji Akeda and Version 2 by Rita Xu.
Spinal diseases are commonly associated with pain and neurological symptoms, which negatively impact patients’ quality of life. Platelet-rich plasma (PRP) is an autologous source of multiple growth factors and cytokines, with the potential to promote tissue regeneration. PRP has been widely used for the treatment of musculoskeletal diseases, including spinal diseases, in clinics.
- spinal diseases
- platelet-rich plasma
- intervertebral disc degeneration
1. Introduction
Spinal diseases, including spinal degenerative diseases, the ossification of spinal ligaments, spinal deformities, and spinal cord injury (SCI) cause pain and neurological symptoms. These greatly affect patients’ activity of daily life (ADL) and quality of life (QOL). Low back pain (LBP) is one of the most common complaints of patients with spinal diseases. Disorders of the intervertebral disc, facet joint, sacroiliac arthritis, and lumbar nerve root can cause LBP, which often becomes chronic and intractable. These disorders are generally treated with medications and rehabilitation, but often with limited efficacy [1]. The development of new, more effective treatments for chronic LBP is desirable.
Lumbar spinal stenosis and lumbar degenerative spondylolisthesis can cause pain, numbness, and muscle weakness in the lower extremities. Conservative treatments, such as medication and rehabilitation, have a certain degree of effectiveness [2]. When conservative treatments are less effective, surgical treatment is recommended; however, the further development of conservative treatments is desirable. In the surgical setting, spinal fusion is often indicated in cases of high instability or degeneration. Spinal fusion is often performed in conjunction with bone grafting, but the grafted bone may fail to fuse, resulting in a pseudoarthrosis. The incidence of pseudoarthrosis in long instrumented posterior spinal fusion for adult spinal deformities is estimated to be from 25 to 35% [3], and the establishment of adjuvant therapies to increase the rate of bone fusion is needed. Thus, there are many areas in the field of spinal diseases where new treatment methods are desired.
Biological and/or cellular therapies have been utilized in a variety of regenerative medicine treatments [4][5][6][7][4,5,6,7]. Platelet-rich plasma (PRP) has been used clinically for tissue regeneration and repair [8]. In recent years, especially in the field of orthopedics, the regenerative capabilities of PRP have been shown to repair damaged tissues, such as tendons, ligaments, and cartilage [9][10][9,10]. Recently, a number of studies have reported on the treatment of spinal diseases with PRP [11].
2. Biology of Platelets
2.1. Platelet Activation and Secretion
Upon vessel injury, circulating platelets are exposed to the vascular wall and soluble agonists, which induce platelet activation, leading to clot formation. Platelets contain several types of secretory inclusions, such as dense granules, α-granules, and lysosomes [12]. Among them, α-granules are the most abundant, with approximately 50–80 granules per platelet, ranging in size from 200 to 500 nm. α-granules contain membrane-bound and soluble proteins. Membrane-bound proteins include integrins, immunoglobulin family receptors, and leucine-rich repeat family receptors [13]. Following platelet activation, membrane-bound proteins are expressed on the platelet surface, whereas soluble proteins are released into the extracellular compartment. Importantly, α-granules contain small vesicles called exosomes [14], which can also be released following platelet activation. Proteomic studies revealed that more than 300 soluble proteins are released from activated α-granules [15][16][15,16]. These bioactive proteins released from α-granules play diverse roles in hemostasis, inflammation, antimicrobial host defense, angiogenesis, and wound healing [12]. Specific examples of these proteins are shown in Table 1.Table 1. Bioactive proteins released from α-granule.
| Factor | Examples |
|---|---|
| Adhesive proteins | Von Willebrand factor, fibrinogen, fibronectin, vitronectin, thrombospondin-1 and -2, laminin-8 |
| Clotting factors and inhibitors | Factor V/Va, factor XI, multimerin, protein S, high-molecular-weight kininogen, protease nexin-1 and -2, tissue factor pathway inhibitor, protein C inhibitor |
| Fibrinolytic factors and inhibitors | Plasminogen, plasminogen activator inhibitor-1, urokinase-type plasminogen activator (u-PA), α2-antiplasmin, histidine-rich glycoprotein, thrombin-activatable fibrinolysis inhibitor (TAFI,) α2-macroglobulin |
| Proteases and antiproteases | Metalloproteinases (MMP)-1, -2, -4, -9, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) 10, -13, TIMPs 1–4, platelet inhibitor of FIX, C1 inhibitor, α1-antitrypsin |
| Growth and mitogenic factors | transforming Growth Factor (TGF)-β1, -β2, platelet-derived growth factor (PDGF) -A, -B, and -C, epithelial growth factor (EGF), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF) -A, -C, basic fibroblast growth factor (bFGF)-2, hepatocyte growth factor (HGF), bone morphometric protein (BMP)-2, -4, -6, connective tissue growth factor (CTGF), signal peptide, CUB domain and EGF-like domain containing 1 (SCUBE1), insulin-like growth factor binding protein 3 (IGFBP3) |
| Chemokines, cytokines and others | Interleukin (IL)-1, RANTES (CCL5), IL-8 (CXCL8), macrophage inflammatory protein (MIP)-1α (CCL3), MIP-2 (CXCL2), LIX (CXCL6) GRO-α (CXCL1), ENA-78 (CXCL5), stromal cell-derived factor (SDF)-1α (CXCL12), MCP-1 (CCL2), MCP-3 (CCL7), platelet factor 4 (PF4) (CXCL4), pro-platelet basic protein (PBP), β-thromboglobulin (β-TG), neutrophil activating protein-2 (NAP-2), connective-tissue activating peptide III T(CXCL7), thymus and activation-regulated chemokine (TARC) (CCL17), angiopoietin-1, high mobility group box 1 (HMGB1), interleukin-6 soluble receptor (IL-6sR), bone sialoprotein, dickkopf-1, osteoprotegerin |
| Others | Chondroitin 4-sulfate, albumin, immunoglobulins G and M, amyloid β-protein precursor, disabled-2, complement factor H, bile salt-dependent lipase (BSDL), semaphorin 3A |
2.2. Platelet Extracellular Vesicles
Extracellular vesicles (EVs) include exosomes (30–100 nm in diameter) and micro-vesicles ([MV] 100–1000 nm in diameter). EVs contain membrane proteins, messenger RNA (mRNA), microRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA); they are generated and released from the vast majority of cell types into the extracellular space [17]. It has been reported that EVs are also released from activated platelets [18][19][18,19], and play an essential role in coagulation, the immune response, inflammation, angiogenesis, and tissue repair [20].2.3. PRP
PRP is a fraction of plasma with a high concentration of platelets obtained by centrifugation. Theoretically, PRP with supra-physiological concentrations of bioactive proteins, including platelet EVs, have the potential to stimulate regenerative and/or reparative effects in the target tissues and/or organs. In particular, PRP has been used in clinical settings for repairing tissues in the musculoskeletal system, including bone, cartilage, intervertebral disc, tendons, joints, and in the nervous system [21].3. PRP Classification
There are a wide variety of methods used in the purification of PRP; depending on the centrifugal conditions and extraction method, the concentration of platelets, white blood cells, and growth factors varies. Additionally, there are many commercially available kits that aim to efficiently purify highly stable PRP, but the quality of the purified PRP varies depending on the kit used. This is one of the obstacles for increasing the efficacy of PRP therapy. There are two main PRP purification methods: the open and closed techniques. In the open technique, the blood is in contact with the environment in the working area during PRP purification. Pipettes and tubes are sterilized separately and used in the PRP purification process. In contrast, the closed technique uses commercially available equipment and kits, and the blood and PRP are not exposed to the environment during the preparation process [22]. The open technique has the advantage of being low cost, but there is a risk of bacterial contamination. The closed technique has a lower contamination risk, but is more costly; additionally, certain kits provide lower yield in terms of platelet concentration. Kushida et al. [23] compared seven systems and evaluated the purified PRP in detail. Centrifugation was performed two times in four of the systems and once in three of the systems, each system following the original protocol for PRP preparation. PRP was separated by tube centrifugation in four systems, gel separation in two systems, and fully automated centrifugation in one system. The required whole blood volume ranged from 8 to 60 mL, the final volume of PRP ranged from 0.6 to 3 mL, and the average platelet concentration of PRP varied widely from 8.8 × 104/µL to 152.1 × 104/µL, depending on the system. Although PRP containing more than a specific concentration of platelets tends to have higher concentrations of platelet-derived growth factor-AB (PDGF-AB), the relationship was not always directly proportional. The concentrations of transforming growth factor beta-1 (TGF-β1) and vascular endothelial growth factor (VEGF) vary widely from system to system. Platelet concentration ratios from less than 2-fold to an 8.5-fold increase have been reported over baseline; however, reports suggest a 3- to 5-fold increase is desirable [23][24][23,24]. A certain concentration of platelets is necessary because a low platelet concentration tends to reduce the number of growth factors. As mentioned above, the content of PRP is considered to have a significant impact on treatment efficacy, and evaluating the content and quality of PRP is essential to validate its efficacy. DeLong et al. proposed a classification system based on Platelet concentration, Activation, or not, and leukocyte (White blood cell) concentration (PAW classification), which can be used to quickly evaluate the PRP preparations used in multiple studies and clinical practice [25]. The activation of PRP namely refers to two main processes: degranulation and cleavage-released growth factors from platelets. This process turns liquid plasma into a solid clot or membrane [26]. Exogenous activation techniques of PRP have been used for in vivo and clinical studies. PRP is usually activated by the addition of calcium chloride and/or thrombin, freezing and thawing, or exposure to collagen [11]. In a systematic review and meta-analysis, activated PRP was reported to be more effective for improving pain and functionality in patients with knee OA compared with non-activated PRP [27]. Additionally, Gentile reported that non-activated PRP was more useful for hair growth than activated PRP [28]. When PRP is injected into soft tissue, activation prior to administration is not always necessary because natural collagen type I acts as the activator [28]. Various basic and clinical studies have reported on the role of leukocyte content in the efficacy of PRP, but no consensus has been reached [29]. High concentrations of leukocytes may negatively affect PRP therapy, as leukocytes (especially neutrophils) act as inflammatory mediators. Nevertheless, leukocytes play an important role in the wound healing process, and their bactericidal activity has been reported to be beneficial for the treatment of bedsores and extensive soft tissue injuries [25]. Jia et al. reported that the presence of leukocytes in PRP may stimulate an inflammatory response at the cellular level [30]. Yan et al. reported that Leukocyte-poor PRP (Lp-PRP) significantly induced tendon regeneration compared to Leukocyte-rich PRP (Lr-PRP) in animal studies [31]. The results of clinical trials on patellar tendonitis [32], Achilles tendinopathy [33], and lateral epicondylitis [34] suggest that there was no difference in treatment outcomes between the Lr-PRP and Lp-PRP groups. Dohan et al. used a simpler classification: Pure Platelet-Rich Plasma (P-PRP), Leukocyte-and Platelet-Rich Plasma (L-PRP), and Pure Platelet-Rich Fibrin (P-PRF), depending on whether the preparations were plasma or fibrin products and whether they contained white blood cells [35]. PRF is purified by collecting blood in dry glass or glass-coated plastic tubes and immediately centrifuging it at a low RPM. PRF preparations have a high-density fibrin network, meaning they can be handled as if they are a solid material [36]. Mishra et al. proposed dividing PRP preparations into eight categories based on white blood cell (WBC) count, activation or lack of, and platelet concentration (subtype), as follows [37]. Type 1: Increased WBCs without activation; Type 2: Increased WBCs with activation; Type 3: Minimal or no WBCs without activation; Type 4: Minimal or no WBCs with activation. Subtype A contains an increased platelet concentration at or above five times the baseline. Subtype B contains an increased platelet concentration less than five times the baseline. This classification is simple and best reflects the characteristics of PRP.4. Basic Studies
4.1. Basic Studies on PRP for Intervertebral Disc Degeneration
Since the study examining the effects of PRP on intervertebral disc (IVD) cells was first reported in 2006 [38], several in vitro studies have been published. Many studies have used human IVD cells while others have used porcine, bovine, and rabbit cells to investigate the effects of PRP on cell growth and matrix metabolism [11]. Akeda et al. reported that PRP releasate increased the activity of the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads [38]. Concurrently, Chen et al. concluded that growth factors in PRP, including transforming growth factor-beta1, could effectively act as a growth factor cocktail to promote the proliferation and differentiation of human nucleus pulposus cells and tissue-engineered NP formation [39]. In terms of molecular mechanisms, Kim et al. reported that PRP was effective in reducing the expression of the proteolytic enzymes matrix metalloproteinase-3 (MMP3) and cyclooxygenase-2 (COX-2), which were increased by the stimulation of inflammatory cytokines, in human intervertebral disc cells [40]. Xu et al. recently reported that PRP secreted exosomal miR-141-3p to activate the Keap1-NF-E2-related factor 2 pathway, which was found to prevent IVD degeneration [41]. In addition, PRP-derived exosomes were reported to alleviate IVD degeneration-associated inflammation by regulating the ubiquitination and autophagic degradation of the NLRP3 inflammasome [42]. Thus, exosomes have recently attracted attention in relation to PRP function, and in the study of mesenchymal stem cells (MSC) [43]. These mechanisms were summarized in Figure 1.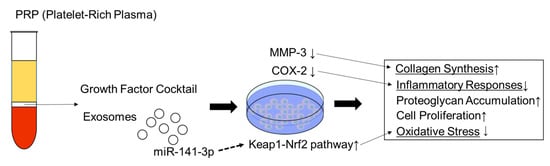
Figure 1. Schematic model for mechanism of PRP on intervertebral disc cells. ↑: increase; ↓: decrease.
