Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ayaz Ali Shah and Version 4 by Jessie Wu.
Hydrothermal liquefaction (HTL) of biomass is establishing itself as one of the leading technologies for the conversion of virtually any type of biomass feedstock into drop-in biofuels and renewable materials.
- HTL
- catalyst
1. Catalysis during Hydrothermal Liquefaction
The role of a catalyst for biomass processing in hydrothermal liquefaction (HTL) to enhance biocrude yield and quality is intensely dependent upon several factors such as temperature, residence time, reactor system, etc. [1][2][3][12,19,20]. Among them, temperature is the most dominant operating parameter for HTL. Many studies have proven that temperature strongly affects the biocrude yield and elemental composition [4][5][6][7][8][21,22,23,24,25]. The overall temperature range for HTL varies from 270 to 400 °C [9][10]. Regardless of feedstock type, many studies show that biocrude yield increases with the rise in temperature (from 280 to 350 °C); however, a further increase in temperature generally decreases the biocrude yield [4][10][11][12][21,26,27,28]. In the majority of cases, the catalysts were applied in a subcritical temperature range (270−350 °C) [13][14][15][16][17][18][29,30,31,32,33,34], except in a few studies, where the catalyst effects in supercritical temperature (>373.94 °C) were explored [12][19][20][21][22][23][24][25][28,35,36,37,38,39,40,41]. All these authors stated different conclusions depending on the nature and type of catalyst employed.
Moderate temperatures (300−350 °C) facilitate the hydrolysis of biomass, condensation, and repolymerization of reactive substances to form biocrude [14][26][30,42]. However, temperatures above the critical point (373.94 °C) improve the degree of deoxygenation and offer higher HHVs [20][36]. Retention time (RT) is another important parameter, longer residence time, higher than 10 min, mostly increases the biocrude yield, however, above the threshold level, biocrude yield decreases on account of higher organic loss in the form of water-soluble organics to the aqueous phase or gases by cracking reactions [27][14]. Xu et al. stated the possible reasons behind the leveling off or decreasing the biocrude yield at prolonging retention times, which include cracking of biocrude components to gases, repolymerization to form char, and condensation to aqueous products [28][43]. Malins et al. used a catalyst (FeSO4) for sewage sludge at 300 °C in an autoclave under reaction times of 10 to 100 mins and reported a maximum biocrude yield of 48% at 40 min. On the other hand, prolonged RTs enhance the gaseous products, and the biocrude quality is improved through intermingling tar substances with biocrude that could positively affect the HHV [10][26]. Seehar et al. derived a different conclusion by conducting a catalytic (K2CO3) reaction time study on eucalyptus at 350 °C from 10 min to 25 mins and reported that 15 min is the best reaction time for the eucalyptus conversion [11][27]. Another study indicated that 10 min is the optimum RT for the HTL of lignocellulosic biomass (Cunninghamia lanceolata) at 320 °C [29][44].
The reactor system also influences the overall energy recovery of the HTL system, typically, longer RTs are selected for autoclave-based reactors that give slightly lower yields due to lower heating rates [6][7][10][23,24,26]. Alternatively, improved biocrude productivity has been observed by many studies adopting micro-batch reactors. Even so, shorter RTs in the range of 10 to 20 min are declared as ideal for biomass liquefaction in all micro-batch reactor-oriented systems [11][12][27][30][14,27,28,45].
2. Catalysis for Hydrothermal TLiquefaction Biocrude Upgrading
HTL biocrude is a diverse pool of unsaturated organics containing significant amounts of contaminants such as oxygen, nitrogen, and inorganics in higher amounts and sulfur in lower amounts. Inevitably, the presence of these organic contaminants makes HTL biocrude an intermediate product with high TAN, high viscosity/density, low H/C, and poor thermal stability. Therefore, a downstream refining step is essential before HTL biocrudes can be utilized for the production of drop-in fuels. To date, the removal of organic contaminants via catalytic hydrotreatment has been a widely explored research area. During catalytic hydrotreatment, the removal of inorganics, O, N, and S takes place with reactions involving hydrodemetallization (HDM), hydrodeoxygenation (HDO), decarboxylation, decarbonylation, hydrodenitrogenation (HDN), hydrodesulfurization (HDS), and hydrogenation (HYD) [31][32][33][54,130,131]. In the literature, researchers have largely utilized both non-sulfided and sulfided catalysts. Most of these studies have been carried out in batch units. The main purpose of these efforts was to demonstrate the practicability of hydroprocessing for the treatment of HTL biocrudes from different feedstocks (such as lignocellulosic residues, algae, sewage sludge etc.) toward the production of drop-in fuels. Moreover, these batch hydrotreating studies also documented the effect of different sulfided/non-sulfided catalysts and operating conditions. Likewise, both families of catalysts have also been tested to some extent in continuous units. Hereafter, this section will comprehensively discuss and compare the effect of both non-sulfided and sulfided catalysts on the properties of hydrotreated oils (i.e., H/C, O/C, and N/C atomic ratios). Details of batch hydrotreating studies that utilized different non-sulfided and sulfided catalysts are listed in Table 1 and Table 2, respectively. The documented results of these batch hydrotreating studies are discussed and compared based on the fuel properties (such as H/C, O/C, and N/C atomic ratios) in this section.Table 1.
Batch hydrotreatment of different HTL biocrudes in the presence of non-sulfided catalysts.
| HTL Biocrude | Non-Sulfided Catalysts | T | PH2 | Time | Hydrotreated Oils | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| (°C) | (MPa) | (h) | H/C | N/C | O/C | |||
| Chlorella pyrenoidosa | Pt/C | 400 | 6 | 4 | 1.76 | 0.026 | 0.029 | [34][132] |
| Chlorella pyrenoidosa | Ru/C | 400 | 6 | 4 | 1.68 | 0.026 | 0.010 | [34][132] |
| Chlorella pyrenoidosa | Pd/C | 400 | 6 | 4 | 1.64 | 0.027 | 0.043 | [34][132] |
| Chlorella pyrenoidosa | Activated carbon | 400 | 6 | 4 | 1.63 | 0.031 | 0.054 | [34][132] |
| Chlorella pyrenoidosa | Raney-Ni | 400 | 6 | 4 | 1.77 | 0.017 | 0.031 | [34][132] |
| Chlorella pyrenoidosa | Ru/C + Raney-Ni | 400 | 6 | 4 | 1.74 | 0.021 | 0.018 | [34][132] |
| Chlorella pyrenoidosa | Ru/C:Pd/C | 400 | 6 | 4 | 2.06 | 0.020 | 0.030 | [35][133] |
| Chlorella pyrenoidosa | Ru/C:Pt/C | 400 | 6 | 4 | 1.83 | 0.026 | 0.008 | [35][133] |
| 1.51 | ||||||||
| 0.013 | ||||||||
| 0.018 | ||||||||
| [ | ||||||||
| 45 | ||||||||
| ] | ||||||||
| [ | ||||||||
| 143 | ||||||||
| ] | ||||||||
2.1. Non-Sulfided Catalysts in Batch Hydrotreating
Bai et al. [34][132] carried out an extensive catalytic screening study by employing a wide range of different non-sulfided catalysts (such as Pt/C, Ru/C, Pd/C, activated carbon, Raney-Ni, and Ru/C + Raney-Ni) to HTL biocrude from Chlorella pyrenoidosa algae under hydrotreating conditions. Their results showed that Pt/C has the highest HYD activity (resulting in an increase in H/C in the biocrude), Ru/C has the highest HDO, and Raney-Ni has the highest HDN. However, Ru/C + Raney-Ni (two-component catalyst) exhibited optimal HYD, HDO, and HDN [34][132]. Based on these results, Xu et al. [35][133] further investigated two-component catalysts (Ru/C + others metals) in 1:1 mass fraction. Ru/C was employed in all experiments because of its proven ability of achieving higher HDO. In comparison to a single-component catalyst (i.e., Ru/C), the two-component catalysts (Ru/C:Pd/C, Ru/C:Pt/C, Ru/C:Pt/γ-Al2O3, Ru/C:Rh/γ-Al2O3, Ru/C:Mo2C, Ru/C:Raney-Ni, Ru/C:Activated carbon, and Ru/C:Alumina) showed reduced coke yield, reduced gas formation, and increased HYD. Duan et al. [36][134] studied the influence of catalyst loading on the properties of hydrotreated oils. They found out that with 40% catalyst loading, high HDN (low N/C) and high HDO (low O/C) were achieved [36][134]. Moreover, Barreiro et al. [37][135] reported the hydrotreatment of two different microalgae HTL biocrudes with Pt/γ-Al2O3 catalyst. They noticed a reduction in the heteroatom content and an increase in volatility of both microalgae HTL biocrudes [37][135]. Shakya et al. [38][136] also reported the hydrotreatment of Nannochloropsis sp. algae with several non-sulfided catalysts (Ni/C, Ru/C and Pt/C). Ru/C and Pt/C resulted in a better oil quality in terms of HHV, HDN, and TAN. However, Ni/C showed the highest upgraded oil yields. They also observed a significant decrease in the pore volume and surface area of the catalysts (Ni/C, Ru/C, and Pt/C), primarily because of coke formation [38][136]. Patel et al. [39][137] carried out the hydrotreatment of algae biocrude in the presence of noble metal catalysts (Pt, Pd, and Ru) with both carbon and γ-Al2O3 supports. They documented an improvement in HDO when the γ-Al2O3 support was added to Pt and Ru [39][137]. Xu et al. [40][138] investigated the hydrotreatment of algae biocrude with the Ni-Ru/CeO2 and Ni/CeO2 catalysts. They recorded higher HDS for Ni-Ru/CeO2 and considered it as an optimal catalyst for the hydrotreatment of algal biocrude [40][138]. Xu et al. [41][139] explored the applicability of multi-metallic catalysts (NiMoW/γ-Al2O3, CoMoW/γ-Al2O3, and CoNiMoW/γ-Al2O3) during the hydrotreatment of Chlorella microalgae HTL biocrude. They noted that both CoMoW/γ-Al2O3 and CoNiMoW/γ-Al2O3 effectively reduced both the molecular weight distribution and boiling point distribution. Guo et al. [42][140] investigated the hydrotreatment of Chlorella vulgaris and Nannochloropsis gaditana HTL biocrudes in the NiW/γ-Al2O3 catalyst and reported higher HDS activity in comparison to the conventional hydrotreating catalyst. Yu et al. [43][141] also explored the NiW/γ-Al2O3 catalyst during the hydrotreatment of aspen wood HTL biocrude and recorded an increase in H/C, HHV, and HDO activity. Yue et al. [44][142] presented the hydrotreatment of sweet sorghum bagasse by utilizing Ru/C as a HDO catalyst under mild operating conditions (350 °C and 3.5 MPa). Furthermore, Duan et al. [45][143] utilized Ru on activated carbon (Ru/C) and successfully upgraded the duckweed HTL biocrude by reducing the heteroatom content and increasing the overall H/C and HHV.2.2. Sulfided Catalysts in Batch Hydrotreating
Sulfided catalysts represent the state-of-the-art in hydrotreating and have been widely employed in fossil oil refineries for the desulfurization of oil fractions [46][144]. Sulfided catalysts are often represented by supported CoMo and NiMo. Although sulfur removal is generally not the main issue in biocrude hydrotreating, sulfided catalysts have also proven to be effective for the removal of other heteroatoms such as O and N as well as for hydrogenation. Bai et al. [34][132] investigated sulfided CoMo/γ-Al2O3 and MoS2 catalysts during the hydrotreatment of Chlorella pyrenoidosa algae HTL biocrude. Both sulfided catalysts reduced the heteroatom content and increased the H/C and HHV of hydrotreated oils. During their investigation, they found that CoMo/γ-Al2O3 tends to reduce coke formation compared to other non-sulfided catalysts [34][132]. Biller et al. [47][145] reported the hydrotreatment of HTL biocrude from Chlorella microalgae with conventional sulfided catalysts (CoMo/γ-Al2O3 and NiMo/γ-Al2O3). They achieved higher HDN activity with sulfided NiMo and higher HDO activity with sulfided CoMo at given hydrotreating conditions (405 °C and 6.6 MPa) [47][145]. Jensen et al. [48][146] carried-out the hydrotreatment of hardwood biocrude with a commercial NiMo/γ-Al2O3 catalyst. They found that the operating temperature and hydrogen to oil ratio had a positive influence on overall HYD and HDO. However, operating pressure mostly affects the HYD and HDO of low reactivity oxygenates [48][146]. Guo et al. [42][140] and Yu et al. [43][141] explored both non-sulfided NiW/γ-Al2O3 and commercial sulfided NiMo/γ-Al2O3 catalysts with Chlorella vulgaris/Nannochloropsis gaditana and aspen wood HTL biocrudes, respectively. Both of these separate studies showed higher HDN and HDO when the sulfided NiMo/γ-Al2O3 catalyst was employed [42][43][140,141]. Zhao et al. [49][50][147,148] extensively investigated sulfided NiMo/γ-Al2O3, both alone [49][147] and combined with the guard bed NiMo catalyst [50][148]. They evaluated a two-stage approach for effective catalytic hydrotreatment and successfully achieved higher HDY, HDO, and HDN. Haider et al. [51][149] employed a sulfided NiMo/γ-Al2O3 catalyst during the hydrotreatment of Spirulina microalgae biocrude and carried out a statistical analysis to evaluate the significance of the different process conditions. It was revealed that, up to 350 °C, HDO is mainly temperature driven, while HDN is affected by both initial H2 pressure and pressure–temperature interaction. They also documented complete HDO at 350 °C and 8 MPa [51][149]. Castello et al. [52][150] utilized sulfided NiMo/γ-Al2O3 catalyst and studied the effect of different operating parameters on three different HTL biocrudes (Spirulina algae, sewage sludge, and Miscanthus). They achieved complete HDO for Spirulina algae and sewage sludge HTL biocrudes and reported a high extent of HDN. They found that higher hydrogen pressure is needed to prevent extensive coking and undesired decarboxylation reactions [52][150]. Rathsack et al. [53][151] and Zuber et al. [54][152] reported the hydrotreating of Chlorella vulgaris with sulfided NiMo/γ-Al2O3 catalyst. They also achieved complete HDO, lower N/C (0.003), and higher H/C (1.91). Thanks to FT-ICR MS (Fourier-transform ion cyclotron resonance with mass spectrometry), they were able to conclude that N1 species are difficult to remove compared to N2 species [54][152]. Subagyono et al. [55][153] investigated sulfided NiMo/Al-SBA-15 as a catalyst for the hydrotreatment of microalgae HTL biocrude. During catalytic hydrotreatment with NiMo/Al-SBA-15, they attained high HYD, HDO, and HDN. In addition, they also realized that the acidity of the support material is directly related to product yield, while product quality is assured when NiMo is incorporated in the support material [55][153]. An important aspect is represented by the potential thermal instability of biocrude, which can seriously affect hydrotreating operations. Haider et al. [56][154] showed that HTL biocrudes are thermally unstable at high temperatures (400 °C) and they reported extensive coke formation upon directly subjecting these HTL biocrudes at these temperatures. They utilized a sulfided NiMo/γ-Al2O3 catalyst in two-stages and ensured higher oil yields, higher HDN, complete HDO, and remarkable fuel properties with respect to H/C and HHV [56][154].Table 2.
Batch hydrotreatment of different HTL biocrudes in the presence of sulfided catalysts.
| HTL Biocrude | Sulfided Catalysts | T | PH2 | Time | Hydrotreated Oils | Ref. | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (MPa) | (h) | H/C | N/C | O/C | ||||||||||||||||||||
| Chlorella pyrenoidosa | MoS2 | 400 | 6 | 4 | 1.68 | 0.031 | 0.044 | [34][132] | |||||||||||||||||
| Chlorella pyrenoidosa | CoMo/γ-Al2O3 | 400 | 6 | 4 | 1.74 | 0.028 | 0.045 | [34][132] | |||||||||||||||||
| Chlorella | NiMo/γ-Al2O3 | 405 | 6.6 | 2 | 1.64 | 0.024 | 0.013 | [47][145] | |||||||||||||||||
| Chlorella | Nannochloropsis–Celana HLCoMo/γ-Al2O3 | 405 | 6.6 | 2 | 1.68 | 0.027 | 0.009 | [47 | CoMo/fluorinated-Al2O3][145] | ||||||||||||||||
| 405 | 13.6 | 0.20 LHSV | 1.98 | 0.001 | 0.015 | [ | 61 | ][158] | Hard wood | NiMo/γ-Al2O3 | 350 | 14.7max | 2 | 1.67 | 0.004 | 0.003 | [48][146] | ||||||||
| Nannochloropsis oceanica | NiMo/γ-Al2O3 + NiMo/γ-Al2O3 | 350 + 350 | 4 + 4 | 2 + 2 | 1.85 | 0.021 | 0.007 | [49][147] | |||||||||||||||||
| Nannochloropsis oceanica | NiMo guard catalyst with NiMo/γ-Al2O3 | 350 + 350 | 6 + 6 | 1 + 1 | 1.98 | 0.001 | 0.005 | [50][148] | |||||||||||||||||
| Chlorella–Standard Lipid | CoMo guard bed with CoMo/Al2O3 | 400 | 10.3 | 0.20 LHSV | 1.97 | 0.001 | 0.015 | [62][159] | |||||||||||||||||
| Chlorella–High Lipid | CoMo guard bed with CoMo/Al2O3 | 400 | 10.3 | 0.20 LHSV | 2.02 | 0.0005 | 0.015 | [62][159] | |||||||||||||||||
| Primary sludge | CoMo guard bed with CoMo/Al2O3 | 400 | 10.6 | 0.16 LHSV | 2.00 | 0.0003 | 0.010 | [63][160] | Aspen wood | NiMo/γ-Al2O3 | 350 | 10 | 4 | 1.25 | 0.005 | 0.006 | [43][141] | Chlorella pyrenoidosa | Ru/C:Pt/γ-Al2O3 | 400 | 6 | 4 | 1.73 | 0.019 | 0.040 |
| Spirulina | [ | 35 | ] | [ | 133 | ] | |||||||||||||||||||
| NiMo/γ-Al | 2O3 | 375 | 7 | 3 | 1.79 | 0.031 | 0.000 | [51][149] | Chlorella pyrenoidosa | Ru/C:Rh/γ-Al2O3 | 400 | ||||||||||||||
| Spirulina | NiMo/γ-Al2O | ||||||||||||||||||||||||
| Digested solids | CoMo guard bed with CoMo/Al2O3 | 400 | 10.6 | 0.16 LHSV | 1.93 | 0.0006 | 0.008 | [63][160] | |||||||||||||||||
| Corn stover | CoMo guard bed with CoMo/Al2O3 | 400 | 10.3 | 6 | 4 | 1.80 | 0.025 | 0.028 | 3[35][133] | ||||||||||||||||
| 400 | 8 | 4 | 1.76 | 0.042 | 0.000 | [ | 52 | ][150] | Chlorella pyrenoidosa | Ru/C:Mo2C | 400 | 6 | |||||||||||||
| 0.21 LHSV | 2.00 | 0.002 | 0.010 | [ | 64 | ] | [ | 161] | |||||||||||||||||
| Forestry residues | CoMo/γ-Al2O3 | 350 | 9.5 | 0.3 WHSV | 1.52 | - | 0.056 | [60][157] | Sewage sludge | NiMo/γ-Al2O3 | 4004 | 1.85 | 0.032 | 0.001 | 8 | 4[ | 1.95 | 0.00935 | 0.000][133] | ||||||
| [ | 52 | ] | [ | 150 | ] | Chlorella pyrenoidosa | Ru/C:Raney-Ni | 400 | |||||||||||||||||
| Forestry residues | CoMo/γ-Al2O3 + NiMo/γ-Al2O3 | 350 + 350 | 9.5 + 9.5 | 0.3 + 0.3 WHSV | 1.62 | - | 0.032 | [60][157] | 6 | 4 | 1.73 | 0.020 | 0.017 | ||||||||||||
| Pine wood | [ | 35 | NiMo/γ-Al2 | ] | [ | 133 | ] | ||||||||||||||||||
| Miscanthus | NiMo/γ-Al2O3 | O3400 | 8 | 4 | 1.45 | 0.015 | 0.007 | 400 | 12.4[ | 0.10 LHSV52 | 1.60][150] | 0.0005 | 0.004 | [65][162] | Chlorella pyrenoidosa | Ru/C:Activated carbon | 400 | 6 | 4 | 1.96 | 0.024 | ||||
| Chlorella vulgaris | 0.033 | NiMo/γ-Al2O3 | [ | 400 | 12 | 35 | 9.9 | ||||||||||||||||||
| Sludge/Fog–GLWA | CoMo guard bed with NiMo/Al2O3 | ] | [ | 1.91 | 133 | ] | |||||||||||||||||||
| 0.003 | 400 | 0.000 | 10.3 | [ | 0.39 WHSV | 2.03 | 0.001 | 0.00953] | [66][[16354][151,152] | ] | Chlorella pyrenoidosa | Ru/C:Alumina | 400 | 6 | 4 | 1.87 | 0.026 | 0.004 | [35][133 | ||||||
| Sludge–CCCSD | ] | ||||||||||||||||||||||||
| Chlorococcum sp. | NiMo/Al-SBA-15 | CoMo guard bed with NiMo/Al425 | 3 | 0.15 | 1.62 | 20.031 | 0.032 | O3[55][153] | 400 | 10.3 | 0.39 WHSV | 2.00 | 0.007 | 0.004 | [66][163] | Chlorella pyrenoidosa | Pt/γ-Al2O3 | 400 | |||||||
| Spirulina | NiMo/γ-Al2O3 + NiMo/γ-Al2O3 | 350 + 400 | 6 | 1 | 1.48 | 0.051 | 0.053 | [36][134] | |||||||||||||||||
| 8 + 8 | 4 + 4 | ||||||||||||||||||||||||
| Sludge/Fog–CCCSD | 2.07 | CoMo guard bed with NiMo/Al2 | 0.006 | O | 0.000 | 3 | [ | 56][154] | 400 | 10.3 | 0.39 WHSV | 2.12 | 0.001 | 0.004 | [66][ | Scenedesmus almeriensis | Pt/γ-Al2O3 | 400 | |||||||
| 163 | ] | Sewage sludge | NiMo/γ-Al2O3 + NiMo/γ-Al2O3 | 350 + 4008 | 4 | 1.59 | 0.044 | 0.017 | [37][135] | ||||||||||||||||
| 8 + 8 | 4 + 4 | 2.16 | 0.003 | Nannochloropsis gaditana | |||||||||||||||||||||
| Microalga Spirulina | MoS2 + NiMo/Al2O3 | 340 | 10.0 | 0.5 WHSV | 1.99 | 0.057 | - | [67][164] | Pt/γ-Al2O3 | 400 | 8 | 4 | 1.67 | 0.024 | 0.014 | [37][135] | |||||||||
| Nannochloropsis sp. | Ni/C | 350 | 6.9 | 10 | 1.59 | 0.018 | 0.003 | [38][136] | |||||||||||||||||
| Nannochloropsis sp. | Ru/C | 350 | 6.9 | 10 | 1.72 | 0.014 | 0.009 | [38][136] | |||||||||||||||||
| Nannochloropsis sp. | Pt/C | 350 | 6.9 | 10 | 1.59 | 0.013 | 0.005 | [38][136] | |||||||||||||||||
| Nannochloropsis sp. | Ru/γ-Al2O3 | 400 | 5 | 1 | 1.66 | 0.033 | 0.04 | [39][137] | |||||||||||||||||
| Nannochloropsis sp. | Pt/γ-Al2O3 | 400 | 5 | 1 | 1.77 | 0.030 | 0.014 | [39][137] | |||||||||||||||||
| Nannochloropsis sp. | Pd/γ-Al2O3 | 400 | 5 | 1 | 1.72 | 0.035 | 0.025 | [39][137] | |||||||||||||||||
| 0.000 | Nannochloropsis sp. | Pt/C | 400 | 5 | 1 | 1.76 | 0.034 | 0.018 | [39][137] | ||||||||||||||||
| Nannochloropsis sp. | Ru/C | 400 | 5 | 1 | 1.76 | 0.037 | 0.034 | [39][137] | |||||||||||||||||
| Nannochloropsis sp. | Pd/C | 400 | 5 | 1 | 1.75 | 0.034 | 0.058 | [39][137] | |||||||||||||||||
| [ | 15 | Nannochloropsis sp. | Ni-Ru/CeO2 | 450 | 2 | 1 | 1.42 | 0.044 | 0.045 | [40][138] | |||||||||||||||
| Nannochloropsis sp. | Ni/CeO2 | 450 | 2 | 1 | 1.33 | 0.049 | 0.055 | [40][138] | |||||||||||||||||
| Chlorella | NiMoW/Al2O3 | 400 | 3.4 | 4 | 1.38 | 0.059 | 0.067 | [41][139] | |||||||||||||||||
| Chlorella | CoMoW/Al2O3 | 400 | 3.4 | 4 | 1.31 | 0.063 | 0.072 | [41][139] | |||||||||||||||||
| Chlorella | CoNiMoW/Al2O3 | 400 | 3.4 | 4 | 1.43 | 0.056 | 0.065 | [41][139] | |||||||||||||||||
| Chlorella vulgaris | NiW/Al2O3 | 400 | 13.9 | 4 | 1.62 | 0.042 | 0.013 | [42][140] | |||||||||||||||||
| Nannochloropsis gaditana | NiW/Al2O3 | 400 | 13.9 | 4 | 1.75 | 0.047 | 0.024 | [42][140] | |||||||||||||||||
| Aspen wood | NiW/Al2O3 | 350 | 7.5 | 2 | 1.20 | 0.006 | 0.062 | [43][141] | |||||||||||||||||
| Sweet sorghum bagasse | Ru/C | 350 | 3.5 | 4 | 1.24 | 0.006 | 0.02 | [44][142] | |||||||||||||||||
| Duckweed (Lemna minor) | Ru/C | 400 | 6 | 1 | |||||||||||||||||||||
Figure 1 illustrates the effect of non-sulfided and sulfided catalysts on the properties of the hydrotreated HTL biocrudes by means of van Krevelen-like plots. Sulfided catalysts perform better compared to non-sulfided ones under given hydrotreated conditions. HTL biocrudes treated with sulfided catalysts are on the extreme left side of both diagrams, meaning that the upgraded oil possesses a higher degree of HYD (highest H/C atomic ratio) along with the highest HDO (lowest O/C atomic ratio) and HDN (lowest N/C atomic ratio) activity. In contrast, non-sulfided noble metal catalysts were comparatively not conducive to enhanced HYD, HDO, and HDN activity. Thereby, non-sulfided noble metal catalysts retain a lower drop-in fuel properties. The lower efficiency of non-sulfided noble metal catalysts is probably due to the rapid catalyst deactivation due to sulfur molecules [57][95]. Similar concerns regarding sulfur poisoning of precious noble metal catalysts (Pt, Pd, Rh, Ru, etc.) are also suggested during the catalytic HDO of pyrolysis bio-oils where sulfur concentrations up to a few hundred ppm are found [58][155]. However, the short-term nature of batch hydrotreating HTL experiments does not allow for a correct evaluation of the deactivation mechanism by sulfur poisoning.
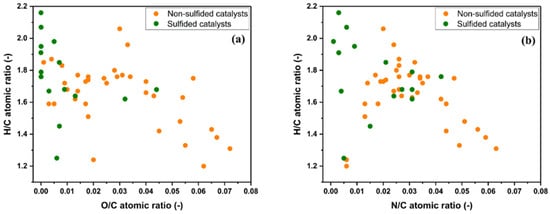

Figure 1. Van Krevelen diagram showing the atomic ratio of H/C as a function of O/C (a) and modified van Krevelen diagram with H/C as a function of N/C (b) of hydrotreated biocrudes with non-sulfided and sulfided catalysts. The atomic ratios are those reported in Table 1 and Table 2.
2.3. Catalysis in Continuous Hydrotreating
Only a handful of continuous hydrotreating studies on HTL biocrudes are present in the open literature. Continuous processing indeed requires more complex facilities than batch units and, normally, also higher volumes of catalysts and biocrude feed. Continuous operations are, however, more significant toward the scale up of the process. Processing in a continuous unit may be substantially different from the batch, and results are often difficult to compare. Indeed, batch units often experience significant equilibrium and mass transfer limitations, which lead to lower performance compared to continuous operations in fixed beds or trickle beds. All of the available continuous studies are carried-out in the presence of sulfided CoMo/γ-Al2O3 and NiMo/γ-Al2O3 hydrotreating catalysts (Table 1). However, only one continuous hydrotreating study [59][156] based on two-stage non-sulfided noble metal catalysts (NiW/SiO2/Al2O3 and Pd/Al2O3) is available in the open literature. In his work, Jensen [59][156] carried out the catalytic hydrotreatment of HTL biocrude from forestry residue and employed two individual reactors, the former filled with NiW/SiO2/Al2O3 and the latter with the Pd/Al2O3 catalyst. Overall results showed poor HDO, large exothermicity, and immediate reactor plugging. Subsequently, in the presence of the sulfided NiMo catalyst, Jensen was able to successfully operate for 660 hours on stream and also obtained complete HDO and higher H/C (1.73). Similarly, Haghighat et al. [60][157] also performed hydrotreatment of forestry residue HTL biocrude in a two-stage continuous unit. They utilized CoMo/γ-Al2O3 in the first-stage and NiMo/γ-Al2O3 in the second-stage and achieved 83.5% HDO and 1.62 H/C. Recently, Haider et al. conducted continuous upgrading of microalga Spirulina biocrude on NiMo/Al2O3 by using MoS2 as a HDM catalyst in the upper part of the bed. Results showed complete deoxygenation and a large extent of denitrogenation. The proper selection of catalyst bed and operating conditions allowed for a continuous campaign to be conducted for around 335 h on stream, which was stopped only due to feed exhaustion. Researchers from the Pacific Northwest National Laboratory (PNNL) accomplished the continuous hydrotreatment of HTL biocrudes by utilizing sulfided CoMo and NiMo catalysts. Elliott et al. [61][158] comprehensively investigated four different algal HTL biocrudes in a fixed-bed hydrotreating unit operating at continuous mode. After employing sulfided CoMo with fluorinated γ-Al2O3 support, they achieved good HYD (1.91–1.99 H/C) and HDO (0.007–0.016 O/C), along with much higher HDN (0.001–0.003 N/C). Afterward, Albrecht et al. [62][159], Marrone et al. [63][160], and Collet et al. [64][161] employed CoMo as both the guard-bed HDM and bulk catalyst during the continuous hydrotreatment of algae, wastewater solids, and corn stover HTL biocrudes, respectively. They all achieved remarkable fuel properties with good HDO (0.008–0.015 O/C) and high HYD (1.93–2.02 H/C) and HDN (0.0003–0.002 N/C). Recently, PNNL investigated the continuous hydrotreatment of pine wood HTL biocrude [65][162] in a sulfided NiMo catalyst and sewage sludge HTL biocrude [66][163] in CoMo as the guard-bed HDM and NiMo as the bulk catalyst. By doing so, they obtained impressive drop-in fuel properties in the hydrotreated oils (Table 3).Table 3.
Continuous hydrotreatment of different HTL biocrudes in the presence of sulfided catalysts.
| HTL Biocrude | Process Parameters | Hydrotreated Oils | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Sulfided Catalysts | T | PH2 | WHSV/LHSV i | |||||
| (°C) | (MPa) | (h−1) | H/C | N/C | O/C | |||
| Nannochloropsis–Solix LEA | CoMo/fluorinated-Al2O3 | 405 | 13.6 | 0.14 LHSV | 1.99 | 0.001 | 0.007 | [61][158] |
| Nannochloropsis–NB238 | CoMo/fluorinated-Al2O3 | 405 | 13.6 | 0.20 LHSV | 1.86 | 0.002 | 0.011 | [61][158] |
| Nannochloropsis–Cellana LL | CoMo/fluorinated-Al2O3 | 405 | 13.6 | 0.20 LHSV | 1.91 | 0.003 | 0.016 | [61][158] |
i WHSV = Weight hourly space velocity; LHSV = Liquid hourly space velocity.
