The presence of antibiotic-resistant bacteria in people's environment is a matter of growing concern. The issue of multidrug-resistant bacteria urged the need to elaborate the novel antipathogen agents. The polymers and copolymers modified with bioactive compounds have emerged as a group of highly effective antimicrobial agents that find usage in many fields. The natural polymers have a great advantage over the synthetic ones due to their non-toxicity, biocompatibility, non-immunogenicity, and high stability. On the other hand, they are less effective in biomedical applications in comparison to synthetic polymers. The modifications that provide the natural polymers with desirable industrial activity include chemical treatment processes such as hydroxylation, carboxylation and epoxidation, or in vitro enzyme treatment.
- polymeric materials
- antibacterial activity
- antibiotic-resistant bacteria
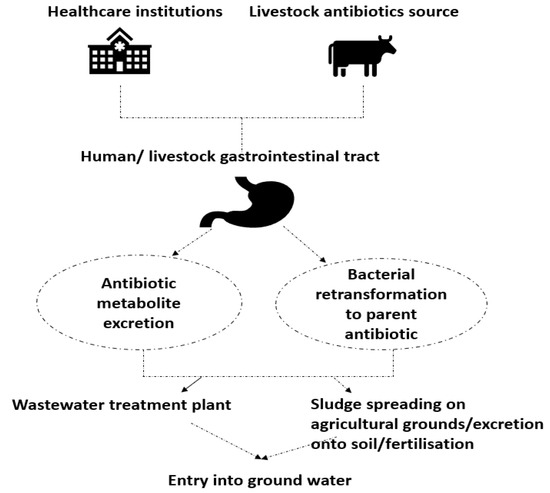
[1]1. Antibiotic Resistant Bacteria in Environment
2. Polymeric Materials with Antibacterial Properties—Mechanism of Action and Medical Applications
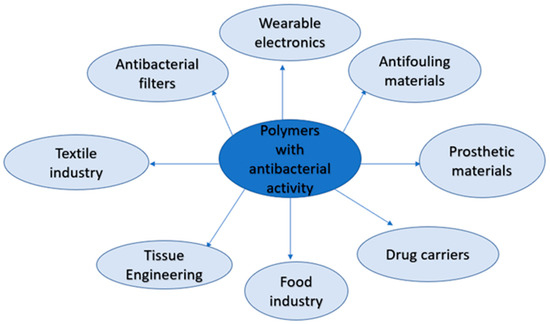
2.1. The Medical Application of Polymeric Materials with Antibacterial Activity
2.2. Polymer Materials as Antifouling Agents
References
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of antibiotics from wastewater by membrane technology: Limitations; successes; and future improvements. Sci. Total Environ. 2022, 838, 156010. Parcheta, Monika; Sobiesiak, Magdalena Preparation and Functionalization of Polymers with Antibacterial Properties—Review of the Recent Developments . Materials 2023, 16, 4411, https://doi.org/10.3390/ma16124411.
- Korzeniewska, E.; Harnisz, M. Sources; occurence and envrionmental risk assessment of antibiotics and antimibrobial-resistant bacteria in aquatic environments of Poland. Pol. River Basins Lakes—Part II 2020, 87, 179–193.
- Mpatani, M.M.; Aryee, A.A.; Kani, A.N.; Han, R.; Li, Z.; Dovi, E.; Qu, L. A review of treatement techniques applied for selective removal of emerging pollutant-trimethoprim from aqueous systems. J. Clean. Prod. 2021, 308, 127359–127379.
- Chen, J.F.; Yang, Y.Y.; Ke, Y.C.; Chen, X.L.; Jiang, X.S.; Chen, C.; Xie, S.G. Anaerobic sulfamethoxazole-degrading bacterial consortia in antibiotic-contaminated wetland sediments identified by DNS-stable isotope probing and metagenomics analysis. Environ. Microbiol. 2022, 24, 3751–3763.
- Omuferen, L.O.; Maseko, B.; Olowoyo, J.O. Occurrence of antibiotics in wastewater from hospial and convectional wastewater treatment plants and their impack on the effluent receiving rivers: Current knowledge between 2010 and 2019. Environ. Monit. Assess. 2022, 194, 306–331.
- Swaminathan, P.; Sen, S.; Mandira, M.A.; Prasad, A.A. Compounds and methods to resesitize Antibiotic-resistant bacteria Mediterr. J. Infect. Microbes Antimicrob. 2021, 10, 46–53.
- Russel, J.N.; Yost, C.K. Anternative; environmentally conscious approaches for removing antibiotics from wastewater treatment systems. Chemosphere 2021, 263, 1281177–1281187.
- Balloudj, J.; BhuvaneswariAssadi, I.; Nasrallah, N.; Jery, A.L.; Khezami, L.; Assadi, A.A. Simultaneus removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process. Eng. 2021, 42, 102089–1021100.
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Shan, X.; Nghiem, L.D.; Nguyen, L.N. Removal process of antibiotics during anaerobic treatment of swine wastewater. Bioresour. Technol. 2020, 300, 122707–122739.
- Monohan, C.; Nag, R.; Morris, D.; Cummins, E. Antibiotic residues in aquatic environment-current perspective and risk consideration. J. Environ. Sci. Health A 2021, 56, 733–751.
- Wu, C.; Ruan, L.; Yau, L. Tracking Epidemiological Characteristics and Risk Factors of Multi-Drug Resistant Bacteria in Intensive Care Units. Infect. Drug Resist. 2023, 16, 1499–1509.
- Fahad, K.H.; Al-Muhana, B.M.M.; Sadiq, J.N.N. Beneficial antimicrobial treatment options forpan-drug-resistant bacterial species. J. Agric. Biol. Sci. 2023, 14, 45–51.
- Geetha, M.; Rajendran, I.; Jayakumar, T.; Dhayalan, S. Prevalence and Detection of Multidrug Resistance Bacterial Strains Isolated from the Different Inanimate Surfaces of the Hospital Environment. Uttar Pradesh J. Zool 2023, 44, 95–104.
- De Kraker, M.A.D.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, 1–6.
- Chen, J.; Wang, F.; Liu, Q.; Du, J. Antibacterial polymeric nanostructures for biomedical applications. Chem. Commun. 2014, 93, 14482–14493.
- Rofeal, M.; Abdelmalek, F.; Steinbüchel, A. Naturally—Sourced Antibacterial Polymeric Nonomaterials with Special Reference to Modified Polymer Variants. Int. J. Mol. Sci. 2022, 23, 4101.
- Arora, A.; Mishra, A. Antibacterial Polymers—A Mini Review. Mater. Today Proc. 2018, 5, 17156–17161.
- Yu, K.; Mei, Y.; Hadjesfandiari, N.; Kizhakkedathu, J.N. Engineering biometrials surfaces to modulate the host response. Colloids Surf. B 2014, 124, 69–79.
- Deka, S.R.; Sharma, A.K.; Kumar, P. Cationic polymers and their self assembly for antibacterial applications. Curr. Top Med. Chem. 2015, 15, 1179–1195.
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655.
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985.
- Zhang, H.; Chiao, M. Anti-fouling Coatings of Poly(dimethylosiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155.
- Jeepery, I.F.; Sudesh, K.; Abe, H. Miscibility and enzymatic degradability of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)-based polyester blends by PHB depolymerase and lipase. Polym. Degrad. Stab. 2021, 192, 109692–109704.
- El-malek, F.A.; Steinbüchel, A. Post-synthetic enzymatic and chemical modifications for novel sustainable polyesters. Front. Bioeng. Biotechnol. 2022, 9, 1460.
- Zhao, H.; Lin, Z.Y.; Yildirimer, L.; Dhinakar, A.; Zhao, X.; Wu, J. Polymer-based nanoparticles for protein delivery: Design; strategies and applications. J. Mater. Chem. 2016, 4, 4060–4071.
- Liu, Y.; van der Mei, H.; Zhao, B.; Zhai, Y.; Cheng, T.; Li, Y.; Zhang, Z.; Busscher, H.; Ren, Y.; Shi, L. Eradication of Multidrug—Resistant Staphylococcal Infections by Light—Activable Micelar Nanocarriers in a Murine Model. Adv. Funct. Mater. 2017, 27, 1701974.
- Imperiale, J.; Acosta, G.; Sosnik Alejandro, B. Polymer-based carriers for ophthalmic drug delivery. J. Control. Release 2018, 285, 106–141.
- Pignatello, R.; Bucolo, C.; Puglisi, G. Ocular tollerability of Eudragit@ and RL100@ nanosuspensions as carriers for ophthalamic controlled drug delivery. J. Pharm. Sci. 2002, 91, 2636–2641.
- Adibkia, K.; Shadbad, M.R.S.; Nokhodchi, A.; Javadzedeh, A.; Barzegar-Jalali, M.; Barar, J.; Omidi, Y. Piroxicam nanoparticles for ocular delivery: Physicochemical characterization and implemantation in endotoxin—Induced uveitis. J. Drug Target. 2007, 15, 407–416.
- Rathod, L.V.; Kapadia, R.; Sawant, K.K. A novel nanoparticles impregnated ocular insert for enhanced bioavailability to posterior segment of eye: In vitro; in vivo and stability studies. Mater. Sci. Eng. C 2017, 71, 529–540.
- Calzoni, E.; Caseretii, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater 2018, 10, 4.
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172.
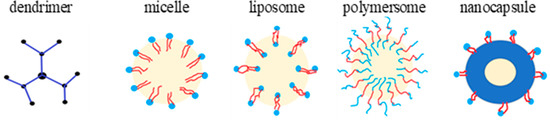
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles; nanospheres; nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269.
- Musyanovytch, A.; Landefster, K. Polymer Micro- and Nanocapsules as biological carriers with multifunctional properties. Macomol. Biosci. 2014, 14, 458–477.
- Hallaj-Nezhadi, S.; Hassan, M. Nanoliposome—Based antibacterial drug delivery. Drug Deliv. 2015, 22, 581–589.
- Buhleier, E.; Wehner, W.; Vögtle, F. Cascade and Nonskid—Chain—Like Syntheses of Molecular Cavity Topologies. Synthesis 1978, 2, 155–158.
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 15, 2106–2129.
- Chen, C.Z.S.; Cooper, S.L. Recent Advances in Antimicrobial Dendrimers. Adv. Mater. 2000, 12, 843–846.
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1073.
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting Dendrimer Multivalency to Combat Emerging and Re-Emerging Infectious Diseases. Mol. Pharm. 2012, 9, 342–354.
- Thiele, A.R.; Abate, H.C.; Shum, S.; Bachtler, S.; Feorster, D.A. Weitz Fabrication of Polymersomes using Double-Emulsion Templates in Glass-Coated Stamped Microfluidic Devices. Small 2010, 6, 1723–1727.
- Mueller, W.; Koyonov, K.; Fischer, K.; Hartmann, S.; Pierrat, S.; Baschee, T.; Maskos, M. Hydrophobic Shell Loading of PB-b-PEO Vesicles. Macromolecules 2008, 42, 357–361.
- Wang, T.; Qin, J.; Cheng, J.; Li, C.; Du, J. Inteligent design of polymersomes for antibacterial and anticancer applications. Wiley Interdiscip. Rev. Nanomed. Nanotechnol. 2022, 14, e1822.
- Kaur, M.; Cohen, Y.; Poverenov, E.; Eltzov, E. Binding selectivity of N-alkylaminated modified chitosan nanoparticles produce a synergistic antibacterial effect against gram-negative strains. React. Funct. Polym. 2023, 186, 105567.
- Ziesmer, J.; Larsson, J.V.; Sotiriou, G.A. Hybrid microneedle arrays for antibiotic and near-IR photothermal synergistic antimicrobial effect against Methicillin-Resistant Staphylococcus aureus. J. Chem. Eng. 2023, 462, 142127.
- Juang, H.; Su, Y.; Wang, C.; Lei, B.; Song, X.; Wang, W.; Wu, P.; Liu, X.; Dong, X.; Zhong, L. Injectable Tissue-Adhesive Hydrogel for Photothermal/ Chemodynamic Synergistic Antibacterial and Wound Healing Promotion. Appl. Mater Interfaces 2023, 15, 2714–2724.
- Li, W.; Cai, J.; Zhou, W.; Zhao, X.; Wang, M.; Zhou, X.; Ren, L. Poly(aspartic acid)-based self-healing hydrogel with precise antibacterial ability for rapid infected-wound repairing. Colloids Surf. B 2023, 221, 112982.
- Feldman, D. Polymer nanocomposytes in medicine. J. Macromol. Sci A 2016, 53, 55–62.
- Escobar, A.; Muzzio, N.; Moya, S. Antibacterial Lyer—By—Lyer Coating for Medical Implants. Pharmaceutics 2021, 13, 16.
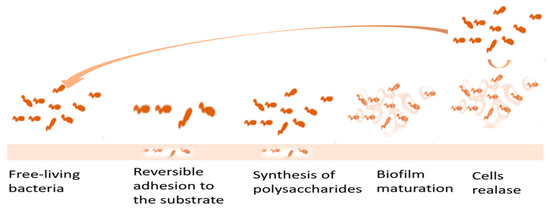
- Quinn, J.; McFadden, R.; Chan, C.-W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. IScience 2020, 23, 101745.
- Goudarzi, M.; Navidinia, M.; Khadembashi, N.; Rasouli, R. Biofilm Matrix Formation in Human: Clinical Significance, Diagnostic Techniques, and Therapeutic Drugs. Arch. Clin. Infect. Dis. 2021, 16, 107919–107929.
- Francolini, I.; Piozzi, A. Polymeric Systems as Antimicrobial or Antifouling Agents. Int. J. Mol. Sci. 2019, 20, 4866.
- Phuong, T.; Nguyen, T. Polymer and Surface Modifications for Antibacterial Purposes. Ph.D. Thesis, Université Paris Saclay, Paris, France, 2019.
- Shmidt Bernhard, V.K.J. Hydrophilic polymers. Polymers 2019, 11, 693.
- Zhang, C.; Chen, J.; Liu, M.; Liu, Y.; Liu, Z.; Chu, H.; Cheng, Q.; Wang, J. Regulation mechanism of biomolecule interaction behaviors on the superlubricity of hydroplilic coatings. Friction 2022, 10, 94–109.
- Brunzel, M.; Majdanski, T.C.; Vitz, J.; Nischang, I.; Schubert, U.S. Fast Screening of Diol Impurities in Methoxy Poly(Ethylene Glycol)s (mPEG)s by Liquid Chromatography on Monolithic Silica Rods. Polymers 2018, 10, 1395.
- Wu, C.; Zhou, Y.; Wang, H.; Hu, J. P4VP Modified Zwitterionic Polymer for the Preparation of Antifouling Functionalized Materials. Nanomaterials 2019, 9, 706.
- Zheng, J.; Li, L.; Tsao, H.K.; Sheng, Y.J.; Chen, S.; Jiang, S. Strong Repulsive Forces between Protein and Oligo (Ethylene Glycol) Self-Assembled Monolayers: A Molecular Simulation Study. Biophys. J. 2005, 89, 158–166.
- Chen, C.H.; Chen, S.H.; Mao, S.H.; Tsai, M.J.; Chou, P.Y.; Liao, C.H.; Chen, J.P. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 2017, 173, 721–731.
- Sun, Q.; Su, Y.; Ma, X.; Wang, Y.; Jiang, Z. Improved antifouling property of zwitterionic ultrafiltration membrane composed of acrylonitrile and sulfobetaine copolymer. J. Membr. Sci. 2006, 285, 299–305.
- Shafi, H.Z.; Khan, Z.; Yang, R.; Gleason, K.K. Surface modification of reverse osmosis membranes with zwitterionic coating for improved resistance to fouling. Desalination 2015, 362, 93–103.
- Leigh, B.L.; Cheng, E.; Xu, L.; Derk, A.; Hansen, M.R.; Guymon, C.A. Antifouling Photograftable Zwitterionic Coatings on PDMS Substrates. Langmuir 2019, 35, 1100–1110.
- Racovita, S.; Trofin, M.A.; Loghin, D.F.; Zaharia, M.M.; Bucatariu, F.; Mihai, M.; Vasiliu, S. Polybetaines in Biomedical Applications. Mol. Sci. 2021, 22, 9321.
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfich, M.H. Antibacterial Fluorinated Silica Colloid Superhydrophobic Surfaces. Langmuir 2011, 27, 9597–9601.
- Manivasagam, V.K.; Perumal, G.; Arora, H.S.; Popat, K.C. Enhanced antibacterial properties on superhydrophobic micronano structured titanium surface. J. Biomed. Mater. 2022, 110, 1314–1328.
- Qianqian, Q.; Xiaogang, B.; Jine, S.; Shu, C.; Yao, X.; Yuan, Y.; Jia, L.; Guohua, X. Fabrication of superhydrophobic composite coating of hydroxyapatite/stearic acid on magnesium alloy and its corrosion resistance; antibacterial adhesion. J. Mater. Sci. 2020, 56, 5233–5249.
- Oki, Y.; Kirita, K.; Ohta, S.; Ohba, S.; Horiguchi, I.; Sakai, Y.; Ito, T. Switching of Cell Proliferation/Differentiation in Thiol−Maleimide Clickable Microcapsules Triggered by in Situ Conjugation of Biomimetic Peptides. Biomacromolecules 2019, 20, 2350–2359.
- Hasoyama, K.; Lazurko, C.; Muñoz, M.; Mc Tiernan, C.D.; Alarcon, E.I. Peptide Based Functional for Soft-Tissue Repair. Front. Bioeng. Biotechnol. 2019, 7, 205.
- Lin, M.; Ding, J.; Sun, J. Photo-triggered polymeric antimicrobial peptide mimics with excellent selectivity and antifouling and antimicrobial hydrogels. Giant 2022, 10, 100097.
- Liu, F.; Qu, W.; Zhang, J.; Liu, J.; Zhu, Q.; Yue, T.; Xu, X.; Ma, N.; Ma, J.; Sun, Y.; et al. Cationic Alternating Polypeptide Fixed on Polyurethane at Multiple Sites for Excellent Antibacterial and Antifouling Properties. Langmuir 2021, 37, 10657–10667.
- Ghosh, S.; Mukherjee, R.; Basak, D.; Haldar, J. One-Step Curable; Covalently Immobilized Coating for Clinically Relevant Surfaces That Can Kill Bacteria; Fungi; and Influenza Virus. Appl. Mater. Interfaces 2020, 12, 27853–27865.
- Bai, Z.; Liu, Q.; Zhang, H.; Liu, J.; Yu, J.; Wang, J. A novel 3D reticular anti-fouling bio-adsorbent for uranium extraction from seawater: Polyethylenimine and guanidyl functionalized hemp fibers. J. Chem. Eng. 2019, 382, 122555.
- Xu, X.; Wang, Q.; Chang, Y.; Peng, H.; Whittaker, A.K.; Fu, C. Antifouling and Antibacterial Surfaces Grafted with Sulfur-Containing Copolymers. Appl. Mater. Interfaces 2022, 14, 41400–41411.
