Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Dieter Rehder and Version 2 by Rita Xu.
In Earth’s regions accessible for living organisms (Earth’s crust, crude oil, water sanctuaries and lower atmosphere), vanadium is present in the oxidation states +III and—essentially—+IV (cationic) and +V (cationic and anionic), with the redox interchange and biochemical recycling often monitored by bacteria. Organisms having available vanadium-containing (bio)molecules with essential functions for life include marine brown algae (haloperoxidases), ascidians and fan worms, as well as terrestrial organisms, viz., nitrogen-fixing bacteria (associated with the roots of legumes), and the fly agaric mushroom.
- halide oxidation
- dinitrogen reduction
- marine organisms
1. Introduction
On Earth, the crustal abundance of vanadium (0.019%; 20th most abundant element) compares to that of zinc; however, vanadium is more dispersed than Zn. The common redox states in the minerals of the Earth’s crust are +V, +IV and +III [1]. Crude oil (asphaltenes) can obtain up to 5 g V per litre; the high concentrations here originate from the coordinative incorporation of vanadium (VO2+) into, essentially, porphinogens. The main sources of atmospheric vanadium are marine aerosols and continental dust originating from geological processes, and due to anthropogenic input, including emissions—VO2, V2O5 and vanadium carbide—from combustion engines. In aqueous media, such as seawater, lakes and rivers, vanadium is present as H2VO4−/HVO42− (depending on the pH); common average concentrations in marine environments (pH~7; where the dominant species is H2VO4− (and HV2O73− at higher concentrations)) amount to 1.8 µg/L, in rivers to 0.7 µg/L. The average amount of vanadium in sediments is 150 mg/kg [2]. In mining areas, vanadium concentrations in aqueous environments can go up by a factor of 106. In human blood plasma, vanadium concentrations are higher by a factor of ~10 with respect to seawater, pointing towards a (possibly essential) role of vanadium in life: In living organisms, vanadate H2VO4− acts as an antagonist/competitor/enhancer of phosphate but tends to be toxic at non-physiological concentrations. Vanadate also forms binary and ternary compounds with carbonate, viz., HVO3CO32− and VO2(CO3)23−, with logK values of 1.09 and 0.17, respectively. At higher concentrations, oligomers such as the dimeric V2O74− can form; reducing conditions are responsible for the generation of VIVO2+·aq (which precipitates to form VO(OH)2 or remains dissolved due to coordination to (organic) substrates) and VIII·aq (present in, e.g., ascidians). The redox interconversion of vanadium (VV ↔ VIV ↔ VIII) in a natural environment is often monitored or exerted by specialised bacteria, such as Pseudomonas vanadiumreducens.
Besides various strains of bacteria, several multicellular organisms resort to vanadium as an essential element, the latter occasionally also in cooperation with bacteria. Noteworthy among these higher organisms are ascidians, marine brown algae and certain mosses, amavadin in Amanita mushrooms and the nodules in the roots of legumes.
2. Vanadium in Haloperoxidases, Nitrogenases, Amanita Mushrooms, Ascidians and Fan Worms
Along with the potentiality of vanadium in many catalytic applications carried out in the frame of industrial processes (examples are oxidation reactions, carbon–carbon bond formation, hydrogenation and dehydrogenation and cyanation [3][7]), the role of vanadium in naturally occurring catalytic processes is noteworthy. These functions include the oxidation of halides catalysed by haloperoxidases (particularly in seawater estuaries) on the one hand, and the reductive conversion of aerial dinitrogen into ammonia/ammonium ions (and hence nitrogen in a form utilisable in bio-physiological processes), catalysed by nitrogenases on the other hand. An additional naturally occurring vanadium compound is amavadin, present in the fly agaric mushroom. Specific brown and red algae, fungi and bacteria—such as the marine brown alga Ascophyllum nodosum, the marine flavobacterium Zobiella galactanivorans [4][8] and the cyanobacterium Synechocosccus (associated with macroalgae and specialised in the oxidation of iodide)—can have available vanadate-dependent haloperoxidases, one of the six known families of halogenating enzymes [5][9]. Actually, the VHPOs apparently derive from a species closely related to bacterial acid phosphatase [4][8], well in agreement with the structural similarity between phosphate and vanadate, and the competitive/comparable behaviour of these two anions in life processes. Iodide, bromide, chloride and cyanide may be subject to oxidation to hypohalite/hypohalous acid and cyanate, respectively. The halide specificity essentially reflects differences in hydrogen-bonding interaction between the active centre and the extended (second) coordination sphere. The oxidising agent commonly is H2O2/peroxide. Hypohalite in turn is involved in the biosynthesis of halogenated organic compounds, such as bromoform, which is released into the atmosphere. Figure 1 (left) shows the active centre of the vanadate-dependent iodo/bromoperoxidase of the marine brown alga Ascophyllum nodosum (Figure 1, right) from which this enzyme had been originally isolated [6][10] and characterised with respect to its reactivity [7][11]. The active centres of the enzyme present in other algal and bacterial species are essentially identical, i.e., they only differ in the second sphere amino acid surroundings of the central penta-coordinated {VO(OH)(O−)His} unit, and in H-bonding interaction between the first and the second coordination sphere. In summary, halogenases are involved in the oxidation of halides (to hypohalous acids) and, in such a way, in the halogenation of organic compounds and the oxidative elimination of (invasive) bacteria via the intermediate formation of reactive oxygen species [8][12].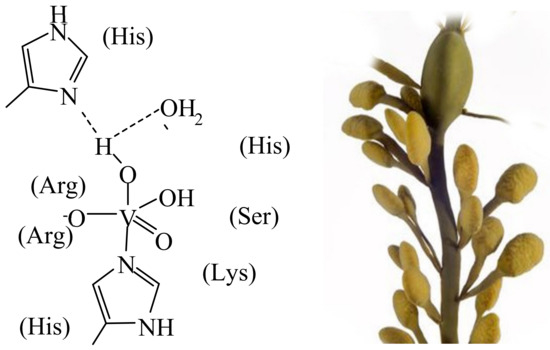
Figure 1.
The active centre (schematised (
left
)) of vanadate-dependent, bromo/iodoperoxidases present in the brown alga
Ascophyllum nodosum
(
right
).
N2 + 8H+ + 6e− → 2NH4+
(powered by: MgATP + H+ ⮩ MgADP + HPO42−)
2CO + 3H2 → C2H4 + H2O (+ C2H6, C3H8, …)
CO2 + 4H2 → CH4 + 2H2O
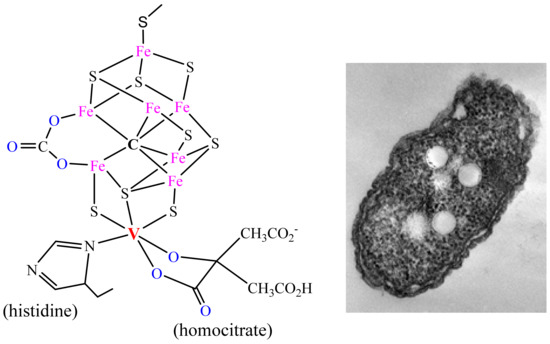
Figure 2. The active centre of vanadium nitrogenase and the soil bacterium Azotobacter vinelandii.
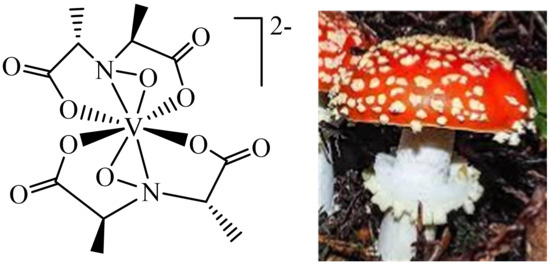
Figure 3. Amavadin present in the fly agaric (Amanita muscaria).
Figure 4.
The fan worm
Pseudopotamilla occelata
(
left
) and the sea squirt
Ascidia sydneiensis
(
right
), the latter with the branchial crown at the upper left.
