Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Müslüm Kaplan.
The COVID-19 pandemic has hugely affected the textile and apparel industry. Besides the negative impact due to supply chain disruptions, drop in demand, liquidity problems, and overstocking, this pandemic was found to be a window of opportunity since it accelerated the ongoing digitalization trends and the use of functional materials in the textile industry.
The COVID-19 pandemic has hugely affected the textile and apparel industry. Besides the negative impact due to supply chain disruptions, drop in demand, liquidity problems, and overstocking, this pandemic was found to be a window of opportunity since it accelerated the ongoing digitalization trends and the use of functional materials in the textile industry.
- smart textiles
- medical textiles
- COVID-19
1. Introduction
The global COVID-19 pandemic spread worldwide quickly, affecting many aspects of daily life, public health, the global economy, and social stability. As a solution in the first phase, many countries took action by using personal protective equipment (PPE), especially masks, which were found to be effective in preventing the spread of the virus [1]. Most countries mandated the wearing of masks in public spaces. These efforts included severe measures, such as banning citizens from leaving their homes in many countries and effectively suspending all social and economic activities.
Just over three years have passed since the coronavirus outbreak, as on March 11, 2020, the World Health Organization (WHO) declared the global spread of SARS-CoV-2 a pandemic. More than 550 million people were infected, and more than 6,300,000 died as a consequence of the infection [2].
The epidemiological situation improved and relaxations of COVID-19 rules were implemented worldwide [3]. Still, the emergence of the pandemic irreversibly changed the world, as it is the first pandemic in the digital age. Yet, even the most developed parts of the world were found to be unprepared with no action strategy in place.
Textiles, whether regular, advanced, or smart, played an essential role in tackling this pandemic, mainly as part of technical and medical textiles (e.g., personal protective equipment (PPE), sensors, and telemedicine), which mirrors the importance of textiles in tackling past pandemics [4].
As a major contributor to the European economy, with a current annual turnover of EUR 162 billion and employing over 1.9 million people [5], the textile industry has been hit hard by the COVID-19 crisis due to supply chain disruptions, a drop in demand, liquidity problems, and overstocking. In Europe, production fell by 16.8% in the period between January and April 2020 in comparison with 2019. Nevertheless, the pandemic also created an urgent new demand for technical textiles in the field of personal protective equipment (PPE) and opened a window of opportunity for the sector to rationalize production, revise supply chains, and push for more digitalization. Personal protective equipment with functional textile materials and devices for combating coronavirus infections and smart e-textile wearables for remote diagnosis [5] and monitoring saw rapid development. These technological innovations played an important part in solving the current crisis and might be used as a reference for managing future severe situations.
2. Medical Textiles in Pandemic Control and Prevention
From the cradle to the grave, medical textiles are an indispensable part of our life. Surgical gowns, caps, isolation gowns, masks, and coveralls are key items to achieve success in medical operations, personal protection, or daily hygiene. Several prevention techniques were devised after it was found that the SARS-CoV-2 coronavirus is transmitted in respiratory particles from an infected person’s mouth or nose [6]. Physical distancing strategies, i.e., maintaining a safe distance between individuals, is the most effective prevention technique against disease spread via the respiratory route [7,8][7][8]. When a safe distance is not practicable, personal protective equipment (PPE) is the standard form of self-protection. Masks and respirators are conclusively essential items of PPE. They cover the nose and mouth of the user, and thus, act as a physical barrier to the droplets/particles (carrying virus) during inhalation and exhalation [9,10][9][10].2.1. Face Masks—Types and Efficiency
Medical face masks and respirators are of various material types, costs, and protection levels [11]. Medical face masks and respirators come under the category of medical devices; therefore, regulatory authorities around the world already defined their specifications and requirements and also advised which mask should be used in which specific condition. For instance, type IIR masks are surgical face masks, as they have splash-resistant properties and are tested per the conditions defined in EN 14683: 2019. Type I and type II medical face masks are advised for patients and other persons to reduce the spreading of infections. A type II medical face mask is most promising, as it has a bacterial filtration efficiency of ≥98% with good breathability. The material used for the construction and design of medical face masks is advised not to disintegrate, split, or tear during intended use. Medical face masks generally have three layers (spunbond–melt-blown–spunbond) and are made of synthetic fibers, such as polypropylene. Natural fibers are generally not recommended, as they are hydrophilic and may provide sites for bacteria and viruses to grow. Although medical face masks have good filtration, ranging from 95 to 98%, they have a loose fit on the face and air leaks from the sides. To counter this issue, respirators are advised, as they have a close and tight fit to the wearer’s face and decrease the wearer’s exposure to pollutants in the air, such as particles, gases, and vapors. Their specifications and requirements are also defined, as per EN 149:2001, and they may have inhalation or exhalation valves. FFP2 and FFP3 are the most promising respirators, as they filter 95–99% of the particles ranging from 0.1 to 5 μm. Cloth masks are reusable and made of knitted or woven textiles. Despite providing less protection than surgical or non-surgical face masks, they are common in use mainly by the public, and homemade versions are also available [13][12]. Perhaps the essential basic parameters determining face masks’ effectiveness and wear comfort are defined as air permeability and filtration efficiency [14][13]. Depending on the reference standard, masks usually fall into distinct areas within these categories in accordance with the employed filter layer [15][14] (Figure 1A). While the general principles of air filtration are known to be complex [16][15], a high filtration efficiency commonly means a low air permeability and vice versa [17][16]. Despite the accessibility of advanced materials [18][17], confusion on actual testing parameters persisted [13[12][18][19],19,20], hence diminishing the confidence of manufacturers in such innovations. Instead, several manufacturers and studies revisited the efficacy of improvised filter materials [19,21][18][20]; however, the overall advice remained against material repurposing due to, e.g., biocompatibility and leakage concerns. At the same time, further research showed that non-woven materials commonly provided better air permeability and filtration efficiency combined than woven materials alone [22][21], as well as strong resistance to mechanical or sweat exposure [23][22], which explains the wide application of melt-blown non-woven fabrics in air filtration [24][23]. A quality factor and other models based on structural parameters of woven textiles were developed, which indicated that nanofibers in a less densely packed formation achieve an optimal compromise of both values, as opposed to the macro-fibrous structure of typical woven textiles [25,26][24][25] (Figure 1B). Certainly accelerated by the pandemic, electrospinning and similar methods were identified as promising techniques to produce nanofibrous filter materials, which not only satisfy the filtration efficiency and permeability criteria [20,27,28,29][19][26][27][28] but also offer other benefits, such as transparency [30][29], reuse after washing or disinfection [31[30][31],32], and antimicrobial or antiviral functionalization [33,34,35][32][33][34]. Eventually, during 2021 and convinced by the research and increasing availability [20][19], a growing number of manufacturers successfully integrated not just melt-blown but also highly efficient nanofibrous non-woven fabrics as filter layers in their community mask compositions (Figure 1C,D) [20,36][19][35].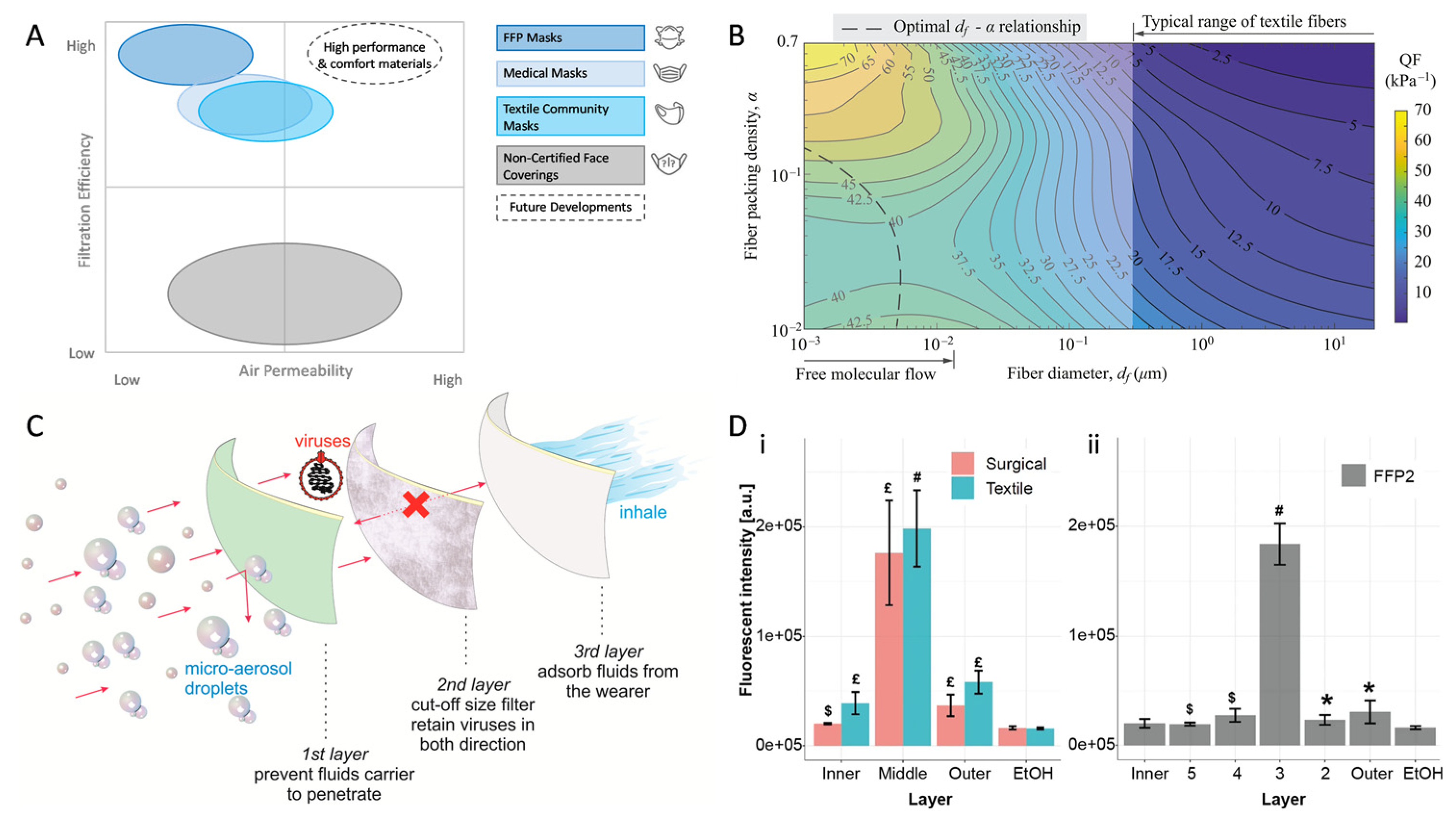
Figure 1. Filtration efficiency and air permeability as driving factors for community mask development. Categorization of face mask types (A), air permeability limited by fiber architecture (B), the established layer structure of community masks with nanofibrous non-woven fabrics as a filter (C), and viral distribution per layer after simulated use in common face mask types (D). (A,B,D) Reproduced under CC BY 4.0 (C) Reproduced with permission from the American Physical Society.
2.2. Improvement of Medical Textiles toward Enhanced Anti-Viral Properties
Apart from the general improvement of filtration efficiency and breathability in masks, additional measures targeting SARS-CoV-2 specifically remained imperative. The virus spread was reduced in most countries by enforcing regulations such as social distancing, limiting social activities and events, and compulsory use of protective equipment such as facemasks, which were summarized as non-pharmaceutical measures [37][36]. However, antiviral functionalization of materials prone to contact with viral particles promised to further reduce the number of infections more reliably [38][37], with a minimal impact on people’s personal lives and without hampering economic growth.2.2.1. Metal-Derived and Carbon-Based Nanomaterials for (Nano)Textile Modification in the Fight against SARS-CoV-2 Infection
It is well known that different types of metal-derived nanoparticles exhibit antiviral properties against various groups of viruses; recently, special attention was paid to checking the effect of various nanomaterials on SARS-CoV-2 [39][38]. In parallel to this activity, the study and application of metal-derived nanomaterials for (nano)textile modification to develop advanced protective clothing in the fight against SARS-CoV-2 infection are highly important [40][39]. More detailed information about these topics can be found in recent review papers [41,42,43,44,45][40][41][42][43][44]. Selected metal-derived nanoparticles, due to their high reactive surface-area-to-volume ratio and unique chemical properties of metal-based nanoparticles, enable their potent inactivation of viruses, thus forming efficient antiseptic coatings to prevent pathogen transmission and infection. Nanoparticles exert their virucidal action through various mechanisms, including inhibition of virus–cell receptor binding, reactive oxygen species oxidation, and destructive displacement bonding with key viral structures [41][40]. Silver-based nanoparticles (AgNPs) have an extensive history of medical uses due to their unique microbicidal properties. They can be prepared using a wide variety of strategies [46][45]. AgNPs are known for their high antimicrobial activity, biocompatibility, and low toxicity in eukaryotic cells [41,47][40][46]. In one of the in vitro studies that investigated the use of AgNPs against SARS-CoV-2, a large number of AgNPs of different sizes and concentrations were tested. It was observed that particles with a diameter of approximately 10 nm effectively inhibited extracellular SARS-CoV-2 at concentrations ranging between 1 and 10 ppm, while the cytotoxic effect was observed at concentrations of 20 ppm and above. AgNPs potently inhibited the viral entry step by disrupting the viral integrity [48][47]. In addition, mouthwash and nose rinse containing AgNPs were tested on hospital health workers to prevent SARS-CoV-2 infection. The incidence of SARS-CoV-2 infection was significantly lower in the group testing AgNPs (1.8%) in comparison with the group using regular mouth and nasal rinse (28.2%) [49][48]. The antiviral potential of AgNPs is further enhanced by their bactericidal effect against important multi-drug-resistant bacteria, such as methicillin-resistant Streptococcus aureus (MRSA), Pseudomonas aeruginosa, ampicillin-resistant Escherichia coli O157:H7, and erythromycin-resistant Streptococcus pyogenes [41][40]. Blosi et al. [50][49] developed electrospun nanowebs and found a filtration efficiency of 97.7% for nanosized aerosol particles. They found excellent antimicrobial activity as a result of the presence of AgNPs in the electrospun nanowebs. Selvam et al. [51][50] developed PA/AgNP-based electrospun nanowebs and found a 99% bacterial filtration efficiency, plus they found these nanowebs to be effective against Gram-positive and Gram-negative bacteria. Ahmed et al. [40][39] suggested that AgNPs can inhibit glycine and alanine of S-proteins along with other proteins from SARS-CoV-2. When incorporated into PPEs, these can effectively protect the attack by the user from SARS-CoV-2. HeiQ®® Viroblock [52][51] is a silver-based antimicrobial finish that is claimed to be 99.9% effective in 30 min against SARS-CoV-2. Copper, its alloys, and specific insoluble copper compounds are promising materials for use in fighting against SARS-CoV-2 due to their excellent antiviral and antimicrobial properties [53,54][52][53]. Copper(I) iodide has a white color and exhibits high antiviral properties, which is why this material was integrated into masks, filters, and other surfaces [55][54]. A molecular docking study was also carried out to understand the interaction of Cu(I) with the SARS-CoV-2 main protease [56][55]. Furthermore, copper nanoparticles, as well as Cu(I) and Cu(II) oxides, were shown to be effective antiviral agents, again after deposition on non-woven polypropylene fabrics that were used as the outer and inner fabric layers in commercial respirators [57][56]. Kumar et al. [58][57] developed a reusable and self-sterilizable surgical face mask by spraying a hybrid of shellac (a natural hydrophobic polymer)/copper nanoparticles on a non-woven surgical facemask. They found that it also increased the hydrophobicity of the layer of the face mask, thus stopping the flow of droplets that contain SARS-CoV-2. Jung et al. [59][58] also developed an antiviral KN94 mask by depositing a 20 nm copper layer using a vacuum deposition method. They found that copper-deposited polypropylene KN94 facemasks have a particle filtration efficiency of 95.1% for NaCl and 91.6% efficiency for paraffin oil. They also showed a 75% reduction in SARS-CoV-2 when the virus came in contact with the copper-coated face mask. Kumar et al. [60][59] developed a medical face mask by incorporating copper@ZIF-8 core–shell nanowires on a non-woven polypropylene surface. They found a 55% inhibition of virus replication after 48 h by 1 μg of Cu@ZIF-8 NW per well. Despite the fact that copper is an essential trace element for regular metabolism in the human body [61][60], and the declaration that small amounts of copper-derived particles used for textile coating will not cause health problems during wearing, the potentially toxic and sub-toxic effects of copper nanoparticles, possible allergic reactions, and occurrence of other long-term issues cannot be overlooked [62,63][61][62]. Zinc oxide nanoparticles (ZnO NPs) exhibit antibacterial properties against an extensive range of microorganisms, including Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Pseudomonas vulgaris, and Campylobacter jejuni. In addition, nanoparticles of zinc oxide are considered safe for human contact [41][40]. Unfortunately, there are not so many studies on the antiviral properties of ZnO NPs. In a recent study, the efficacy of ZnO nanoparticles against SARS-CoV-2 was evaluated. ZnO NPs were produced using an ecofriendly and scalable electrochemical procedure and were fully characterized. Their antiviral activity was tested in vitro against SARS-CoV-2, showing a decrease in viral load between 70% and 90%, as a function of the material’s composition ZnO NPs would be best suited as coatings for commonly touched surfaces [64][63]. Researchers developed eco-friendly nanowebs of PVA/Aloe vera/ZnO that can be used in the inner protective layer of face masks. These composite nanomembranes have excellent antimicrobial properties, killing almost 100% of Gram-positive bacteria and almost 99.2% of Gram-negative bacteria with 4% ZnO nanoparticles in the nanowebs [65][64]. A similar approach was also taken to obtain an antimicrobial effect with Aloe vera/PVA nanofibers [66][65]. Multifunctional electrospun poly(methyl methacrylate) (PMMA) nanofibers decorated with ZnO nanorods and Ag nanoparticles (PMMA/ZnO Ag NF) were also developed, and these nanowebs showed antimicrobial properties against Gram-positive and Gram-negative bacteria, antiviral properties against SARS-CoV-2, and self-cleaning properties against pollutants [67][66]. ZnO-loaded PVDF nanofibers have excellent antiviral properties, and 5% ZnO/PVDF nanofibers inhibited virus growth and inhibited viral entry due to the nanoweb structure. These nanowebs have potential applications in filter-embedded half-face respirators [68][67]. As it is difficult to separate the very thin layer of nanofibers from the electrospinning machine, researchers used a non-woven sheet on the collector so that the nanofibers are collected directly on the surface of the non-woven sheet. Pardo-Figuerez et al. [69][68] developed and upscaled polyacrylonitrile (PAN)/ZnO nanofibers onto non-woven spunbonded polypropylene (SPP) with different weight bases of 0.4 g/m2 and 0.8 g/m2. They suggested that a symmetric structure based on SPP/PAN/PAN/SPP was the optimal one, as it reduced SPP consumption while maintaining an FFP2-type filtration efficiency and reducing breathing resistance, especially at high air flow rates, such as those mimicking FFP2 exhalation conditions. They also found excellent antimicrobial properties because of the presence of ZnO nanoparticles in their sheets. Titanium dioxide has the required properties, including low toxicity to humans and excellent UV-activated viral inhibition [70][69]. The antiviral mechanism involves the photocatalytic production of reactive oxygen species (ROS), which are highly unstable and rapidly react with biomolecules in reactions that exchange electrons. This process results in alterations in the structure of biopolymers and lipids, making ROS cytotoxic to a wide variety of organisms [41][40]. TiO2 and polyvinylpyrrolidone nanofibers were also shown to have excellent filtration efficiency. The antimicrobial behavior of TiO2 made this nanoweb an excellent material for filtration media, especially in face masks and respirators. PAN/TiO2/Ag nanofibers were also developed with a filtration efficiency of particulate matter 2.5. These nanofibers have excellent antimicrobial and UV-resistant properties, making them suitable for filtration media in facemasks and respirators [71][70]. Iron oxide nanoparticles (IONPs) found interesting medical applications (cancer treatment, magnetic drug targeting, contrast agents for MRI) due to their high biocompatibility and magnetic properties; these nanoparticles were approved by the FDA and various European Union agencies as nanoparticle-based medicines [72][71]. IONPs exhibit antiviral activity against various viruses due to a broad range of antiviral mechanisms, including ROS generation, lipid peroxidation, and binding to viral surface proteins to impair attachment to host cells. Coating of IONPs with appropriate polymers, including polyvinylpyrrolidone or polyethylene glycol, can enhance their antiviral activity, stability, and safety [41][40]. A recent theoretical molecular docking study showed the specific interactions of IONPs with the viral glycoproteins of SARS-CoV-2; both maghemite and magnetite interacted efficiently [73][72]. The advantages of metal-based nanoparticles can be summarized as follows: these nanoparticles exhibit a versatile range of antiviral mechanisms that target important viral components, including structural proteins and the lipid envelope. It is expected that the probability of the development of resistance against metal-based NP is low. Different NPs can attack viruses through diverse mechanisms, including oxidative stress, protein disruption, or lipid envelope and capsid damage. In addition, the presence of the antibacterial activity of metal nanoparticles is of high importance. The properties of metal nanoparticles can be finely tuned (e.g., the size and surface modification), even on a larger scale, using green synthesis. The NPs are usually stable [41][40]; however, their safety in human beings after inhalation is still contradictorily discussed. Carbon-based nanomaterials, such as graphene, showed utility in numerous applications besides metallic and oxide-based nanoparticles. Graphene and graphene oxide are capable of inhibiting the infective capacity of SARS-CoV-2. When they are incorporated into polyurethane or cotton, the resulting fabric maintains these properties, completely reducing the infectivity of the virus [74][73]. Complex mechanisms respond to graphene’s capacity for inactive viruses that are dependent on intrinsic material properties and environmental conditions. The physical interaction of graphene or graphene oxide is able to promote structural changes in the virus capsid and envelope [75][74]. Other forms of carbon, such as carbon nanotubes or carbon dots, also showed their activity. Carbon nanotubes were incorporated into a polyester substrate, giving rise to a filter with efficiency similar to HEPA that can be disinfected by applying resistive heating. In addition to offering other technical opportunities, carbon dots in poly(vinylidene fluoride) membranes confer solar-induced self-sterilization via sunlight absorption and concomitant heat dissipation [76][75]. However, as for NPs, the safety aspects, e.g., of graphene-related materials is also a matter of current research [77][76]. The nanomaterials contained in the fabrics possess the risk of leaching during their use and the subsequent management as waste. Studies suggest that the exposure to silver and graphene nanoparticles derived from the continued use of a functionalized mask is much lower than the maximum recommended exposure levels. Estevan et al. [78][77] indicated systemic (internal) exposure derived from silver nanoparticle facemasks would be between 7.0 × 10–5 and 2.8 × 10–4 mg/kg bw/day. In addition, conservative systemic no-effect levels between 0.075 and 0.01 mg/kg bw/day were estimated. In the case of graphene, research conducted in the context of the Graphene Flagship and by other investigators in the past several years showed that the hazard potential for different members of the graphene-based materials family may vary considerably and a systematic collection of data on the safety or biocompatibility is yet to be done [78][77]. However, in the case of its management as waste, this is an aspect to take into account since some of the nanomaterials can show cellular toxicity and damage to the environment [79][78]. To avoid leaching, the nature of the fabric, the surface functionalization of the nanoparticles, and the method of impregnation are important. Among them, the growth of the particles inside the fibers and electrospinning are techniques with the capacity to prevent leaching as the particles are occluded in the polymeric matrix. Gonzalez et al. [80][79] presented masks prepared from fabrics that included zinc oxide nanoparticles. This work presented a new approach to the synthesis of nanocomposites. It is based on a process known as crescoating (coating via the growth of [-cresco]), which relies on an in situ growth process from the thermal treatment of a dissolved ionic precursor solution. The solution is impregnated into a support material followed by heating so as to begin the nucleation and growth of nanoparticles within the support. The polymers tested were polypropylene and cotton, and relatively heterogeneous distributions of ZnO particles between 5 and 500 nm in diameter were obtained. The authors claimed that the textile-doped nanoparticles thus obtained are non-irritating and hypoallergenic and are capable of achieving a reduction of 3 logs (greater than 99.9%) in the presence of coronavirus, and they maintain their activity even after 100 wash cycles. Nanomaterials (metallic, carbon-based) can have antiviral properties per se, blocking viral replication and diffusion, or their antiviral properties can be tailored by playing with surface chemistry. However, a huge effort should be undertaken to translate the research into clinics. Several challenges still need to be overcome before their safe use [81][80]. At the current stage, some nanomaterials were approved only for surface disinfection. For instance, CuNPs have been used in filters for the preparation of highly efficient broad-spectrum antiviral masks [82][81].2.2.2. Development of New Methods to Assess the Efficiency of Antiviral Treatments
A limiting factor in the development of novel antiviral technologies is the characterization of their efficacy at inactivating viruses. It is a slow, expensive, but necessary step in the journey of new technology from the laboratory to commercialization. As of July 2022, several established methods exist to characterize the antiviral properties of materials. For instance, the International Standards Organization (ISO) norms 18,184 and 21,702 for the characterization of antiviral properties of porous and non-porous materials prescribe the use of the traditional plaque assay or the TCID50 methods. Another common antiviral characterization method is the immunofluorescence assay, which relies on fluorescently labeled antibodies [83,84,85,86][82][83][84][85]. A detailed explanation of the established procedures is not within the scope of the present review. However, these require the use of cell cultures; live viruses; trained personnel; incubation times ranging from 2 h to 48 h; and, depending on the targeted virus, laboratory biosafety levels 1, 2, or 3. Since the beginning of the pandemic, several studies investigated novel antiviral materials, such as nanoparticles, fucoidans, other naturally extracted compounds, facemasks [15][14], liquids, and coatings. Most authors characterized their solutions using the plaque assay and/or TCID50 methods [87,88,89,90,91,92][86][87][88][89][90][91]. Less common are cell viability assays (such as MTT and MTS) [93][92], which estimate the viral load reduction indirectly by observing the cell viability, and polymerase chain reaction (PCR) [84[83][89],90], which accurately measures the viral load but presents sampling issues, which makes it impractical in a variety of scenarios. The multitude of studies on antiviral materials and the mentioned drawbacks of existing antiviral characterization procedures highlight the need for novel, fast, and inexpensive antiviral characterization methods. A few months before the COVID-19 pandemic was declared, Wu Liu et al. reported a microfluidic device that allows for single-cell analysis of viral inhibitors and characterized their mechanisms of action [94][93]. This is a significant improvement in terms of scientific research possibilities compared with traditional plaque assay experiments, which measure a reduction in infectivity but do not offer further understanding regarding the antiviral mechanisms in play. Nevertheless, this method involves live viruses and cell cultures; therefore, the challenges of availability and ease of use remain unaddressed. Angel Serrano-Aroca suggested using the bacteriophage phi 6 as a surrogate for enveloped viruses (such as SARS-CoV-2) [95][94]. This would allow researchers without access to biosafety level 3 facilities to perform antiviral characterization and significantly facilitate antiviral developments. However, it does not represent a change in methodology and would still involve the expensive and time-consuming procedure of a plaque assay. Finally, Furer et al. recently proposed an inactivated virus system (InViS), which can detect viral envelope disintegration via simple fluorescent measurement [36][35]. The method is based on an inactivated influenza virus labeled with a fluorescent dye, which is released upon the disintegration of the viral envelope. It does not require specific safety protocols, as the virus is noninfectious; the measurement is a fast (less than 15 min) and inexpensive way to characterize antiviral properties, making it ideal for the rapid testing of all layers of a mask separately (Figure 1D). The InViS system is sensible to viral disintegration, which is one of several ways a virus may be inactivated, and can, therefore, only be used to characterize families of materials that take advantage of this specific mechanism. This method’s advantages could complement the established ISO procedures to facilitate the development of novel antiviral technologies by greatly reducing the feedback loop between laboratory solutions and their antiviral characterization. The development of new methods to assess the efficiency of antiviral treatments should bring us one step closer to determining the filtration properties of medical and technical filtration systems against real viruses in a more relevant exposure scenario. It will facilitate the fast, cheap, and safe pre-screening of a large number of materials and surfaces for potential antiviral properties and will be a valuable tool to support the development of novel antiviral materials, coatings, and facemasks.2.3. PPE Disposal—An Environmental Issue
The COVID-19 pandemic has affected not only human health but also the environment due to the large volume of discarded personal protective equipment (PPE). While personal protective equipment, especially face masks, was recognized as an effective measure to prevent the spread of the virus that causes COVID-19, the remarkable increase in the global usage of face masks and their inappropriate disposal has led to a serious environmental challenge [96,97,98,99,100,101][95][96][97][98][99][100]. Many countries and the World Health Organization (WHO) issued regulations and guidelines on the waste management of PPE and plastics [102][101] and accordingly handle the PPE discarded by hospitals; however, the main problem is due to the wide use of face masks by ordinary citizens and their careless disposal. The improper disposal of face masks not only spreads the disease but also negatively affects the environment. Various environmental risks that originate from the face masks’ inappropriate disposal were recognized and discussed in recent papers, which also proposed possible mitigation strategies. Carelessly discarded face masks can be fatal to the ecosystem; the dyes, inks, and additives leaching from the PPE pose a risk to human health and the environment; and their degradation contributes to microplastic pollution. Face masks can also become a substrate for microorganisms. Additionally, face masks themselves can also act as pollutant carriers and provide a stable environment for pathogenic bacteria and viruses to propagate further since they can adsorb heavy metals and organics [96,97,98,101,103][95][96][97][100][102]. All these risks require urgent zero-waste approaches, which may generally be based upon reuse, appropriate disposal, recycling, incineration, and design for recycling, as presented in Figure 2.
Figure 2. Zero-waste approaches to address COVID-19 waste.
References
- Loeb, M.; Bartholomew, A.; Hashmi, M.; Tarhuni, W.; Hassany, M.; Youngster, I.; Somayaji, R.; Larios, O.; Kim, J.; Missaghi, B.; et al. Medical Masks versus N95 Respirators for Preventing COVID-19 among Health Care Workers. Ann. Intern. Med. 2022, 175, 1629–1638.
- COVID Live—Coronavirus Statistics—Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 21 July 2022).
- Timeline COVID-19 Coronavirus—Consilium. Available online: https://www.consilium.europa.eu/en/policies/coronavirus/timeline/ (accessed on 21 July 2022).
- Ivanoska-Dacikj, A.; Stachewicz, U. Smart Textiles and Wearable Technologies—Opportunities Offered in the Fight against Pandemics in Relation to Current COVID-19 State. Rev. Adv. Mater. Sci. 2020, 59, 487–505.
- European Commission, Executive Agency for Small and Medium-sized Enterprises; Izsak, K.; Shauchuk, P. Advanced Technologies for Industry: Sectoral Watch: Technological Trends in the Textiles Industry; Publications Office: Brussels, Belgium, 2021; Available online: https://data.europa.eu/doi/10.2826/69367 (accessed on 21 July 2022).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207.
- Aquino, E.M.L.; Silveira, I.H.; Pescarini, J.M.; Aquino, R.; de Souza-Filho, J.A.; dos Santos Rocha, A.; Ferreira, A.; Victor, A.; Teixeira, C.; Machado, D.B.; et al. Medidas de Distanciamento Social No Controle Da Pandemia de COVID-19: Potenciais Impactos e Desafios No Brasil. Ciênc. Saúde Coletiva 2020, 25, 2423–2446.
- Kissler, S.; Tedijanto, C.; Lipsitch, M.; Grad, Y.H. Social distancing strategies for curbing the COVID-19 epidemic. medRxiv, 2020; preprint.
- Kunstler, B.; Newton, S.; Hill, H.; Ferguson, J.; Hore, P.; Mitchell, B.G.; Dempsey, K.; Stewardson, A.J.; Friedman, D.; Cole, K.; et al. P2/N95 Respirators & Surgical Masks to Prevent SARS-CoV-2 Infection: Effectiveness & Adverse Effects. Infect. Dis. Health 2022, 27, 81–95.
- World Health Organization. Rational Use of Personal Protective Equipment for COVID-19 and Considerations during Severe Shortages: Interim Guidance, 23 December 2020. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/338033 (accessed on 21 July 2022).
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 286735.
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347.
- Ogbuoji, E.A.; Zaky, A.M.; Escobar, I.C. Advanced Research and Development of Face Masks and Respirators Pre and Post the Coronavirus Disease 2019 (COVID-19) Pandemic: A Critical Review. Polymers 2021, 13, 1998.
- Batt, T.; Herwig, G.; Annaheim, S.; Clement, P.; Furrer, L.; Hirsch, C.; Varanges, V.; Caglar, B.; Michaud, V.; Wang, J.; et al. Community Masks—From an Emergency Solution to an Innovation Booster for the Textile Industry. CHIMIA 2022, 76, 249.
- Shou, D.; Fan, J.; Ye, L.; Zhang, H.; Qian, X.; Zhang, Z. Inverse Problem of Air Filtration of Nanoparticles: Optimal Quality Factors of Fibrous Filters. J. Nanomater. 2015, 2015, 168392.
- Pandey, L.K.; Singh, V.V.; Sharma, P.K.; Meher, D.; Biswas, U.; Sathe, M.; Ganesan, K.; Thakare, V.B.; Agarwal, K. Screening of Core Filter Layer for the Development of Respiratory Mask to Combat COVID-19. Sci. Rep. 2021, 11, 10187.
- Liu, H.; Cao, C.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. Progress on Particulate Matter Filtration Technology: Basic Concepts, Advanced Materials, and Performances. Nanoscale 2020, 12, 437–453.
- Kwong, L.H.; Wilson, R.; Kumar, S.; Crider, Y.S.; Reyes Sanchez, Y.; Rempel, D.; Pillarisetti, A. Review of the Breathability and Filtration Efficiency of Common Household Materials for Face Masks. ACS Nano 2021, 15, 5904–5924.
- Wibisono, Y.; Fadila, C.R.; Saiful, S.; Bilad, M.R. Facile Approaches of Polymeric Face Masks Reuse and Reinforcements for Micro-Aerosol Droplets and Viruses Filtration: A Review. Polymers 2020, 12, 2516.
- Bagheri, H.; Aghakhani, A. Polyaniline-Nylon-6 Electrospun Nanofibers for Headspace Adsorptive Microextraction. Anal. Chim. Acta 2012, 713, 63–69.
- Bourrous, S.; Barrault, M.; Mocho, V.; Poirier, S.; Bardin-Monnier, N.; Charvet, A.; Thomas, D.; Bescond, A.; Fouqueau, A.; Mace, T.; et al. A Performance Evaluation and Inter-Laboratory Comparison of Community Face Coverings Media in the Context of COVID-19 Pandemic. Aerosol Air Qual. Res. 2021, 21, 200615.
- Varanges, V.; Caglar, B.; Lebaupin, Y.; Batt, T.; He, W.; Wang, J.; Rossi, R.M.; Richner, G.; Delaloye, J.-R.; Michaud, V. On the Durability of Surgical Masks after Simulated Handling and Wear. Sci. Rep. 2022, 12, 4938.
- Shim, E.; Jang, J.-P.; Moon, J.-J.; Kim, Y. Improvement of Polytetrafluoroethylene Membrane High-Efficiency Particulate Air Filter Performance with Melt-Blown Media. Polymers 2021, 13, 4067.
- Mao, X.; Hosoi, A.E. Estimating the Filtration Efficacy of Cloth Masks. Phys. Rev. Fluids 2021, 6, 114201.
- Drouillard, K.G.; Tomkins, A.; Lackie, S.; Laengert, S.; Baker, A.; Clase, C.M.; Lannoy, C.F.D.; Cavallo-Medved, D.; Porter, L.A.; Rudman, R.S. Fitted Filtration Efficiency and Breathability of 2-Ply Cotton Masks: Identification of Cotton Consumer Categories Acceptable for Home-Made Cloth Mask Construction. PLoS ONE 2022, 17, e0264090.
- Kang, L.; Liu, Y.; Wang, L.; Gao, X. Preparation of Electrospun Nanofiber Membrane for Air Filtration and Process Optimization Based on BP Neural Network. Mater. Res. Express 2021, 8, 115010.
- Yu, J.; Tian, X.; Xin, B.; Xu, J. Preparation and Characterization of PMIA Nanofiber Filter Membrane for Air Filter. Fibers Polym. 2021, 22, 2413–2423.
- Yang, Y.; He, R.; Cheng, Y.; Wang, N. Multilayer-Structured Fibrous Membrane with Directional Moisture Transportability and Thermal Radiation for High-Performance Air Filtration. e-Polymers 2020, 20, 282–291.
- Avossa, J.; Batt, T.; Pelet, T.; Sidjanski, S.P.; Schönenberger, K.; Rossi, R.M. Polyamide Nanofiber-Based Air Filters for Transparent Face Masks. ACS Appl. Nano Mater. 2021, 4, 12401–12406.
- Lu, H.; Yao, D.; Yip, J.; Kan, C.-W.; Guo, H. Addressing COVID-19 Spread: Development of Reliable Testing System for Mask Reuse. Aerosol Air Qual. Res. 2020, 20, 2309–2317.
- Ullah, S.; Ullah, A.; Lee, J.; Jeong, Y.; Hashmi, M.; Zhu, C.; Joo, K.I.; Cha, H.J.; Kim, I.S. Reusability Comparison of Melt-Blown vs Nanofiber Face Mask Filters for Use in the Coronavirus Pandemic. ACS Appl. Nano Mater. 2020, 3, 7231–7241.
- Robert, B.; Nallathambi, G. A Concise Review on Electrospun Nanofibres/Nanonets for Filtration of Gaseous and Solid Constituents (PM2.5) from Polluted Air. Colloid Interface Sci. Commun. 2020, 37, 100275.
- Lyu, C.; Zhao, P.; Xie, J.; Dong, S.; Liu, J.; Rao, C.; Fu, J. Electrospinning of Nanofibrous Membrane and Its Applications in Air Filtration: A Review. Nanomaterials 2021, 11, 1501.
- Han, S.; Kim, J.; Ko, S.H. Advances in Air Filtration Technologies: Structure-Based and Interaction-Based Approaches. Mater. Today Adv. 2021, 9, 100134.
- Furer, L.A.; Clement, P.; Herwig, G.; Rossi, R.M.; Bhoelan, F.; Amacker, M.; Stegmann, T.; Buerki-Thurnherr, T.; Wick, P. A Novel Inactivated Virus System (InViS) for a Fast and Inexpensive Assessment of Viral Disintegration. Sci. Rep. 2022, 12, 11583.
- Kupferschmidt, K.; Cohen, J. Can China’s COVID-19 Strategy Work Elsewhere? Science 2020, 367, 1061–1062.
- Machida, M.; Nakamura, I.; Saito, R.; Nakaya, T.; Hanibuchi, T.; Takamiya, T.; Odagiri, Y.; Fukushima, N.; Kikuchi, H.; Amagasa, S.; et al. Incorrect Use of Face Masks during the Current COVID-19 Pandemic among the General Public in Japan. Int. J. Environ. Res. Public Health 2020, 17, 6484.
- Maduray, K.; Parboosing, R. Metal Nanoparticles: A Promising Treatment for Viral and Arboviral Infections. Biol. Trace Elem. Res. 2021, 199, 3159–3176.
- Ahmed, T.; Ogulata, R.T.; Sezgin Bozok, S. Silver Nanoparticles against SARS-CoV-2 and Its Potential Application in Medical Protective Clothing—A Review. J. Text. Inst. 2022, 113, 2825–2838.
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral Nanoparticles for Sanitizing Surfaces: A Roadmap to Self-Sterilizing against COVID-19. Nano Today 2021, 40, 101267.
- Pemmada, R.; Zhu, X.; Dash, M.; Zhou, Y.; Ramakrishna, S.; Peng, X.; Thomas, V.; Jain, S.; Nanda, H.S. Science-Based Strategies of Antiviral Coatings with Viricidal Properties for the COVID-19 Like Pandemics. Materials 2020, 13, 4041.
- Rai, M.; Bonde, S.; Yadav, A.; Bhowmik, A.; Rathod, S.; Ingle, P.; Gade, A. Nanotechnology as a Shield against COVID-19: Current Advancement and Limitations. Viruses 2021, 13, 1224.
- Ramaiah, G.B.; Tegegne, A.; Melese, B. Developments in Nano-Materials and Analysing Its Role in Fighting COVID-19. Mater. Today Proc. 2021, 47, 4357–4363.
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martín-Sampedro, R.; del Real, G.; Aranda, P. Nanotechnology Responses to COVID-19. Adv. Healthc. Mater. 2020, 9, 2000979.
- Lekha, D.C.; Shanmugam, R.; Madhuri, K.; Dwarampudi, L.P.; Bhaskaran, M.; Kongara, D.; Tesfaye, J.L.; Nagaprasad, N.; Bhargavi, V.L.N.; Krishnaraj, R. Review on Silver Nanoparticle Synthesis Method, Antibacterial Activity, Drug Delivery Vehicles, and Toxicity Pathways: Recent Advances and Future Aspects. J. Nanomater. 2021, 2021, e4401829.
- Pilaquinga, F.; Morey, J.; Torres, M.; Seqqat, R.; de las Nieves Piña, M. Silver Nanoparticles as a Potential Treatment against SARS-CoV-2: A Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1707.
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent Antiviral Effect of Silver Nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200.
- Almanza-Reyes, H.; Moreno, S.; Plascencia-López, I.; Alvarado-Vera, M.; Patrón-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A.; et al. Evaluation of Silver Nanoparticles for the Prevention of SARS-CoV-2 Infection in Health Workers: In Vitro and in vivo. PLoS ONE 2021, 16, e0256401.
- Blosi, M.; Costa, A.L.; Ortelli, S.; Belosi, F.; Ravegnani, F.; Varesano, A.; Tonetti, C.; Zanoni, I.; Vineis, C. Polyvinyl Alcohol/Silver Electrospun Nanofibers: Biocidal Filter Media Capturing Virus-Size Particles. J. Appl. Polym. Sci. 2021, 138, 51380.
- Selvam, A.K.; Nallathambi, G. Polyacrylonitrile/Silver Nanoparticle Electrospun Nanocomposite Matrix for Bacterial Filtration. Fibers Polym. 2015, 16, 1327–1335.
- HeiQ Viroblock—HeiQ Materials, AG. Available online: https://www.heiq.com/products/functional-textile-technologies/heiq-viroblock/?gclid=Cj0KCQjw06OTBhC_ARIsAAU1yOW6Cvnc9VjbTVG1Ml_0NezyOZD7Qth6JKvINb6Zmy0kU6RE2u1ah6kaAspdEALw_wcB (accessed on 5 July 2022).
- Román, L.E.; Gomez, E.D.; Solís, J.L.; Gómez, M.M. Antibacterial Cotton Fabric Functionalized with Copper Oxide Nanoparticles. Molecules 2020, 25, 5802.
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.R.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral Properties of Copper and Its Alloys to Inactivate COVID-19 Virus: A Review. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2021, 34, 1217–1235.
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel Antiviral Characteristics of Nanosized Copper(I) Iodide Particles Showing Inactivation Activity against 2009 Pandemic H1N1 Influenza Virus. Appl. Environ. Microbiol. 2012, 78, 951–955.
- Archana, K.M.; Rajagopal, R.; Krishnaswamy, V.G.; Aishwarya, S. Application of Green Synthesised Copper Iodide Particles on Cotton Fabric-Protective Face Mask Material against COVID-19 Pandemic. J. Mater. Res. Technol. 2021, 15, 2102–2116.
- Delumeau, L.-V.; Asgarimoghaddam, H.; Alkie, T.; Jones, A.J.B.; Lum, S.; Mistry, K.; Aucoin, M.G.; DeWitte-Orr, S.; Musselman, K.P. Effectiveness of Antiviral Metal and Metal Oxide Thin-Film Coatings against Human Coronavirus 229E. APL Mater. 2021, 9, 111114.
- Kumar, S.; Karmacharya, M.; Joshi, S.R.; Gulenko, O.; Park, J.; Kim, G.-H.; Cho, Y.-K. Photoactive Antiviral Face Mask with Self-Sterilization and Reusability. Nano Lett. 2021, 21, 337–343.
- Jung, S.; Yang, J.-Y.; Byeon, E.-Y.; Kim, D.-G.; Lee, D.-G.; Ryoo, S.; Lee, S.; Shin, C.-W.; Jang, H.W.; Kim, H.J.; et al. Copper-Coated Polypropylene Filter Face Mask with SARS-CoV-2 Antiviral Ability. Polymers 2021, 13, 1367.
- Kumar, A.; Sharma, A.; Chen, Y.; Jones, M.M.; Vanyo, S.T.; Li, C.; Visser, M.B.; Mahajan, S.D.; Sharma, R.K.; Swihart, M.T. Core-Shell Nanowires for Reusable Antimicrobial Face Masks. Adv. Funct. Mater. 2021, 31, 2008054.
- Turnlund, J.R. Human Whole-Body Copper Metabolism. Am. J. Clin. Nutr. 1998, 67, 960S–964S.
- Naz, S.; Gul, A.; Zia, M. Toxicity of Copper Oxide Nanoparticles: A Review Study. IET Nanobiotechnol. 2020, 14, 1–13.
- Hejazy, M.; Koohi, M.K.; Bassiri Mohamad Pour, A.; Najafi, D. Toxicity of Manufactured Copper Nanoparticles—A Review. Nanomed. Res. J. 2018, 3, 1–9.
- Sportelli, M.C.; Izzi, M.; Loconsole, D.; Sallustio, A.; Picca, R.A.; Felici, R.; Chironna, M.; Cioffi, N. On the Efficacy of ZnO Nanostructures against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 3040.
- Munir, M.U.; Mikucioniene, D.; Khanzada, H.; Khan, M.Q. Development of Eco-Friendly Nanomembranes of Aloe Vera/PVA/ZnO for Potential Applications in Medical Devices. Polymers 2022, 14, 1029.
- Khanzada, H.; Salam, A.; Qadir, M.B.; Phan, D.-N.; Hassan, T.; Munir, M.U.; Pasha, K.; Hassan, N.; Khan, M.Q.; Kim, I.S. Fabrication of Promising Antimicrobial Aloe Vera/PVA Electrospun Nanofibers for Protective Clothing. Materials 2020, 13, 3884.
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690.
- Nageh, H.; Emam, M.H.; Ali, F.; Abdel Fattah, N.F.; Taha, M.; Amin, R.; Kamoun, E.A.; Loutfy, S.A.; Kasry, A. Zinc Oxide Nanoparticle-Loaded Electrospun Polyvinylidene Fluoride Nanofibers as a Potential Face Protector against Respiratory Viral Infections. ACS Omega 2022, 7, 14887–14896.
- Pardo-Figuerez, M.; Chiva-Flor, A.; Figueroa-Lopez, K.; Prieto, C.; Lagaron, J.M. Antimicrobial Nanofiber Based Filters for High Filtration Efficiency Respirators. Nanomaterials 2021, 11, 900.
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Elem. Res. 2016, 172, 1–36.
- Hartati, S.; Zulfi, A.; Maulida, P.Y.D.; Yudhowijoyo, A.; Dioktyanto, M.; Saputro, K.E.; Noviyanto, A.; Rochman, N.T. Synthesis of Electrospun PAN/TiO2/Ag Nanofibers Membrane As Potential Air Filtration Media with Photocatalytic Activity. ACS Omega 2022, 7, 10516–10525.
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387.
- Abo-Zeid, Y.; Ismail, N.S.M.; McLean, G.R.; Hamdy, N.M. A Molecular Docking Study Repurposes FDA Approved Iron Oxide Nanoparticles to Treat and Control COVID-19 Infection. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 153, 105465.
- De Maio, F.; Palmieri, V.; Babini, G.; Augello, A.; Palucci, I.; Perini, G.; Salustri, A.; Spilman, P.; De Spirito, M.; Sanguinetti, M.; et al. Graphene Nanoplatelet and Graphene Oxide Functionalization of Face Mask Materials Inhibits Infectivity of Trapped SARS-CoV-2. iScience 2021, 24, 102788.
- Toledo, G.G.; Toledo, V.H.; Lanfredi, A.J.C.; Escote, M.; Champi, A.; Silva, M. Promising Nanostructured Materials against Enveloped Virus. An. Acad. Bras. Ciências 2020, 92, e20200718.
- Dahanayake, M.H.; Athukorala, S.S.; Jayasundera, A.C.A. Recent Breakthroughs in Nanostructured Antiviral Coating and Filtration Materials: A Brief Review. RSC Adv. 2022, 12, 16369–16385.
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620.
- Estevan, C.; Vilanova, E.; Sogorb, M.A. Case Study: Risk Associated to Wearing Silver or Graphene Nanoparticle-Coated Facemasks for Protection against COVID-19. Arch. Toxicol. 2022, 96, 105–119.
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver Nanoparticles in the Environment: Sources, Detection and Ecotoxicology. Sci. Total Environ. 2017, 575, 231–246.
- Gonzalez, A.; Aboubakr, H.A.; Brockgreitens, J.; Hao, W.; Wang, Y.; Goyal, S.M.; Abbas, A. Durable Nanocomposite Face Masks with High Particulate Filtration and Rapid Inactivation of Coronaviruses. Sci. Rep. 2021, 11, 24318.
- Reina, G.; Peng, S.; Jacquemin, L.; Andrade, A.F.; Bianco, A. Hard Nanomaterials in Time of Viral Pandemics. ACS Nano 2020, 14, 9364–9388.
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295.
- Elizondo-Gonzalez, R.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. In Vitro Characterization of the Antiviral Activity of Fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012, 9, 307.
- de Godoi, A.M.; Faccin-Galhardi, L.C.; Rechenchoski, D.Z.; Arruda, T.B.M.G.; Cunha, A.P.; de Almeida, R.R.; Rodrigues, F.E.A.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. Structural Characterization and Antiviral Activity of Pectin Isolated from Inga Spp. Int. J. Biol. Macromol. 2019, 139, 925–931.
- Ng, C.S.; Kasumba, D.M.; Fujita, T.; Luo, H. Spatio-Temporal Characterization of the Antiviral Activity of the XRN1-DCP1/2 Aggregation against Cytoplasmic RNA Viruses to Prevent Cell Death. Cell Death Differ. 2020, 27, 2363–2382.
- Park, J.-G.; Ávila-Pérez, G.; Nogales, A.; Blanco-Lobo, P.; de la Torre, J.C.; Martínez-Sobrido, L. Identification and Characterization of Novel Compounds with Broad-Spectrum Antiviral Activity against Influenza A and B Viruses. J. Virol. 2020, 94, e02149-19.
- Dong, C.-X.; Hayashi, K.; Lee, J.-B.; Hayashi, T. Characterization of Structures and Antiviral Effects of Polysaccharides from Portulaca oleracea L. Chem. Pharm. Bull. 2010, 58, 507–510.
- Sun, Q.-L.; Li, Y.; Ni, L.-Q.; Li, Y.-X.; Cui, Y.-S.; Jiang, S.-L.; Xie, E.-Y.; Du, J.; Deng, F.; Dong, C.-X. Structural Characterization and Antiviral Activity of Two Fucoidans from the Brown Algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487.
- Ibrahim, N.; Moussa, A.Y. A Comparative Volatilomic Characterization of Florence Fennel from Different Locations: Antiviral Prospects. Food Funct. 2021, 12, 1498–1515.
- Chen, R.; Zhang, W.; Gong, M.; Wang, F.; Wu, H.; Liu, W.; Gao, Y.; Liu, B.; Chen, S.; Lu, W.; et al. Characterization of an Antiviral Component in Human Seminal Plasma. Front. Immunol. 2021, 12, 580454.
- Demchenko, V.; Rybalchenko, N.; Zahorodnia, S.; Naumenko, K.; Riabov, S.; Kobylinskyi, S.; Vashchuk, A.; Mamunya, Y.; Iurzhenko, M.; Demchenko, O.; et al. Preparation, Characterization, and Antimicrobial and Antiviral Properties of Silver-Containing Nanocomposites Based on Polylactic Acid–Chitosan. ACS Appl. Bio Mater. 2022, 5, 2576–2585.
- Uraki, R.; Kiso, M.; Iida, S.; Imai, M.; Takashita, E.; Kuroda, M.; Halfmann, P.J.; Loeber, S.; Maemura, T.; Yamayoshi, S.; et al. Characterization and Antiviral Susceptibility of SARS-CoV-2 Omicron BA.2. Nature 2022, 607, 119–127.
- Hwang, H.-J.; Han, J.-W.; Jeon, H.; Cho, K.; Kim, J.; Lee, D.-S.; Han, J.W. Characterization of a Novel Mannose-Binding Lectin with Antiviral Activities from Red Alga, Grateloupia chiangii. Biomolecules 2020, 10, 333.
- Liu, W.; Caglar, M.U.; Mao, Z.; Woodman, A.; Arnold, J.J.; Wilke, C.O.; Cameron, C.E. More than Efficacy Revealed by Single-Cell Analysis of Antiviral Therapeutics. Sci. Adv. 2019, 5, eaax4761.
- Serrano-Aroca, Á. Antiviral Characterization of Advanced Materials: Use of Bacteriophage Phi 6 as Surrogate of Enveloped Viruses Such as SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 5335.
- Li, B.; Huang, Y.; Guo, D.; Liu, Y.; Liu, Z.; Han, J.C.; Zhao, J.; Zhu, X.; Huang, Y.; Wang, Z.; et al. Environmental Risks of Disposable Face Masks during the Pandemic of COVID-19: Challenges and Management. Sci. Total Environ. 2022, 825, 153880.
- Ray, S.S.; Lee, H.K.; Huyen, D.T.T.; Chen, S.S.; Kwon, Y.N. Microplastics Waste in Environment: A Perspective on Recycling Issues from PPE Kits and Face Masks during the COVID-19 Pandemic. Environ. Technol. Innov. 2022, 26, 102290.
- Abedin, M.J.; Khandaker, M.U.; Uddin, M.R.; Karim, M.R.; Ahamad, M.S.U.; Islam, M.A.; Arif, A.M.; Sulieman, A.; Idris, A.M. PPE Pollution in the Terrestrial and Aquatic Environment of the Chittagong City Area Associated with the COVID-19 Pandemic and Concomitant Health Implications. Environ. Sci. Pollut. Res. 2022, 29, 27521–27533.
- Chen, Z.; Zhang, W.; Yang, H.; Min, K.; Jiang, J.; Lu, D.; Huang, X.; Qu, G.; Liu, Q.; Jiang, G. A Pandemic-Induced Environmental Dilemma of Disposable Masks: Solutions from the Perspective of the Life Cycle. Environ. Sci. Process. Impacts 2022, 24, 649–674.
- Hatami, T.; Rakib, M.R.J.; Madadi, R.; De-la-Torre, G.E.; Idris, A.M. Personal Protective Equipment (PPE) Pollution in the Caspian Sea, the Largest Enclosed Inland Water Body in the World. Sci. Total Environ. 2022, 824, 153771.
- Pizarro-Ortega, C.I.; Dioses-Salinas, D.C.; Fernández Severini, M.D.; Forero López, A.D.; Rimondino, G.N.; Benson, N.U.; Dobaradaran, S.; De-la-Torre, G.E. Degradation of Plastics Associated with the COVID-19 Pandemic. Mar. Pollut. Bull. 2022, 176, 113474.
- Asim, N.; Badiei, M.; Sopian, K. Review of the Valorization Options for the Proper Disposal of Face Masks during the COVID-19 Pandemic. Environ. Technol. Innov. 2021, 23, 101797.
- Anastopoulos, I.; Pashalidis, I. Single-Use Surgical Face Masks, as a Potential Source of Microplastics: Do They Act as Pollutant Carriers? J. Mol. Liq. 2021, 326, 115247.
- Wang, D.; Sun, B.C.; Wang, J.X.; Zhou, Y.Y.; Chen, Z.W.; Fang, Y.; Yue, W.H.; Liu, S.M.; Liu, K.Y.; Zeng, X.F.; et al. Can Masks Be Reused after Hot Water Decontamination during the COVID-19 Pandemic? Engineering 2020, 6, 1115–1121.
- Zhong, H.; Zhu, Z.; Lin, J.; Cheung, C.F.; Lu, V.L.; Yan, F.; Chan, C.-Y.; Li, G. Reusable and Recyclable Graphene Masks with Outstanding Superhydrophobic and Photothermal Performances. ACS Nano 2020, 14, 6221.
More
