Coronavirus disease 2019 (COVID-19), the pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which counts more than 650 million cases and more than 6.6 million of deaths worldwide, affects the respiratory system with typical symptoms such as fever, cough, sore throat, acute respiratory distress syndrome (ARDS), and fatigue. Uumbilical-cord-derived mesenchymal stromal cells (UC-MSCs) could be a potent tool against phlogistic status, not only during the last pandemic caused by SARS-CoV-2, but also for other future pandemic or respiratory tract inflammatory diseases. However, a series of challenges still lies ahead, comprising the accomplishment of all the tests required by the regulatory bodies.
- COVID-19
- SARS-CoV-2
- mesenchymal stromal cells
- umbilical-cord-derived mesenchymal stromal cells
1. Introduction
2. Characteristics of UC-MSCs in In Vitro and Preclinical Experimental Evidence Supporting Anti-Inflammatory, Immunomodulation, and Therapeutic Potential
2.1. Adult and Perinatal MSCs: General Features
Mesenchymal stromal cells (MSCs), which derive from the inner mass of the blastocyst, have a high capacity to self-renew, have fibroblastic-like shape when cultured in plastic surface, can differentiate into mesodermal derivatives such as osteoblasts, chondrocytes, and adipocytes, and show phenotype and characteristics in accordance with the minimal criteria of the International Society for Cellular Therapy (ISCT) [21]. They have, therefore, shed a new light on treatment of patients suffering from diseases and disorders that do not yet have a definite cure, and have a long history since their discovery to therapy applications [22]. MSCs are present in almost all post-natal/adult organs, i.e., bone marrow [23][24][25], adipose tissue [26][27], dental pulp [28][29], endometrium [30][31], menstrual blood [32][33], peripheral blood [34], salivary gland [35][36], skin and foreskin [37][38][39][40], synovial fluid [41][42], muscle [43][44][45], corneal stroma [46][47], heart [48][49], and lung [50]. Promising sources of MSCs are represented by the extraembryonic/perinatal tissues [51], among which there are the placenta, the chorionic and amniotic membranes [52][53][54], amniotic fluid [55], umbilical cord blood [56], and umbilical cord stroma [57]. Moreover, since MSCs derived from perinatal, as well as adipose tissue (AT-MSCs) and bone marrow (BM-MSCs), do not express ACE2 and TMPRSS2, this demonstrates that they are not permissive to SARS-CoV-2 infection, increasing the interest in the use of MSCs as potential therapy for COVID-19 [58]. However, the methods for obtaining adult tissues are invasive, and the yield of cells gained after isolation is scarce (e.g., 3.5 × 105 to 1 × 106 in 1 g of adipose tissue and from 500 to 5 × 104 from 1 g of bone marrow aspirate [59]). Perinatal tissues provide an interesting source of MSCs as they are usually wasted after birth and, therefore, the collection procedures are without risks for the donor and ethical issues [60]. Importantly, umbilical cord (UC) matrix is a better source of MSCs, in terms of yields, than the umbilical cord blood and adult tissues [61][62][63].2.2. UC-MSCs Properties: Multilineage Differentiation, Immune Tolerance, Angiogenesis/Wound Healing, Matrix Remodeling, and Resistance to Hypoxia
Multilineage differentiation properties—The UC is consisted of two arteries and one vein included in a connective tissue called “Wharton’s jelly” (WJ), mainly composed of sponge-like structure woven with collagen fibers, proteoglycans, and embedded MSCs, and an outer layer of amniotic epithelium [57][64][65]. Scholras focused research on molecular characterization of UC-MSCs, demonstrating that these cells express CD10, CD13, CD29, CD44, CD54, CD73, CD90, CD105, Stro-1, MHC class I (classical HLA-A, -B and -C), mesenchymal markers (vimentin, α-SMA), neuroectodermal markers (Nestin, NSE, GFAP), and early endoderaml markers (GATA-4,-5,-6, HNF4, cytokeratin-8,-18,-19), and lack the major costimulatory molecules responsible for T cell activation, specifically B7-1 (CD80) and B7-2 (CD86), hematopoietic and endothelial markers CD14, CD19, CD31, CD34, CD38, CD45, CD66b, CD80, CD86, CD106, and CD133, [57][66][67][68][69]. UC-MSCs multipotency is formally demonstrated by their in vitro differentiation capability towards cell types of mesodermal origin (chondrocytes, adipocytes, osteoblasts, odontoblast-like cells, dermal fibroblasts, smooth muscle cells, skeletal muscle cells, cardiomyocytes) and endodermal lineages (hepatocyte-like cells, pancreatic endocrine cells), as well as ectodermal and neuroectodermal (sweat gland cells, oligodendrocytes, and dopaminergic neurons) [57][66][70][71][72][73][74][75][76][77][78][79][80][81][82][83]. UC-MSCs also feature “primitive stemness” properties due to their close relation with the embryologic phase, also maintaining the length of telomeric ends even after around 60 population doublings, and having no chromosomal mutation acquisition [66]. Immune tolerance and anti-inflammatory properties—UC-MSCs exert immunomodulation properties [72][84][85], even related to the expression and release of specific factors, such as the nonclassical HLA class I antigen, HLA-G [66][86], HLA-E, CD276/B7-H3, leukemia inhibitory factor (LIF), indolamine 2,3-dioxygenase-1 (IDO-1), galectin-1 (Gal-1), and heat shock protein 10/Early Pregnancy Factor (HSP10/EPF), being able to modulate or inhibit lymphocyte proliferation [84]. These factors are involved in tolerogenic processes occurring at the fetal–maternal interface [87][88][89][90][91][92], permitting, in turn, the semi-allogeneic embryo to escape surveillance of the maternal immune system. Specifically, HLA-G is an inhibitory molecule involved in immune tolerance and exerts its inhibitory functions interacting with inhibitory receptors Ig-like transcript (ILT) receptors, such as ILT-2, ILT-3, and ILT-4, and killer cell immunoglobulin-like receptor (KIR), two Ig domains, and long cytoplasmic tail 4, KIR2DL4, differentially expressed by NK, T, and antigen-presenting cells [93][94]. Its expression is enhanced by Gal-1 [92]. HLA-G also interacts with leukocyte immunoglobulin-like receptor subfamily B member 1 (LILRB1) expressed by CD56brightCD16− natural killer (NK) cells that are enriched in the uterus during pregnancy, are poorly cytotoxic, and produce low amounts of IFN-γ as compared with peripheral blood CD56dimCD16+ NK cells [69]. Lastly, HSP10, commonly known as a heat shock protein of 10 KDa, mainly expressed in the inner membrane of mitochondria, is also known as early pregnancy factor (EPF), discovered in the 1970s as a factor released during pregnancy in the serum of within 24 h after fertilization, preventing T cell-rosette formation and reduction of proinflammatory cytokines release, such as TNF-α (through interaction with TLR4 expressed by macrophages) (see in [95]). Although the underlying mechanism is still unknown, UC-MSCs secrete different prostanoids, such as PGD2, PGF2a, and PGE2, and several molecules, including IL-1R antagonist, IL-6, IL-10, M-CSF, VEGF, TGF-β1, and B7-H4, which could contribute to the differentiation of M2 macrophages [69]. UC-MSCs have been also involved in regulation of the monocyte/macrophage system. In particular, UC-MSCs can prevent the differentiation and maturation of monocytes toward DCs [96]. UC-MSCs also secrete other neuroprotective, angiogenic, and antiapoptotic factors, such as Neurotrophin 3 (NTF3), epidermal growth factor (EGF), neurite growth-promoting factor 2 (NEGF2/MDK), heparin binding EGF-like growth factor (HBEGF), Chemokine ligand 2 (CXCL2), CXCL5, and fibroblast growth factor 9 (FGF9) [97]. Angiogenesis/wound healing—Being mainly involved in WJ remodeling, UC-MSCs are not in contact with capillaries and small blood vessels, excluding the unique three vessels involved in umbilical blood circulation (the two arteries and the one vein), and they produce small amounts of angiogenic factor VEGF-A. Their support on endothelial cell proliferation and vasculogenesis could be related with other VEGF-independent factors, such as IL-8, hepatocyte growth factor (HGF), and MCP-1 [98]. Matrix remodeling—The UC-MSCs are involved in ECM composition of WJ that surrounds the umbilical vessels, expressing vimentin and collagen II [72]. Lo Iacono et al., demonstrated, using mass spectrometry analyses, that UC-MSCs co-cultured with umbilical cord blood–CD34+ hematopoietic stem/progenitor cells are able to secrete collagens, different proteases and their inhibitors, such as MMP-8, TIMP-1, ADAM with thrombospondin type 1 motif 9 (ADAMTS9), secreted protein acidic and cysteine rich (SPARC), plasminogen activator inhibitor-1 (PAI-1), and serine carboxypeptidase 1 involved in ECM remodeling, as well as α-2-HS glycoprotein, which is a TGF-β antagonist and prevents calcification by buffering excess matrix mineralization, in addition to than mimicking a hematopoietic niche [67]. Migration of cells on ECM, remodeling, and degradation of the ECM by MMPs are key regulators of wound repair, since wound healing requires the controlled activity of MMPs, and UC-MSCs may have a great potential in connective tissues and wounds [98][99][100]. Hypoxia resistance—Another aspect worthy of note about UC-MSCs is their high resistance to a stromal environment that is relatively hypoxic, therefore adapting to survive in limited nutrient and oxygen conditions [101]. To this regard, scholars recently demonstrated similar metabolism and survival capability in both normal and hypoxic conditions (in oxygen–glucose deprivation/reperfusion stroke model) exerted by the three different MSC populations isolated from the three different zones of umbilical stromal, Wharton’s jelly (WJ-MSCs), perivascular region (PV-MSCs), and cord lining (CL-MSCs) of human, suggesting that UC-MSCs are suitable for stem-cell-based therapy of ischemic diseases [102]. This provides the idea that tissue function support may be due to the transfer of healthy mitochondria [103], which has been demonstrated, improving oxidative phosphorylation (OXPHOS) and bioenergetics of recipient cells [104]. The paracrine effects of UC-MSCs-derived molecules with immunomodulatory, anti-inflammatory, and regenerative properties are related to their release by MSCs on the extracellular environment not only through the secretion of soluble factors, but also as cargos of EVs, such as exosomes, which also contain lipids, metabolites, DNA fragments, miRNA fragments, and noncoding RNAs, acting locally and/or at distance as a cell-to-cell communication system, both in physiological and pathological conditions [105][106][107]. Due to their structure, and the opportunity to freely circulate into the body fluids, with low immunogenicity, and their bioavailability, they could be useful for drug delivery, and generate a great interest among scientists for cell-free therapies. The roles of perinatal MSCs and their conditioned media, enriched in EVs, were also explored in preventing lung injury across lung transplantation, described by Miceli and coworkers [108][109]. Taken together, all these features, characterized by the surface expression or secretion (through EVs/exosomes or in soluble form) of a series of factors with anti-inflammatory, immunomodulation, tissue repair, antifibrotic, and antihypoxic functions (summarized in Figure 1), support the interest in further studies about the use of UC-MSCs in in vivo experiments in order to move to allogeneic transplantation or develop novel cell-free products that are able to restore tissue functions, such as lung parenchymal functions dampening the damages exerted during COVID-19 disease.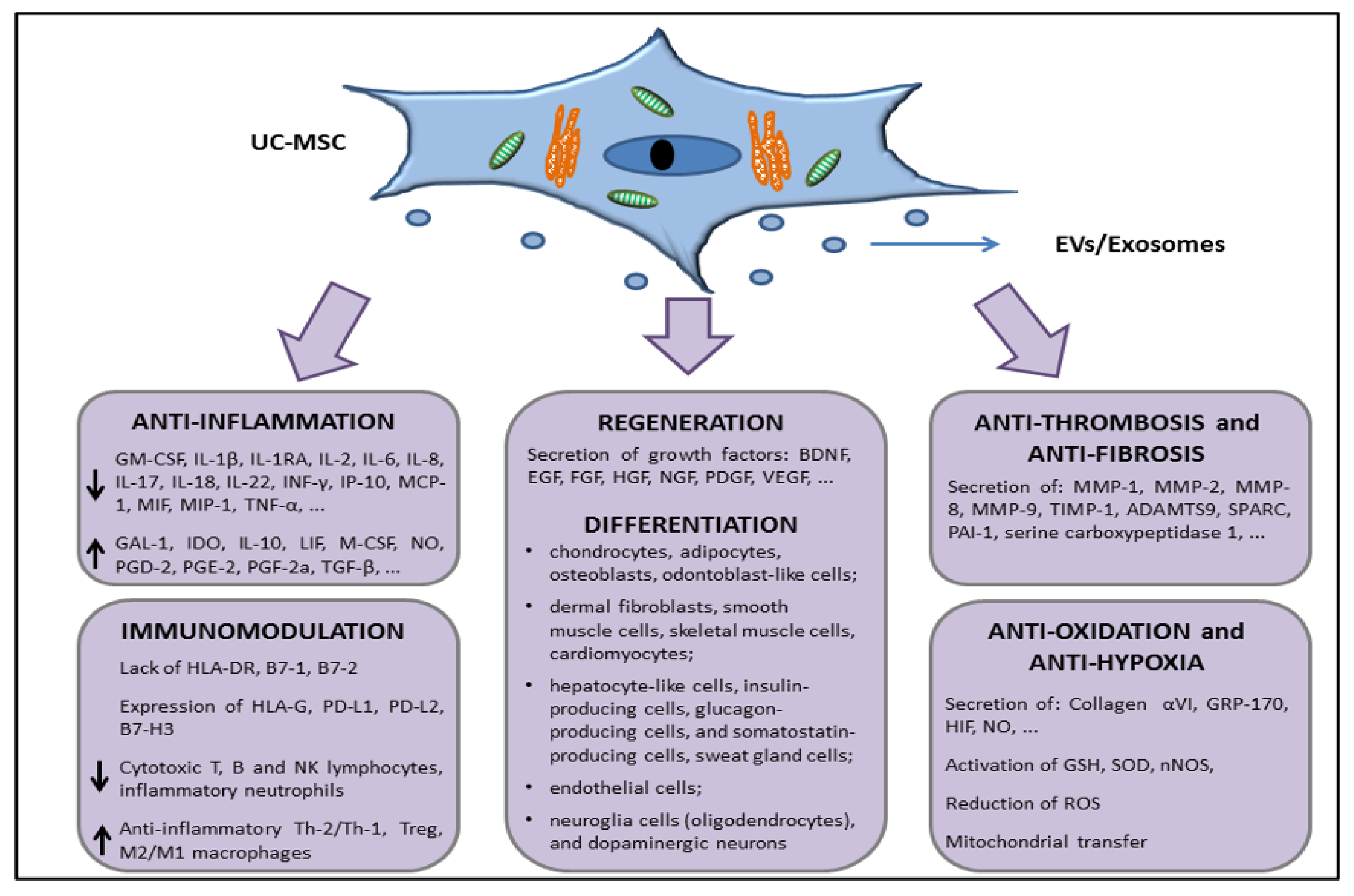
2.3. In Vivo Preclinical Data Supporting the Use of UC-MSCs to Treat Organ Dysfunctions
3. MSCs in COVID-19 Patients
Based on just-reported evidence about the shorter survival time of BM-MSCs compared with UC-MSCs [110], there is still the consensus about the use of BM-MSCs as a “gold-standard” for cell therapy. This is because it is relatively safer to use autologous BM-MSCs (or AT-MSCs) from the same patients, compared to allogeneic UC-MSCs, but the age of the patients, their gender, their health conditions, and the invasive procedure for isolating BM-MSCs (or AT-MSCs) must be taken into account in order to balance pros and cons. Even BM-MSCs have been studied in COVID-19 patients, as reviewed in Yao et al. [117]. BM-MSCs are able to produce and secrete soluble PD-1 ligands (sPD-L1 and sPD-L2) that are responsible for hyporesponsiveness in T cells, arresting the PD-1-mediated AKT pathway, thus inducing immune tolerance [118]. Further, in in vitro and in a humanized mouse model of graft versus host disease (GvHD), it was described that BM-MSCs affected T lymphocyte proliferation more than UC-MSCs, while the latter induced a higher increase of Tregs/Th17 ratio [119]. Since Treg/Th17 ratio imbalance correlates with immune thrombocytopenia (ITP) [120] and this imbalanced ratio was also observed in different cases of COVID-19 disease [121], the reactions of infused MSCs for correcting immune response in such condition should be absolutely taken in consideration. For example, the patients with ITP displayed abnormalities in BM-MSCs, due to defects in mRNA and miRNA that induced downregulation of genes involved in cellular stress machinery, such as the unfolded protein response (UPR), the nuclear protein transcriptional regulator 1 (Nupr1), involved in endoplasmic reticulum pathway, the TGF-β1 signaling, leading to a loss of immunosuppressive properties, and a breakdown of self-tolerance in ITP patients [122]. In this specific case, ITP patients are not eligible for autologous BM-MSCs. In a pilot study involving liver allograft recipients with acute rejection, they also observed a significant increase of Treg/Th17 ratio after 4 weeks of UC-MSC infusion [123]. Moreover, the BM-MSCs revealed a donor’s age-related decrease in colony forming units-fibroblast (CFU-f) in growth rate, in differentiation potential, and in superoxide dismutase (SOD) activity (and an increase of reactive oxygen species production) that could, in turn, affect autologous cell-based therapy [124]. On the contrary, UC-MSCs derived from perinatal tissue of childbearing age population showed no sign of senescence over several passages [66]. An integrated transcriptome-proteome analysis, comparing MSCs from different sources (BM, AT, and UC) revealed that secretome derived from UC-MSCs had a predominantly anti-inflammatory effect enriched in T cell inhibitory interleukins, such as IL-4, IL-13, IL-6, IL-35, IL-2, IL-22, IL-1R1, and IL-25, as well as the colony-stimulating growth factor (CSF) 3, which promoted M2 macrophage polarization, compared with the adult MSCs, while BM-MSCs were more immunosuppressive [125]. The immunosuppression is probably initiated starting from the apoptotic events induced by cytotoxic cells against BM-MSCs infused in GvHD recipients, as a result of a bystander effect of CD56+ natural killer (NK) and CD8+ T cells [126]. Taken together, these findings could highlight that UC-MSCs are more effective in treating symptoms and inflammatory state during the COVID-19-related cytokine storm than BM-MSCs.References
- Kilbourne, E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006, 12, 9–14.
- She, J.; Jiang, J.; Ye, L.; Hu, L.; Bai, C.; Song, Y. 2019 novel coronavirus of pneumonia in Wuhan, China: Emerging attack and management strategies. Clin. Transl. Med. 2020, 9, 19.
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733.
- WHO. Novel Coronavirus (2019-nCoV): Situation Report, 1. Available online: https://apps.who.int/iris/handle/10665/330760 (accessed on 21 December 2022).
- WHO. Novel Coronavirus (2019-nCoV) Report-1. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 (accessed on 21 December 2022).
- WHO. Statement on the Second Meeting of the International Health Regulations. Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). 2005. Available online: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) (accessed on 21 December 2022).
- Nie, J.; Li, Q.; Zhang, L.; Cao, Y.; Zhang, Y.; Li, T.; Wu, J.; Liu, S.; Zhang, M.; Zhao, C.; et al. Functional comparison of SARS-CoV-2 with closely related pangolin and bat coronaviruses. Cell Discov. 2021, 7, 21.
- WHO. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes It. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 21 December 2022).
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269.
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236.
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154.
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 21 December 2022).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- WHO. Living Guidance for Clinical Management of COVID-19. Available online: https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf (accessed on 22 December 2022).
- Samanipour, R.; Tabatabaee, S.; Delyanee, M.; Tavakoli, A. The promising approach of MSCs therapy for COVID-19 treatment. Cell Tissue Bank. 2022, 16, 1–16.
- Cozene, B.M.; Russo, E.; Anzalone, R.; Rocca, G.; Borlongan, C.V. Mitochondrial activity of human umbilical cord mesenchymal stem cells. Brain. Circ. 2021, 7, 33–36.
- Yaghoubi, Y.; Movassaghpour, A.; Zamani, M.; Talebi, M.; Mehdizadeh, A.; Yousefi, M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019, 233, 116733.
- Alzahrani, F.A.; Saadeldin, I.M.; Ahmad, A.; Kumar, D.; Azhar, E.I.; Siddiqui, A.J.; Kurdi, B.; Sajini, A.; Alrefaei, A.F.; Jahan, S. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes as Immunomodulatory Agents for COVID-19 Patients. Stem Cells Int. 2020, 2020, 8835986.
- Russo, E.; Caprnda, M.; Kruzliak, P.; Conaldi, P.G.; Borlongan, C.V.; La Rocca, G. Umbilical Cord Mesenchymal Stromal Cells for Cartilage Regeneration Applications. Stem Cells Int. 2022, 2022, 2454168.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272.
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274.
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650.
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147.
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228.
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295.
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630.
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806.
- Chan, R.W.; Schwab, K.E.; Gargett, C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004, 70, 1738–1750.
- Lenero, C.; Bowles, A.C.; Correa, D.; Kouroupis, D. Characterization and response to inflammatory stimulation of human endometrial-derived mesenchymal stem/stromal cells. Cytotherapy 2022, 24, 124–136.
- Meng, X.; Ichim, T.E.; Zhong, J.; Rogers, A.; Yin, Z.; Jackson, J.; Wang, H.; Ge, W.; Bogin, V.; Chan, K.W.; et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007, 5, 57.
- Chen, L.; Qu, J.; Xiang, C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res. Ther. 2019, 10, 1.
- Pang, Q.M.; Yang, R.; Zhang, M.; Zou, W.H.; Qian, N.N.; Xu, Q.J.; Chen, H.; Peng, J.C.; Luo, X.P.; Zhang, Q.; et al. Peripheral Blood-Derived Mesenchymal Stem Cells Modulate Macrophage Plasticity through the IL-10/STAT3 Pathway. Stem Cells Int. 2022, 2022, 5181241.
- Rotter, N.; Oder, J.; Schlenke, P.; Lindner, U.; Bohrnsen, F.; Kramer, J.; Rohwedel, J.; Huss, R.; Brandau, S.; Wollenberg, B.; et al. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev. 2008, 17, 509–518.
- Moon, J.H.; Kim, H.R.; Lim, J.Y.; Lim, Y.C. Single clonal glandular stem cells derived from human parotid glands do not attain malignant phenotype during long-term in vitro culture. Neoplasma 2021, 68, 1139–1146.
- Tappenbeck, N.; Schroder, H.M.; Niebergall-Roth, E.; Hassinger, F.; Dehio, U.; Dieter, K.; Kraft, K.; Kerstan, A.; Esterlechner, J.; Frank, N.Y.; et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy 2019, 21, 546–560.
- Vander Beken, S.; de Vries, J.C.; Meier-Schiesser, B.; Meyer, P.; Jiang, D.; Sindrilaru, A.; Ferreira, F.F.; Hainzl, A.; Schatz, S.; Muschhammer, J.; et al. Newly Defined ATP-Binding Cassette Subfamily B Member 5 Positive Dermal Mesenchymal Stem Cells Promote Healing of Chronic Iron-Overload Wounds via Secretion of Interleukin-1 Receptor Antagonist. Stem Cells 2019, 37, 1057–1074.
- Najar, M.; Lagneaux, L. Foreskin as a source of immunotherapeutic mesenchymal stromal cells. Immunotherapy 2017, 9, 207–217.
- Najar, M.; Merimi, M.; Faour, W.H.; Lombard, C.A.; Moussa Agha, D.; Ouhaddi, Y.; Sokal, E.M.; Lagneaux, L.; Fahmi, H. In Vitro Cellular and Molecular Interplay between Human Foreskin-Derived Mesenchymal Stromal/Stem Cells and the Th17 Cell Pathway. Pharmaceutics 2021, 13, 1736.
- Neybecker, P.; Henrionnet, C.; Pape, E.; Mainard, D.; Galois, L.; Loeuille, D.; Gillet, P.; Pinzano, A. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 329.
- Amemiya, M.; Tsuji, K.; Katagiri, H.; Miyatake, K.; Nakagawa, Y.; Sekiya, I.; Muneta, T.; Koga, H. Synovial fluid-derived mesenchymal cells have non-inferior chondrogenic potential and can be utilized for regenerative therapy as substitute for synovium-derived cells. BioChem. Biophys. Res. Commun. 2020, 523, 465–472.
- Asakura, A.; Komaki, M.; Rudnicki, M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 2001, 68, 245–253.
- Kang, J.S.; Krauss, R.S. Muscle stem cells in developmental and regenerative myogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 243–248.
- Elashry, M.I.; Kinde, M.; Klymiuk, M.C.; Eldaey, A.; Wenisch, S.; Arnhold, S. The effect of hypoxia on myogenic differentiation and multipotency of the skeletal muscle-derived stem cells in mice. Stem Cell Res. Ther. 2022, 13, 56.
- Funderburgh, J.L.; Funderburgh, M.L.; Du, Y. Stem Cells in the Limbal Stroma. Ocul. Surf. 2016, 14, 113–120.
- Eslani, M.; Putra, I.; Shen, X.; Hamouie, J.; Afsharkhamseh, N.; Besharat, S.; Rosenblatt, M.I.; Dana, R.; Hematti, P.; Djalilian, A.R. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5507–5517.
- Messina, E.; De Angelis, L.; Frati, G.; Morrone, S.; Chimenti, S.; Fiordaliso, F.; Salio, M.; Battaglia, M.; Latronico, M.V.; Coletta, M.; et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004, 95, 911–921.
- Anzalone, R.; Corrao, S.; Lo Iacono, M.; Loria, T.; Corsello, T.; Cappello, F.; Di Stefano, A.; Giannuzzi, P.; Zummo, G.; Farina, F.; et al. Isolation and characterization of CD276+/HLA-E+ human subendocardial mesenchymal stem cells from chronic heart failure patients: Analysis of differentiative potential and immunomodulatory markers expression. Stem Cells Dev. 2013, 22, 1–17.
- Rolandsson Enes, S.; Andersson Sjöland, A.; Skog, I.; Hansson, L.; Larsson, H.; Le Blanc, K.; Eriksson, L. MSC from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived MSC. Sci. Rep. 2016, 6, 29160.
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal 2011, 9, 12.
- Soncini, M.; Vertua, E.; Gibelli, L.; Zorzi, F.; Denegri, M.; Albertini, A.; Wengler, G.S.; Parolini, O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007, 1, 296–305.
- Pampalone, M.; Corrao, S.; Amico, G.; Vitale, G.; Alduino, R.; Conaldi, P.G.; Pietrosi, G. Human Amnion-Derived Mesenchymal Stromal Cells in Cirrhotic Patients with Refractory Ascites: A Possible Anti-Inflammatory Therapy for Preventing Spontaneous Bacterial Peritonitis. Stem Cell Rev. Rep. 2021, 17, 981–998.
- Miceli, V.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Comparative study of the production of soluble factors in human placenta-derived mesenchymal stromal/stem cells grown in adherent conditions or as aggregates in a catheter-like device. BioChem. Biophys. Res. Commun. 2020, 522, 171–176.
- De Coppi, P.; Bartsch, G., Jr.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106.
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675.
- Corrao, S.; La Rocca, G.; Lo Iacono, M.; Corsello, T.; Farina, F.; Anzalone, R. Umbilical cord revisited: From Wharton’s jelly myofibroblasts to mesenchymal stem cells. Histol. Histopathol. 2013, 28, 1235–1244.
- Avanzini, M.A.; Mura, M.; Percivalle, E.; Bastaroli, F.; Croce, S.; Valsecchi, C.; Lenta, E.; Nykjaer, G.; Cassaniti, I.; Bagnarino, J.; et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl. Med. 2021, 10, 636–642.
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109.
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. BioSci. Rep. 2015, 35, e00191.
- Zeddou, M.; Briquet, A.; Relic, B.; Josse, C.; Malaise, M.G.; Gothot, A.; Lechanteur, C.; Beguin, Y. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol. Int. 2010, 34, 693–701.
- Vangsness, C.T., Jr.; Sternberg, H.; Harris, L. Umbilical Cord Tissue Offers the Greatest Number of Harvestable Mesenchymal Stem Cells for Research and Clinical Application: A Literature Review of Different Harvest Sites. Arthroscopy 2015, 31, 1836–1843.
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016, 2016, 6901286.
- Watson, N.; Divers, R.; Kedar, R.; Mehindru, A.; Mehindru, A.; Borlongan, M.C.; Borlongan, C.V. Discarded Wharton jelly of the human umbilical cord: A viable source for mesenchymal stromal cells. Cytotherapy 2015, 17, 18–24.
- Davies, J.E.; Walker, J.T.; Keating, A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2017, 6, 1620–1630.
- La Rocca, G.; Anzalone, R.; Corrao, S.; Magno, F.; Loria, T.; Lo Iacono, M.; Di Stefano, A.; Giannuzzi, P.; Marasà, L.; Cappello, F.; et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: Differentiation potential and detection of new markers. Histochem. Cell Biol. 2009, 131, 267–282.
- Lo Iacono, M.; Russo, E.; Anzalone, R.; Baiamonte, E.; Alberti, G.; Gerbino, A.; Maggio, A. Wharton’s Jelly Mesenchymal Stromal Cells Support the Expansion of Cord Blood–derived CD34+ Cells Mimicking a Hematopoietic Niche in a Direct Cell–cell Contact Culture System. Cell Transpl. 2018, 27, 117–129.
- Silini, A.R.; Di Pietro, R.; Lang-Olip, I.; Alviano, F.; Banerjee, A.; Basile, M.; Borutinskaite, V.; Eissner, G.; Gellhaus, A.; Giebel, B.; et al. Perinatal Derivatives: Where Do We Stand? A Roadmap of the Human Placenta and Consensus for Tissue and Cell Nomenclature. Front. Bioeng. Biotechnol. 2020, 8, 610544.
- Magatti, M.; Abumaree, M.H.; Silini, A.R.; Anzalone, R.; Saieva, S.; Russo, E.; Trapani, M.E.; La Rocca, G.; Parolini, O. Chapter 6. The Immunomodulatory Features of Mesenchymal Stromal Cells Derived from Wharton’s Jelly, Amniotic Membrane, and Chorionic Villi: In Vitro and In Vivo Data. In Placenta: The Tree of Life; Parolini, O., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 91–128.
- Anzalone, R.; Lo Iacono, M.; Loria, T.; Di Stefano, A.; Giannuzzi, P.; Farina, F.; La Rocca, G. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: Extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev. 2011, 7, 342–363.
- Bharti, D.; Shivakumar, S.B.; Park, J.K.; Ullah, I.; Subbarao, R.B.; Park, J.S.; Lee, S.L.; Park, B.W.; Rho, G.J. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018, 372, 51–65.
- La Rocca, G.; Lo Iacono, M.; Corsello, T.; Corrao, S.; Farina, F.; Anzalone, R. Human Wharton’s jelly mesenchymal stem cells maintain the expression of key immunomodulatory molecules when subjected to osteogenic, adipogenic and chondrogenic differentiation in vitro: New perspectives for cellular therapy. Curr. Stem Cell Res. Ther. 2013, 8, 100–113.
- Corrao, S.; La Rocca, G.; Lo Iacono, M.; Zummo, G.; Gerbino, A.; Farina, F.; Anzalone, R. New frontiers in regenerative medicine in cardiology: The potential of Wharton’s jelly mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2013, 8, 39–45.
- Kwon, A.; Kim, Y.; Kim, M.; Kim, J.; Choi, H.; Jekarl, D.W.; Lee, S.; Kim, J.M.; Shin, J.C.; Park, I.Y. Tissue-specific Differentiation Potency of Mesenchymal Stromal Cells from Perinatal Tissues. Sci. Rep. 2016, 6, 23544.
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392.
- Han, Y.; Chai, J.; Sun, T.; Li, D.; Tao, R. Differentiation of human umbilical cord mesenchymal stem cells into dermal fibroblasts in vitro. BioChem. Biophys. Res. Commun. 2011, 413, 561–565.
- Conconi, M.T.; Burra, P.; Di Liddo, R.; Calore, C.; Turetta, M.; Bellini, S.; Bo, P.; Nussdorfer, G.G.; Parnigotto, P.P. CD105(+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int. J. Mol. Med. 2006, 18, 1089–1096.
- Wu, K.H.; Mo, X.M.; Zhou, B.; Lu, S.H.; Yang, S.G.; Liu, Y.L.; Han, Z.C. Cardiac potential of stem cells from whole human umbilical cord tissue. J. Cell Biochem. 2009, 107, 926–932.
- Campard, D.; Lysy, P.A.; Najimi, M.; Sokal, E.M. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 2008, 134, 833–848.
- Anzalone, R.; Iacono, M.L.; Corrao, S.; Magno, F.; Loria, T.; Cappello, F.; Zummo, G.; Farina, F.; La Rocca, G. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: Immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010, 19, 423–438.
- Belame Shivakumar, S.; Bharti, D.; Baregundi Subbarao, R.; Park, J.M.; Son, Y.B.; Ullah, I.; Choe, Y.H.; Lee, H.J.; Park, B.W.; Lee, S.L.; et al. Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton’s jelly mesenchymal stem cells using small molecules. J. Cell Physiol. 2019, 234, 3933–3947.
- Sarang, S.; Viswanathan, C. Umbilical Cord Derived Mesenchymal Stem Cells Useful in Insulin Production—Another Opportunity in Cell Therapy. Int. J. Stem Cells 2016, 9, 60–69.
- Mitchell, K.E.; Weiss, M.L.; Mitchell, B.M.; Martin, P.; Davis, D.; Morales, L.; Helwig, B.; Beerenstrauch, M.; Abou-Easa, K.; Hildreth, T.; et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells 2003, 21, 50–60.
- Corsello, T.; Amico, G.; Corrao, S.; Anzalone, R.; Timoneri, F.; Lo Iacono, M.; Russo, E. Wharton’s Jelly Mesenchymal Stromal Cells from Human Umbilical Cord: A Close-up on Immunomodulatory Molecules Featured In Situ and In Vitro. Stem Cell Rev. Rep. 2019, 15, 900–918.
- Tesarova, L.; Jaresova, K.; Simara, P.; Koutna, I. Umbilical Cord-Derived Mesenchymal Stem Cells Are Able to Use bFGF Treatment and Represent a Superb Tool for Immunosuppressive Clinical Applications. Int. J. Mol. Sci. 2020, 21, 5366.
- Weiss, M.L.; Anderson, C.; Medicetty, S.; Seshareddy, K.B.; Weiss, R.J.; VanderWerff, I.; Troyer, D.; McIntosh, K.R. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells 2008, 26, 2865–2874.
- Yang, Y.; Wang, W.; Weng, J.; Li, H.; Ma, Y.; Liu, L.; Ma, W. Advances in the study of HLA class Ib in maternal-fetal immune tolerance. Front. Immunol. 2022, 13, 976289.
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal-Fetal Immunity. Front. Immunol. 2020, 11, 458.
- Sawai, K.; Matsuzaki, N.; Kameda, T.; Hashimoto, K.; Okada, T.; Shimoya, K.; Nobunaga, T.; Taga, T.; Kishimoto, T.; Saji, F. Leukemia inhibitory factor produced at the fetomaternal interface stimulates chorionic gonadotropin production: Its possible implication during pregnancy, including implantation period. J. Clin. Endocrinol. Metab. 1995, 80, 1449–1456.
- Hamelin-Morrissette, J.; Dallagi, A.; Girouard, J.; Ravelojaona, M.; Oufqir, Y.; Vaillancourt, C.; Van Themsche, C.; Carrier, C.; Reyes-Moreno, C. Leukemia inhibitory factor regulates the activation of inflammatory signals in macrophages and trophoblast cells. Mol. Immunol. 2020, 120, 32–42.
- Kudo, Y.; Koh, I.; Sugimoto, J. Localization of Indoleamine 2,3-Dioxygenase-1 and Indoleamine 2,3-Dioxygenase-2 at the Human Maternal-Fetal Interface. Int. J. Tryptophan. Res. 2020, 13, 1178646920984163.
- Tirado-Gonzalez, I.; Freitag, N.; Barrientos, G.; Shaikly, V.; Nagaeva, O.; Strand, M.; Kjellberg, L.; Klapp, B.F.; Mincheva-Nilsson, L.; Cohen, M.; et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013, 19, 43–53.
- Rouas-Freiss, N.; Goncalves, R.M.; Menier, C.; Dausset, J.; Carosella, E.D. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA 1997, 94, 11520–11525.
- LeMaoult, J.; Zafaranloo, K.; Le Danff, C.; Carosella, E.D. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 2005, 19, 662–664.
- Corrao, S.; Campanella, C.; Anzalone, R.; Farina, F.; Zummo, G.; Conway de Macario, E.; Macario, A.J.; Cappello, F.; La Rocca, G. Human Hsp10 and Early Pregnancy Factor (EPF) and their relationship and involvement in cancer and immunity: Current knowledge and perspectives. Life Sci. 2010, 86, 145–152.
- Tsai, P.J.; Wang, H.S.; Lin, G.J.; Chou, S.C.; Chu, T.H.; Chuan, W.T.; Lu, Y.J. Undifferentiated Wharton’s Jelly Mesenchymal Stem Cell Transplantation Induces Insulin-Producing Cell Differentiation and Suppression of T-Cell-Mediated Autoimmunity in Nonobese Diabetic Mice. Cell Transpl. 2015, 24, 1555–1570.
- Hsieh, J.Y.; Wang, H.W.; Chang, S.J.; Liao, K.H.; Lee, I.H.; Lin, W.S.; Wu, C.H.; Lin, W.Y.; Cheng, S.M. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS ONE 2013, 8, e72604.
- Arutyunyan, I.; Fatkhudinov, T.; Kananykhina, E.; Usman, N.; Elchaninov, A.; Makarov, A.; Bolshakova, G.; Goldshtein, D.; Sukhikh, G. Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: In vitro study. Stem Cell Res. Ther. 2016, 7, 46.
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound. Care 2015, 4, 225–234.
- Edwards, S.S.; Zavala, G.; Prieto, C.P.; Elliott, M.; Martinez, S.; Egana, J.T.; Bono, M.R.; Palma, V. Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton’s jelly in dermal regeneration. Angiogenesis 2014, 17, 851–866.
- Lavrentieva, A.; Majore, I.; Kasper, C.; Hass, R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun. Signal. 2010, 8, 18.
- Russo, E.; Lee, J.Y.; Nguyen, H.; Corrao, S.; Anzalone, R.; La Rocca, G.; Borlongan, C.V. Energy Metabolism Analysis of Three Different Mesenchymal Stem Cell Populations of Umbilical Cord Under Normal and Pathologic Conditions. Stem Cell Rev. Rep. 2020, 16, 585–595.
- Russo, E.; Napoli, E.; Borlongan, C.V. Healthy mitochondria for stroke cells. Brain Circ. 2018, 4, 95–98.
- Lin, H.Y.; Liou, C.W.; Chen, S.D.; Hsu, T.Y.; Chuang, J.H.; Wang, P.W.; Huang, S.T. Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion 2015, 22, 31–44.
- Alberti, G.; Russo, E.; Corrao, S.; Anzalone, R.; Kruzliak, P.; Miceli, V.; Conaldi, P.G.; Di Gaudio, F.; La Rocca, G. Current Perspectives on Adult Mesenchymal Stromal Cell-Derived Extracellular Vesicles: Biological Features and Clinical Indications. Biomedicines 2022, 10, 2822.
- Alberti, G.; Sánchez-López, C.M.; Andres, A.; Santonocito, R.; Campanella, C.; Cappello, F.; Marcilla, A. Molecular Profile Study of Extracellular Vesicles for the Identification of Useful Small “Hit” in Cancer Diagnosis. Appl. Sci. 2021, 11, 10787.
- Stefano, F.; Mariantonia, L.; Giusi, A.; Claudia, C. Exosomal Hsp60: A tumor biomarker? In Heat Shock Protein 60 in Human Diseases and Disorders; Asea, A., Kaur, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 107–116.
- Miceli, V.; Bertani, A.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Carcione, C.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 510.
- Miceli, V.; Bertani, A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells 2022, 11, 826.
- Deuse, T.; Stubbendorff, M.; Tang-Quan, K.; Phillips, N.; Kay, M.A.; Eiermann, T.; Phan, T.T.; Volk, H.D.; Reichenspurner, H.; Robbins, R.C.; et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transpl. 2011, 20, 655–667.
- Donders, R.M.; Vanheusden, R.M.; Bogie, J.F.; Ravanidis, S.; Thewissen, K.; Stinissen, P.; Gyselaers, W. Human Wharton’s jelly-derived stem cells display immunomodulatory properties and transiently improve rat experimental autoimmune encephalomyelitis. Cell Transpl. 2015, 24, 2077–2098.
- Janssens, K.; Van den Haute, C.; Baekelandt, V.; Lucas, S.; van Horssen, J.; Somers, V.; Van Wijmeersch, B. Leukemia inhibitory factor tips the immune balance towards regulatory T cells in multiple sclerosis. Brain. Behav. Immun. 2015, 45, 180–188.
- Santos Nascimento, D.; Mosqueira, D.; Sousa, L.M.; Teixeira, M.; Filipe, M.; Resende, T.P.; Araujo, A.F.; Valente, M.; Almeida, J.; Martins, J.P.; et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res. Ther. 2014, 5, 5.
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R. Human Umbilical Cord Mesenchymal Stem Cells Reduce Fibrosis of Bleomycin-Induced Lung Injury. Am. J. Pathol. 2009, 175, 303–313.
- Lelek, J.; Zuba-Surma, E.K. Perspectives for Future Use of Extracellular Vesicles from Umbilical Cord- and Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells in Regenerative Therapies-Synthetic Review. Int. J. Mol Sci. 2020, 21, 799.
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116.
- Yao, W.; Shi, L.; Zhang, Y.; Dong, H.; Zhang, Y. Mesenchymal stem/stromal cell therapy for COVID-19 pneumonia: Potential mechanisms, current clinical evidence, and future perspectives. Stem Cell Res. Ther. 2022, 13, 124.
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776.
- Grégoire, C.; Ritacco, C.; Hannon, M.; Seidel, L.; Delens, L.; Belle, L.; Dubois, S.; Vériter, S.; Lechanteur, C.; Briquet, A.; et al. Comparison of Mesenchymal Stromal Cells from Different Origins for the Treatment of Graft-vs.-Host-Disease in a Humanized Mouse Model. Front. Immunol. 2019, 10, 619.
- Ji, L.; Zhan, Y.; Hua, F.; Li, F.; Zou, S.; Wang, W.; Song, D.; Min, Z.; Chen, H.; Cheng, Y. The ratio of Treg/Th17 cells correlates with the disease activity of primary immune thrombocytopenia. PLoS ONE 2012, 7, e50909.
- Lapietra, G.; Ferretti, A.; Baldacci, E.; Chistolini, A.; Santoro, C. Immune thrombocytopenia management during COVID-19 pandemic: An Italian monocentric experience. EJHaem 2022, 3, 453–456.
- Zhang, J.M.; Zhu, X.L.; Xue, J.; Liu, X.; Long Zheng, X.; Chang, Y.J.; Liu, K.Y.; Huang, X.J.; Zhang, X.H. Integrated mRNA and miRNA profiling revealed deregulation of cellular stress response in bone marrow mesenchymal stem cells derived from patients with immune thrombocytopenia. Funct. Integr. Genom. 2018, 18, 287–299.
- Shi, M.; Liu, Z.; Wang, Y.; Xu, R.; Sun, Y.; Zhang, M.; Yu, X.; Wang, H.; Meng, L.; Su, H.; et al. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl. Med. 2017, 6, 2053–2061.
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173.
- Ganguly, A.; Swaminathan, G.; Garcia-Marques, F.; Regmi, S.; Yarani, R.; Primavera, R.; Chetty, S.; Bermudez, A.; Pitteri, S.J.; Thakor, A.S. Integrated transcriptome-proteome analyses of human stem cells reveal source-dependent differences in their regenerative signature. Stem Cell Rep. 2023, 18, 190–204.
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828.
 Encyclopedia
Encyclopedia
