Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Dieter Rehder.
In Earth’s regions accessible for living organisms (Earth’s crust, crude oil, water sanctuaries and lower atmosphere), vanadium is present in the oxidation states +III and—essentially—+IV (cationic) and +V (cationic and anionic), with the redox interchange and biochemical recycling often monitored by bacteria. Organisms having available vanadium-containing (bio)molecules with essential functions for life include marine brown algae (haloperoxidases), ascidians and fan worms, as well as terrestrial organisms, viz., nitrogen-fixing bacteria (associated with the roots of legumes), and the fly agaric mushroom.
- halide oxidation
- dinitrogen reduction
- marine organisms
1. Introduction
On Earth, the crustal abundance of vanadium (0.019%; 20th most abundant element) compares to that of zinc; however, vanadium is more dispersed than Zn. The common redox states in the minerals of the Earth’s crust are +V, +IV and +III [1]. Crude oil (asphaltenes) can obtain up to 5 g V per litre; the high concentrations here originate from the coordinative incorporation of vanadium (VO2+) into, essentially, porphinogens. The main sources of atmospheric vanadium are marine aerosols and continental dust originating from geological processes, and due to anthropogenic input, including emissions—VO2, V2O5 and vanadium carbide—from combustion engines. In aqueous media, such as seawater, lakes and rivers, vanadium is present as H2VO4−/HVO42− (depending on the pH); common average concentrations in marine environments (pH~7; where the dominant species is H2VO4− (and HV2O73− at higher concentrations)) amount to 1.8 µg/L, in rivers to 0.7 µg/L. The average amount of vanadium in sediments is 150 mg/kg [2]. In mining areas, vanadium concentrations in aqueous environments can go up by a factor of 106. In human blood plasma, vanadium concentrations are higher by a factor of ~10 with respect to seawater, pointing towards a (possibly essential) role of vanadium in life: In living organisms, vanadate H2VO4− acts as an antagonist/competitor/enhancer of phosphate but tends to be toxic at non-physiological concentrations. Vanadate also forms binary and ternary compounds with carbonate, viz., HVO3CO32− and VO2(CO3)23−, with logK values of 1.09 and 0.17, respectively. At higher concentrations, oligomers such as the dimeric V2O74− can form; reducing conditions are responsible for the generation of VIVO2+·aq (which precipitates to form VO(OH)2 or remains dissolved due to coordination to (organic) substrates) and VIII·aq (present in, e.g., ascidians). The redox interconversion of vanadium (VV ↔ VIV ↔ VIII) in a natural environment is often monitored or exerted by specialised bacteria, such as Pseudomonas vanadiumreducens.
Besides various strains of bacteria, several multicellular organisms resort to vanadium as an essential element, the latter occasionally also in cooperation with bacteria. Noteworthy among these higher organisms are ascidians, marine brown algae and certain mosses, amavadin in Amanita mushrooms and the nodules in the roots of legumes.
2. Vanadium in Haloperoxidases, Nitrogenases, Amanita Mushrooms, Ascidians and Fan Worms
Along with the potentiality of vanadium in many catalytic applications carried out in the frame of industrial processes (examples are oxidation reactions, carbon–carbon bond formation, hydrogenation and dehydrogenation and cyanation [7][3]), the role of vanadium in naturally occurring catalytic processes is noteworthy. These functions include the oxidation of halides catalysed by haloperoxidases (particularly in seawater estuaries) on the one hand, and the reductive conversion of aerial dinitrogen into ammonia/ammonium ions (and hence nitrogen in a form utilisable in bio-physiological processes), catalysed by nitrogenases on the other hand. An additional naturally occurring vanadium compound is amavadin, present in the fly agaric mushroom. Specific brown and red algae, fungi and bacteria—such as the marine brown alga Ascophyllum nodosum, the marine flavobacterium Zobiella galactanivorans [8][4] and the cyanobacterium Synechocosccus (associated with macroalgae and specialised in the oxidation of iodide)—can have available vanadate-dependent haloperoxidases, one of the six known families of halogenating enzymes [9][5]. Actually, the VHPOs apparently derive from a species closely related to bacterial acid phosphatase [8][4], well in agreement with the structural similarity between phosphate and vanadate, and the competitive/comparable behaviour of these two anions in life processes. Iodide, bromide, chloride and cyanide may be subject to oxidation to hypohalite/hypohalous acid and cyanate, respectively. The halide specificity essentially reflects differences in hydrogen-bonding interaction between the active centre and the extended (second) coordination sphere. The oxidising agent commonly is H2O2/peroxide. Hypohalite in turn is involved in the biosynthesis of halogenated organic compounds, such as bromoform, which is released into the atmosphere. Figure 1 (left) shows the active centre of the vanadate-dependent iodo/bromoperoxidase of the marine brown alga Ascophyllum nodosum (Figure 1, right) from which this enzyme had been originally isolated [10][6] and characterised with respect to its reactivity [11][7]. The active centres of the enzyme present in other algal and bacterial species are essentially identical, i.e., they only differ in the second sphere amino acid surroundings of the central penta-coordinated {VO(OH)(O−)His} unit, and in H-bonding interaction between the first and the second coordination sphere. In summary, halogenases are involved in the oxidation of halides (to hypohalous acids) and, in such a way, in the halogenation of organic compounds and the oxidative elimination of (invasive) bacteria via the intermediate formation of reactive oxygen species [12][8].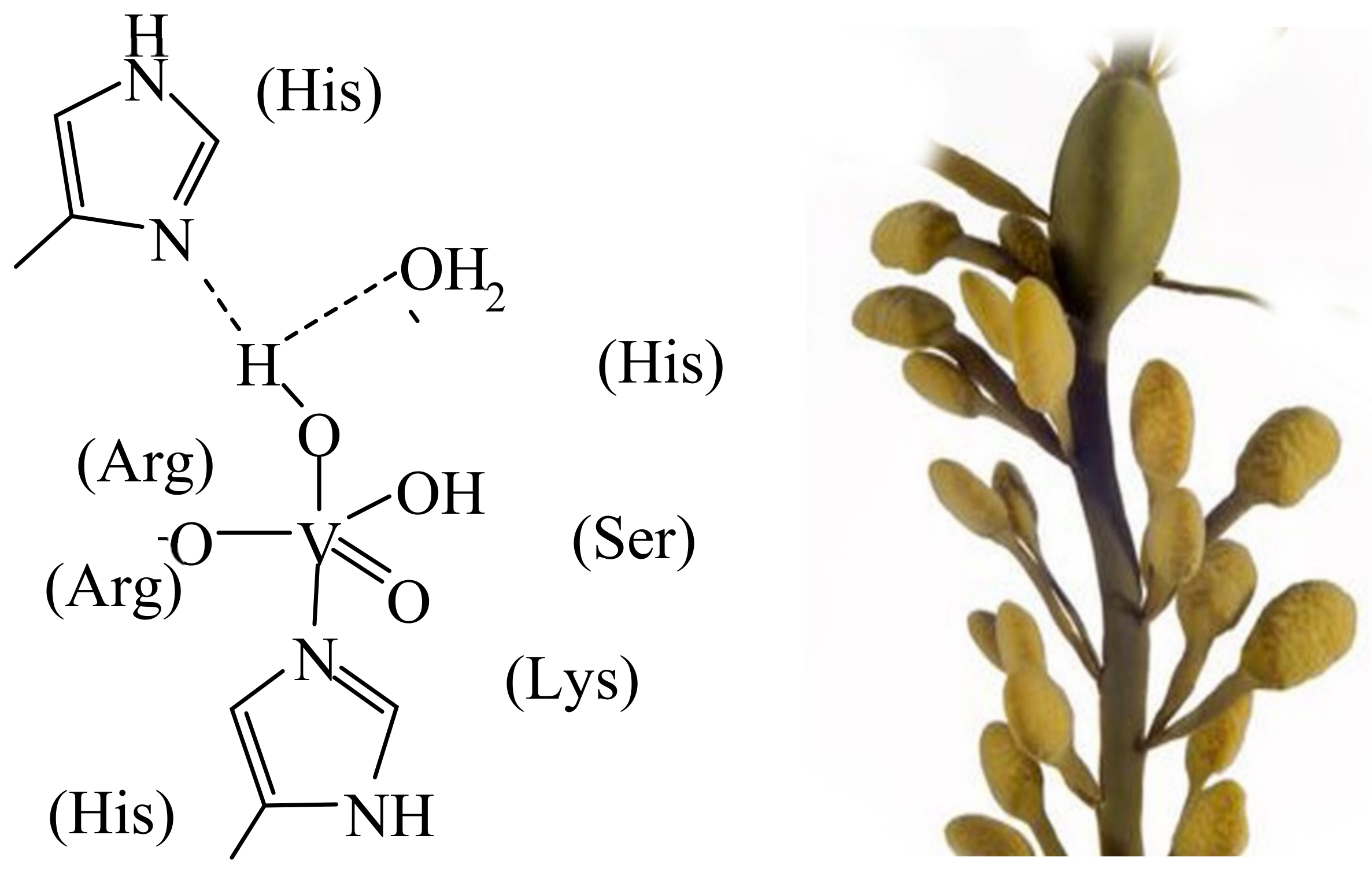
Figure 1.
The active centre (schematised (
left
)) of vanadate-dependent, bromo/iodoperoxidases present in the brown alga
Ascophyllum nodosum
(
right
).
N2 + 8H+ + 6e− → 2NH4+
(powered by: MgATP + H+ ⮩ MgADP + HPO42−)
2CO + 3H2 → C2H4 + H2O (+ C2H6, C3H8, …)
CO2 + 4H2 → CH4 + 2H2O
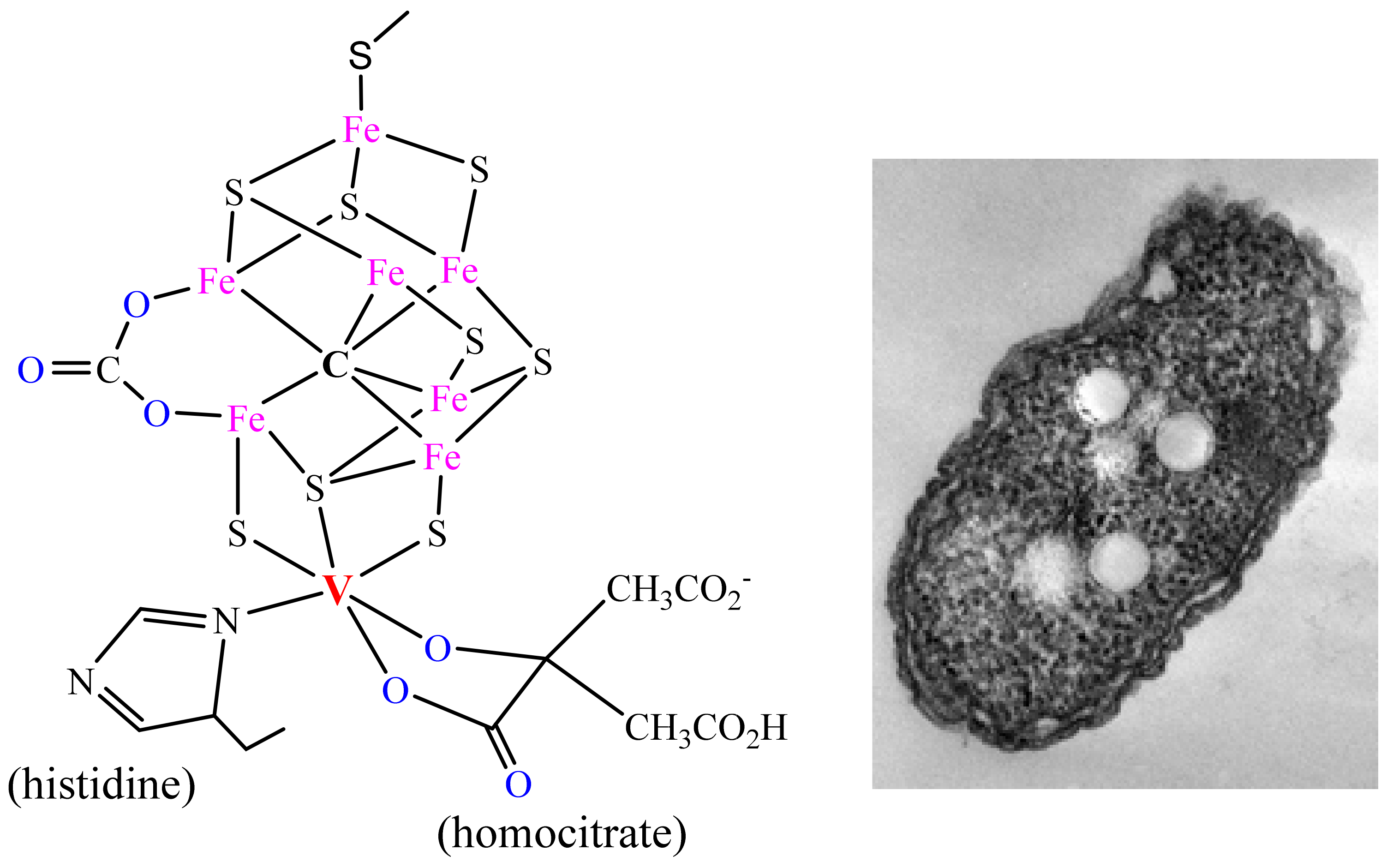
Figure 2. The active centre of vanadium nitrogenase and the soil bacterium Azotobacter vinelandii.
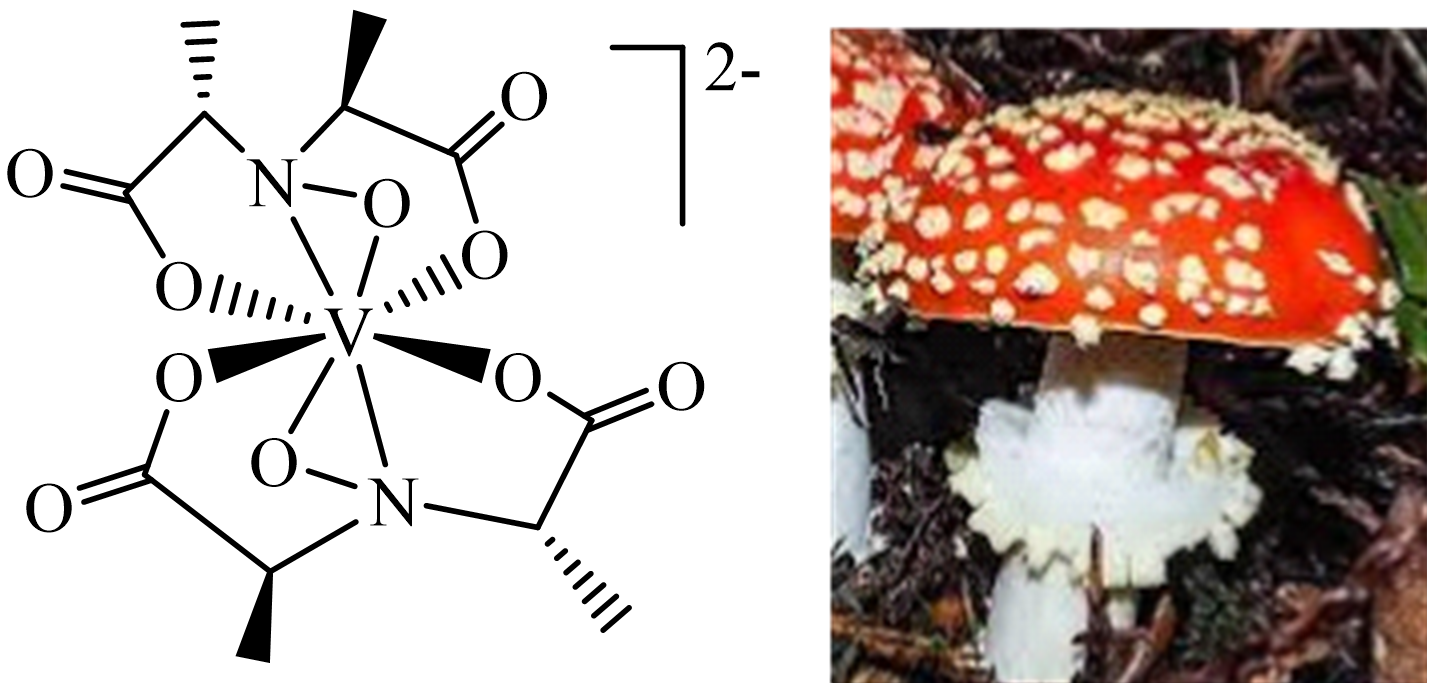
Figure 3. Amavadin present in the fly agaric (Amanita muscaria).
Figure 4.
The fan worm
Pseudopotamilla occelata
(
left
) and the sea squirt
Ascidia sydneiensis
(
right
), the latter with the branchial crown at the upper left.
3. Bacterial Issues
Matters concerning bacteria have already briefly been mentioned in ch. 3 in the context of N2 fixation (see also cyanobacteria in rice fields [25][21]), haloperoxidases, amanita mushrooms and ascidians. Other bacterial activities aiming at redox interconversion (essentially reduction of vanadium(V)) concern several strains of bacteria—belonging to the eucarya—that are able to reduce vanadate H2VO4− to oxidovanadium(IV) VO2+ (which commonly precipitates in the form of VO(OH)2) and thus can contribute to the removal of vanadate(V) from drinking water. Vanadate—being a phosphate antagonist—is toxic at higher, non-physiological concentrations. Examples of these bacteria are Pseudomonas vanadiumreductans, Shewanella oneidensis, Geobacter metallireducens and Saccharomyces cerevisiae [26][22]. Reduction of vanadate to VO2+ can also be achieved by mesophilic bacteria (an archaeal bacterial strain) such as Methanosarcina mazei, which is active at 37 °C, and the thermophile Methanothermobacter termautropicus, which is thriving at an optimum temperature of 65 °C [27][23] and further—at concentrations < 5 mM, by Thiobacillus thiooxidans [25][21]. This bio-reduction of VV to VIV (VO(OH)2↓) in a special anoxic growth medium inhibits methanogenesis (CO2/acetate →→ CH4, the reductant usually is H2), the otherwise common domain of activity for these bacteria.References
- Huang, J.-H.; Huang, F.; Evans, L.; Glasauer, S. Vanadium: Global (bio)geochemistry. Chem. Geol. 2015, 417, 68–89.
- Awan, R.S.; Liu, C.; Yang, S.; Wu, Y.; Zang, Q.; Khan, A.; Li, G. The occurrence of vanadium in nature: Its biogeochemical cycling and relationship with organic matter—A case study of the early Cambrian black rocks of the niutitang formation, western Hunan, China. Acta Geochim. 2021, 40, 973–997.
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic applications of vanadium: A mechanistic perspective. Chem. Rev. 2019, 119, 2128–2191.
- Fournier, J.B.; Rebuffet, E.; Delage, L.; Grijol, R.; Meslet-Gladièr, L.; Rzonca, J.; Potin, P.; Michel, G.; Czjzek, M.; Leblanc, C. The vanadium iodoperoxidases from the marine Flavobacteriaceae Species Zobiella galactanivorans reveals novel molecular and evolutionary features of halide specifity in the vanadium haloperoxidase enzyme family. Appl. Environ. Microbiol. 2014, 80, 7561–7573.
- Xu, G.; Wang, B.-G. Independent evolution of six families of halogenating enzymes. PLoS ONE 2016, 11, e0154619.
- Vilter, H.; Glombitza, K.-W.; Grave, A. A role for vanadium in ascidians and marine algae. Bot. Mar. 1983, 26, 331–340.
- Butler, A. Vanadium peroxidases. Curr. Opin. Chem. Biol. 1998, 2, 279–285.
- Baumgartner, J.T.; McKinnie, S.M.K. Investigating the role of vanadium-dependent haloperoxidase enzymology in microbial secondary metabolism and chemical ecology. mSystems 2021, 6, e00780-21.
- Harwood, C.S. Iron-only and vanadium nitrogenase: Fail-safe enzymes or something more? Ann. Rev. 2020, 74, 247–266.
- Darnajoux, R.; Bradley, R.; Bellenger, J.-P. In Vivo temperature dependence of molybdenum and vanadium nitrogenase activity in the heterocystous cyanobacteria Anabaena variabilis. Environ. Sci. Technol. 2022, 56, 2760–2769.
- Hodkinson, B.P.; Allen, J.L.; Forrest, L.L.; Goffinet, B.; Sérusiaux, E.; Andrésson, Ó.S.; Miao, V.; Bellenger, J.P.; Lutzoni, F. Lichen-symbiontic cyanobacteria associated with Peltigera have an alternative vanadium-dependent nitrogen fixing system. Eur. J. Phycol. 2014, 49, 11–19.
- Rohde, M.; Laun, K.; Einsle, O. Two ligands binding sites in CO-reducing V nitrogenase reveal a general mechanistic principle. Sci. Adv. 2021, 7, eabg4474.
- Zheng, Y.; Harrsi, D.F.; Yu, Z.; Fu, Y.; Poudel, S.; Ledbetter, R.N.; Fixen, K.R.; Yang, Z.-Y.; Boyd, E.S.; Lidstrom, M.E.; et al. A pathway for biological methane production using bacterial iron-only nitrogenase. Nat. Microbiol. 2018, 3, 281–286.
- Braeuer, S.; Walenta, M.; Steiner, L.; Goessler, W. Determination of the naturally occurring vanadium complex amavadin in Amanita muscaria with HPLC-ICPMS. J. Anal. At. Spectrom. 2021, 36, 954–967.
- Dias, L.; Bekhti, N.; Kuznetsov, M.L.; Ferreira, J.A.B.; Bacariza, M.C.; da Silva, J.A.L. Nitrite reduction in aqueous solution mediated by amavadin homologues: N2O formation and water oxidation. Chem. Eur. J. 2017, 24, 2474–2482.
- Yamaguchi, N.; Yoshinaga, M.; Kamino, K.; Ueki, T. Vanadium-binding ability of nucleoside diphosphate kinase from the vanadium-rich fan worm, Pseudopotomilla occelata. Zool. Sci. 2016, 33, 266–271.
- Ueki, T.; Yamaguchi, N.; Romaidi; Isago, Y.; Tanahashi, H. Vanadium accumulation in ascidians: A system overview. Coord. Chem. Rev. 2015, 301–302, 300–308.
- Romaidi; Ueki, T. Bioaccumulation of Vanadium by Vanadium-Resistant Bacteria Isolated from the Intestine of Ascidia sydneiensis samea. Mar. Biotechnol. 2016, 18, 359–371.
- Ueki, T.; Fujie, M.; Romaidi; Satoh, N. Symbiotic bacteria associated with ascidian vanadium accumulation identified by 16S rRNA amplicon sequencing. Mar. Genom. 2019, 43, 33–42.
- Lee, C.-H.; Lin, D.-J.; Pan, H.R.; Wu, J.; Liu, H.-K.; Hsu, H.-F. Reversible conversion of disulfide/dithiolate occurring at a vanadium(IV) centre: A biomimetic system for redox exchange in vanabin. Inorg. Chem. 2022, 61, 19882–19889.
- Boison, G.; Steingen, C.; Stal, L.J.; Bothe, H. The rice field cyanobacteria Anabaena azotica and Anabaena sp. CH1 express vanadium-dependent nitrogenase. Arch. Microbiol. 2006, 186, 367–376.
- Zhang, J.; Dong, H.; Zhao, L.; McCarrick, R.; Agrawal, A. Microbial reduction and precipitation of vanadium by mesophilic and thermophilic methanogens. Chem. Geol. 2014, 370, 29–39.
- Wang, S.; Zhang, B.; Li, T.; Li, Z.; Fu, J. Soil vanadium(V) reducing related bacteria drive community response to vanadium pollution from a smelting plant over multiple gradients. Environ. Int. 2020, 138, 105630.
More
