Among therapeutically valuable opioids, morphinans are of the utmost clinical importance as analgesic drugs because of their agonistic actions to the mu-opioid receptor. They include powerful pain relieving agents, such as naturally occurring alkaloids (e.g., morphine and codeine), semisynthetic analogues (e.g., hydrocodone, hydromorphone, oxycodone, oxymorphone and buprenorphine), and synthetic derivatives (e.g., levorphanol).
- pain
- analgesia
- opioid receptors
- peripheral analgesia
- peripherally restricted opioids
- morphinans
- morphine
1. Introduction

2. Peripheralization Strategies Applied to Morphinans
Different chemical strategies have been developed to limit the ability of opioids to cross the BBB, including (a) chemical modifications to the morphinan skeleton to increase hydrophilicity of known and new opioids, and (b) nanocarrier-based approaches to selectively deliver opioids, such as morphine to the peripheral tissue. The following sections discuss significant representatives, including design strategies, synthetical procedures, pharmacology and structure–activity relationships (SAR).2.1. Quaternization of the Morphinan Nitrogen
The first effort to minimize the CNS effects of opioids while retaining their actions in peripheral tissue was the quaternization of the nitrogen in the clinically used morphine, oxymorphone, nalorphine, naloxone and naltrexone (Figure 2) [27][28]. Peripheral selectivity of the quaternary derivative of morphine, N-methylmorphine, was described over 50 years ago [29]. The systemic intravenous (i.v.) administration of N-methylmorphine caused the inhibition of gastrointestinal transit because of its action on the opioid receptors in the gut. In the hot-plate test, centrally mediated antinociception was produced by 15 mg/kg morphine in mice after intraperitoneal (i.p.) administration but not by N-methylmorphine at the same dose [29]. Furthermore, N-methylmorphine proved to be ineffective in the hot-plate test even in a dose 100 mg/kg [30]. In an acetic-acid-induced writhing assay, N-methylmorphine produced antinociceptive effects in mice after i.p. administration in a dose of 45 mg/kg, being 30-fold less potent than morphine [30]. N-methylmorphine was also shown to selectively inhibited phase II in the formalin test following systemic i.p. administration [31]. The antinociceptive effect of N-methylmorphine after central intracerebroventricular (i.c.v.) administration was antagonized by systemically applied naloxone but not by peripheral antagonist N-methylnaloxone, showing the peripheral site of action of N-methylmorphine [31].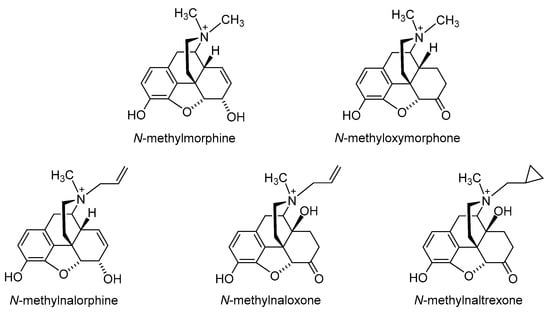
2.2. Introduction of Hydrophilic Substituents at Position 6
Polar or ionizable substitutions are able to increase polarity and inhibit the crossing of the BBB. Therefore, opioids with hydrophilic groups attached to the C-6 position of the morphinan skeleton were designed. The first examples of morphinans having ionizable residues at position 6 were reported more than 30 years ago. They were synthesized from β-oxymorphamine [33], β-naltrexamine [33] and β-funaltrexamine [34]. Such compounds with zwitterionic moieties showed significantly reduced access to the CNS without substantially decreased opioid receptor in vitro and in vivo activity [33][34]. Noteworthy are the 6-amide derivatives of β-oxymorphamine (a–e, Figure 3) reported as the first peripherally selective opioid agonists and effective antinociceptives [33]. All compounds have C-6 moieties that are ionized at the pH of the gut or at physiologic pH, accounting for a more restricted capability to enter the CNS than the unionized molecules. Compounds a, c and d were synthesized from β-oxymorphamine with the appropriate anhydride [33]. The fumaramic acid b was prepared by coupling the half-ester of fumaric acid with β-oxymorphamine and then subjecting the fumaramate esters to hydrolysis. The aspartyl derivative e was obtained through coupling BocAsp γ-benzyl ester with β-oxymorphamine, followed by deprotection with acid to remove the Boc group and hydrogenolysis of the benzyl function [33]. As regards biological activities, the ß-oxymorphamine derivatives a–e were all full agonists in the guinea pig ileum (GPI) bioassay with potencies that were 1.5- to 6-fold higher than the potency of morphine (Table 1) [33]. In a mouse model of acute thermal nociception, the tail-flick assay, all compounds possessed potent antinociceptive activity when administered by the i.c.v route (Table 1). They also were active in inducing antinociception when given systemically by i.v. administration to mice. When compared on a body weight basis, the i.v. ED50 doses were about 1000-fold higher than the i.c.v. ED50 values. Derivatives a and c were also active when given orally (p.o.) (Table 1) [33]. The attachment of polar groups, particularly zwitterionic moieties, at the C-6 position of the morphinan structure is effective in excluding such ligands from the CNS, thereby affording peripheral selectivity.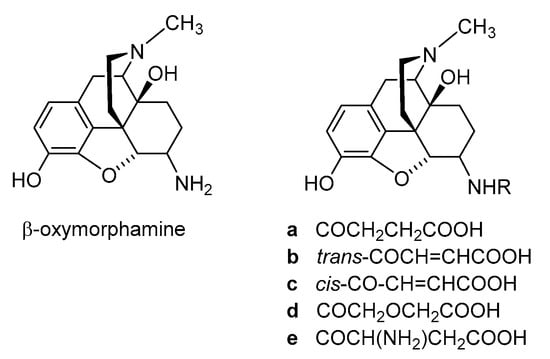
| Compound | Agonist Potencies IC50 × 108 (M) a | Antinociceptive Potencies ED50 b |
|---|---|---|
| a | 14.4 | 0.27 nmol/mouse, i.c.v. 9.8 µmol/kg, i.v. 30.3 µmol/kg, p.o. |
| b | 13.0 | - c |
| c | 3.9 | 0.17 nmol/mouse, i.c.v. 11 µmol/kg, i.v. ~20 µmol/kg, p.o. |
| d | 2.7 | 0.25 nmol/mouse, i.c.v. <20 µmol/kg, i.v. >25 µmol/kg, p.o. |
| e | 3.1 | 0.44 nmol/mouse, i.c.v. <0 µmol/kg, i.v. |
2.2.1. 6-Amino-acid-substituted 14-Alkoxymorphinans
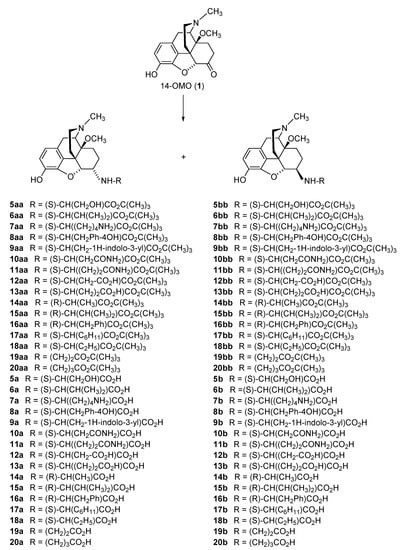
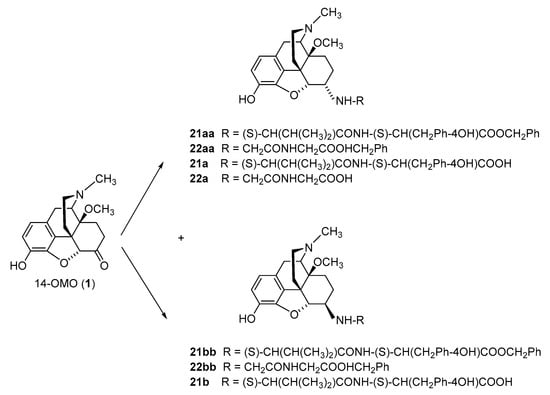
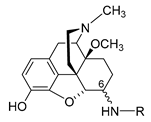
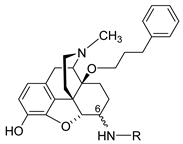
The first synthetic efforts directed towards the development of ionizable molecules in the class of 14-alkoxymorphinans as peripherally acting opioid analgesics started with the series of six 6-amino acids, i.e., Gly-, L-Ala- and L-Phe- substituted derivatives; 2a/b (HS-730/HS-731); 3a/b (HS-935/HS-936); and 4a/b (HS-937/HS-938), respectively, of the highly potent and centrally acting MOR agonist 14-O-methyloxymorphone (14-OMO, 1) (Scheme 1) [35]. A novel synthetic procedure for the synthesis of 6-amino-acid-substituted derivatives in the morphinan series was used. The tert-butyl ester derivatives 2aa/bb, 3aa/bb, and 4aa/bb were prepared from 14-OMO (1) by reductive amination with the respective tert-butyl ester hydrochlorides and sodium cyanoborohydride in ethanol. After separating the diastereoisomers by column chromatography, esters 2aa/bb, 3aa/bb, and 4aa/bb were treated with tetrafluoroboric acid in dichloromethane to afford 6-Gly (2a and 2b), 6-Ala (3a and 3b) and 6-Phe (4a and 4b) substituted derivatives, respectively (Scheme 1) [35].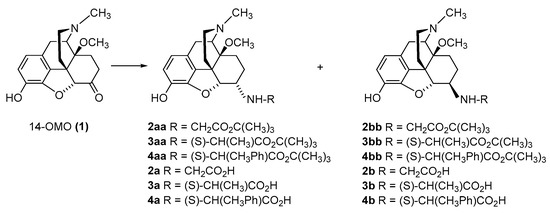
| Morphine | 6053 | 437 | 1613 | 1472 | |
| Fentanyl | 38.6 | ||||
| 14-OMO (1) | 14.9 | 3.26 | |||
| HS-730 (2a) | α-Gly | 58.5 | 35.7 | 72 | 110 |
| HS-731 (2b) | β-Gly | 29.0 | 27.5 | 125 | 204 |
| HS-935 (3a) | α-L-Ala | 68.9 | 16.0 | ||
| HS-936 (3b) | β-L-Ala | 53.4 | 86.3 | ||
| HS-937 (4a) | α-L-Phe | 315 | 31.1 | 171 | 292 |
| HS-938 (4b) | β-L-Phe | >3600 | 579 | 79 | 107 |
| 19a | |||||
| α-β-Ala | |||||
| 31.2 | |||||
| 20a | α-GABA | 41.9 | |||
| 21a | β-L-Val-L-Tyr | 178 | |||
| 22a | β-Gly-Gly | 104 | |||
| 24a | α-Gly | 81.1 | |||
| 24b | β-Gly | 130 |
| Pain Model | Route | ED50 | Reference |
|---|---|---|---|
| Acute nociception Radiant heat tail-flick test (rat) |
i.c.v. s.c. |
0.030 nmol/rat 29.0 nmol/kg |
[40] [40] |
| Trigeminal nociception Eye wiping test (mouse) |
i.p. |
50 µg/kg a |
[43] |
| Compound | Pain Model (Species) | ED50 (Systemic Administration) | ED50 (Central Administration) | Reference |
|---|
| Compound | Pain Model (Species) | ED50 Ratio Peripheral/Central Administration |
Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphine | hot-plate test (mouse) | 4.5 mg/kg, s.c. | ||||||||||
| Fentanyl | radiant heat tail-flick test (rat) | 23 | [50] | |||||||||
| [ | 50 | ] | hot water tail-flick test (rat) | 3.41 mg/kg, i.p. | [64] | Visceral pain | ||||||
| Morphine | radiant heat tail-flick test (rat) | 172 | [40] | |||||||||
| radiant heat tail-flick test (mouse) | 5.5 nmol/mouse, i.c.v. | [59] | ||||||||||
| 159 | [58] | Acetic-acid-induced writhing assay (mouse) | i.c.v. s.c. |
0.49 pmol/mouse 51 nmol/kg |
[ | radiant heat tail-flick test (mouse)42 | 314 ng/mouse, i.c.v. | |||||
| 125 | [53] | ] | [52[42] |
|||||||||
| ] | s.c. | 27.5 µg/kg | ||||||||||
| [ | radiant heat tail-flick test (rat) | 6221 nmol/kg, s.c. | 36 | 38.6 nmol/rat, i.c.v] | ||||||||
| [ | 58 | ] | ||||||||||
| acetic-acid-induced writhing assay (rat) | 118 | [61] | Inflammatory pain Formalin test (rat) Carrageenan-induced thermal and mechanical hyperalgesia (rat) |
i.pl. s.c. s.c. p.o. |
Phase I: 0.2 nmol; Phase II: 0.4 nmol Phase I: 125 nmol/kg; Phase II: 204 nmol/kg 20 µg/kg a 10 mg/kg a |
[44] [40] [41] [ |
5a | α-L-Ser | 32.1 | |||
| 6b | β-L-Val | 117 | ||||||||||
| 7a | α-L-Lys | 20.6 | ||||||||||
| 41 | ] | 8a | α-L-Tyr | 14.6 | ||||||||
| 9b | β-L-Trp | 92.7 | ||||||||||
| 10a | α-L-Asn | 15.2 | ||||||||||
| 12a | α-L-Asp | 38.2 | ||||||||||
| 13a | α-L-Glu | 36.1 | ||||||||||
| 15b | β-D-Val | |||||||||||
| Neuropathic pain, sciatic nerve ligation—Mechanical hyperalgesia (rat) | i.pl. | 441 nmol | [44 | 14.0 | ||||||||
| ] | 16a | α-D-Phe | 18.1 | |||||||||
| 16b | β-D-Phe | 250 | ||||||||||
| 17a | α-L-Chg | 20.6 |
| Compound | R | Opioid Receptor Binding a | Agonist Activity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | b | R | clogD | Opioid Receptor Binding, Ki (nM) a | clogD7.4 b7.4 c | ||||||||
| MOR Ki (nM) |
DOR Ki | ||||||||||||
| MOR | (nM) | DORKOR Ki (nM) |
Ki Ratio MOR/DOR/KOR |
MOR EC50 (nM) |
KORMOR % stim. |
Ki Ratio MOR/DOR/KORDOR EC50 (nM) |
DOR % stim. |
||||||
| radiant heat tail-flick test (rat) | |||||||||||||||||
| 2.68 mg/kg, i.p. | |||||||||||||||||
| 3.51 µg/rat, i.t. | |||||||||||||||||
| [ | 60 | ] | |||||||||||||||
| M6SU | radiant heat tail-flick test (rat) | 25,493 | [58] | radiant heat tail-flick test (rat) | 9.1 mg/kg, p.o. | [60] | |||||||||||
| acetic-acid-induced writhing assay (mouse) | 221,444 | [62] | acetic-acid-induced writhing assay (mouse) | ||||||||||||||
| 14-O | 238.6 nmol/kg, i.p. | 2.02 nmol/mouse, i.c.v. | -MeM6SU | radiant heat tail-flick test (rat) | 11,615[61] | ||||||||||||
| [ | 58 | ] | formalin-induced inflammatory pain (rat) | Phase II: 0.259 mg/kg, i.p. | |||||||||||||
| acetic-acid-induced writhing assay (mouse) | [ | 61] | |||||||||||||||
| 51,177 | [ | 62] | formalin-induced inflammatory pain (rat) | Phase I and II: 3884, 7769, 15,538, 31,075 nmol/kg, s.c. a | |||||||||||||
| 14-O | [ | 65 | ] | ||||||||||||||
| -MeC6SU | radiant heat tail-flick test (rat) | 314 | [53] | neuropathic pain, CCI (rat) hyperalgesia allodynia |
2.65 mg/kg, i.p. 1.45 mg/kg, i.p. |
[60] | |||||||||||
| STZ-induced diabetic neuropathic pain— tail withdrawal test (rat) |
6.47 mg/kg, i.p. | [63] | |||||||||||||||
| STZ-induced diabetic neuropathic pain— paw withdrawal test (rat) |
40,000 nmol/kg, s.c. a | [66] | |||||||||||||||
| Codeine | radiant heat tail-flick test (rat) | 54.01 µmol/kg | [53] | ||||||||||||||
| M6SU | hot-plate test (mouse) | 1.7 mg/kg, s.c. | [50] | ||||||||||||||
| hot water tail-flick (rat) | 0.82 mg/kg, i.p. | [63] | |||||||||||||||
| radiant heat tail-flick test (mouse) | 0.19 nmol/mouse, i.c.v. | [59] | |||||||||||||||
| radiant heat tail-flick test (mouse) | 10.6 ng/mouse, i.c.v. | [52] | |||||||||||||||
| radiant heat tail-flick test (rat) | 9305 nmol/kg, s.c. | 0.356 nmol/rat, i.c.v. | [58] | ||||||||||||||
| radiant heat tail-flick test (rat) | 0.54 mg/kg i.p. | 0.29 μg/rat, i.t. | [60] | ||||||||||||||
| radiant heat tail-flick test (rat) | 4.97 mg/kg, p.o. | [60] | |||||||||||||||
| paw pressure threshold test (rat) | 2.3 mg/kg, i.p. | [64] | |||||||||||||||
| acetic-acid-induced writhing assay (mouse) | 1993 nmol/kg, s.c. | 9 pmol/mouse, i.c.v. | [62] | ||||||||||||||
| formalin-induced inflammatory pain (rat) | Phase II: 0.094 mg/kg, i.p. | [60] | |||||||||||||||
| CFA-induced inflammatory pain—paw pressure test (rat) | 292 nmol/kg, s.c. | [62] | |||||||||||||||
| neuropathic pain, CCI (rat) hyperalgesia allodynia |
0.40 mg/kg, i.p. 0.19 mg/kg, i.p. |
[60] | |||||||||||||||
| STZ-induced diabetic neuropathic pain—tail withdrawal test (rat) | 0.35 mg/kg, i.p. | [63] | |||||||||||||||
| C6SU | radiant heat tail-flick test (mouse) | 200 ng, i.c.v b | [52] | ||||||||||||||
| radiant heat tail-flick test (rat) | weak effect (<20%), s.c. | [53] | |||||||||||||||
| CFA-induced inflammatory pain—paw pressure test (rat) | 6.6 and 13.2 µmol/kg, s.c. a | [53] | |||||||||||||||
| 14-O-MeM6SU | radiant heat tail-flick test (rat) | 182.4 nmol/kg, s.c. | 0.0157 nmol/rat, i.c.v. | [58] | |||||||||||||
| acetic-acid-induced writhing assay (mouse) | 87 nmol/kg, s.c. | 1.7 nmol/mouse, i.c.v. | [62] | ||||||||||||||
| KOR | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | 50 | (nM) | KOR | % stim. | |||||||||||||
| 14-OMO (1) | 0.10 | 4.80 | 10.2 | 1/48/102 | 3.83 | 97 | 37.3 | 106 | 116 | 77 | 0.48 | ||||||
| POMO (23) | 0.073 | 0.13 | 0.30 | 1/1.8/4.1 | 2.89 | ||||||||||||
| HS-730 (2a) | α-Gly | 0.89 | 15.4 | 43.2 | 1/7/49 | 1.16 | |||||||||||
| Compound | R, Amino Acid Substitution at Position 6 |
Radiant Heat Tail-Flick Test (Rat), ED50 | Ratio ED50 (nmol/kg, s.c.)/ ED50 (nmol/rat, i.c.v.). |
||||||||||||||
| s.c. (nmol/kg) | i.c.v. (nmol/rat) | ||||||||||||||||
| Morphine | 6053 | 35.1 | 172 | ||||||||||||||
| 99 | 9.61 | 103 | 399 | 87 | −3.35 | ||||||||||||
| 24a | |||||||||||||||||
| Fentanyl | α-Gly | 0.19 | 38.6 | 1.66 | 23 | HS-731 (2b) | β-Gly | 0.83 | 7.86 | 44.8 | 1/9.5/54 | 3.78 | 98 | ||||
| 14-OMO ( | 7.92 | 1 | 103 | 361 | ) | 82 | −3.35 | ||||||||||
| 14.9 | 0.172 | HS-935 (3a) | α-L-Ala | 0.77 | 26.9 | 142 | 1/35/184 | 1.34 | 97 | 9.55 | 93 | 214 | 51 | −2.81 | |||
| 0.22 | 0.73 | 1/1.2/3.8 | −0.85 | ||||||||||||||
| 45.6 | |||||||||||||||||
| 24b | β-Gly | 0.16 | 0.19 | 0.81 | 1/1.2/5.1 | 87−0.85 | |||||||||||
| HS-730 (2a) | α-Gly | 58.5 | 0.031 | 1887 | HS-936 (3b) | β-L-Ala | 1.90 | 7.71 | 63.7 | 1/4.1/34 | 6.24 | 87 | 5.20 | 104 | 392 | 64 | |
| HS-731 ( | −2.81 | ||||||||||||||||
| 2b) | β-Gly | 29.0 | 0.030 | 967 | HS-937 (4a) | α-L-Phe | |||||||||||
| HS-935 ( | 0.95 | 3a | 3.67 | 28.5 | )1/3.9/30 | 0.38 | 93 | 0.39 | 102 | 219 | 39 | −1.13 | |||||
| α-L-Ala | 68.9 | 0.121 | 569 | HS-938 (4b) | β-L-Phe | 2.58 | 1.03 | 151 | 1/0.4/59 | 6.76 | 99 | 0.48 | 94 | 1172 | 81 | ||
| HS-936 ( | −1.13 | ||||||||||||||||
| 3b) | β-L-Ala | 53.4 | 0.082 | 651 | 5a | α-L-Ser | 2.21 | 5.32 | 196 | 1/2.4/89 | 1.60 | 87 | 13.9 | 101 | 1213 | 44 | −3.89 |
| HS-937 (4a) | α-L-Phe | 315 | 0.063 | 5000 | 5b | β-L-Ser | 2.14 | 5.29 | 152 | 1/2.5/71 | 3.56 | 101 | |||||
| HS-938 (4b | 6.98 | ) | β-L-Phe | 98 | 201 | 88 | −3.89 | ||||||||||
| >3600 | 0.776 | >4600 | 6a | α-L-Val | 3.16 | 3.91 | 325 | 1/1.2/103 | 10.5 | 95 | 33.8 | 91 | 462 | 51 | −1.94 | ||
| 6b | β-L-Val | 3.04 | 3.52 | 305 | 1/1.2/100 | 11.7 | 84 | 5.73 | 96 | 1117 | 24.7 | 93 | 6.23 | 95 | 774 | 60 | −1.41 |
| 68 | −1.94 | ||||||||||||||||
| 7a | α-L-Lys | 0.19 | 1.27 | 12.6 | 1/6.7/66 | 2.25 | 9 | 152 | 106 | 118 | 79 | −5.57 | |||||
| 7b | β-L-Lys | 0.53 | 3.34 | 33.7 | 1/6.3/64 | 6.85 | 90 | 45.1 | 93 | 525 | 62 | −5.57 | |||||
| 8a | α-L-Tyr | 0.83 | 2.18 | 39.5 | 1/2.6/48 | 1.87 | 92 | 1.76 | 88 | 100 | 62 | −1.41 | |||||
| 8b | β-L-Tyr | 3.20 | 3.89 | 186 | 1/1.2/58 | 9a | α-L-Trp | 0.36 | 1.02 | ||||||||
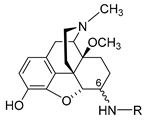
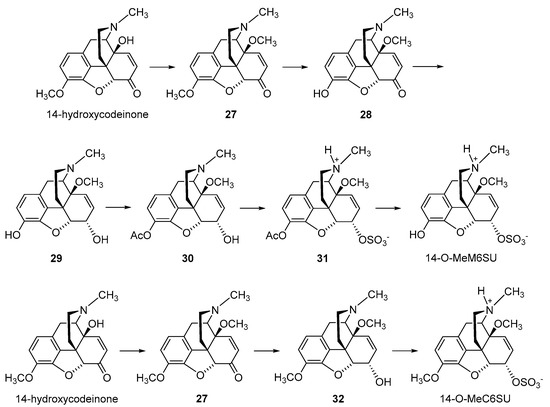
2.2.2. 6-O-Sulfate Esters of Morphine and Codeine
Further chemical strategies to limit the penetration of morphinans from the periphery to the CNS following systemic administration include 6-O-sulfation. Sulfate conjugation generally leads to an increase in the water solubility of the compounds (fully ionized at neutral pH) yet is a biotransformation step in humans to facilitate the excretion of xenobiotics, including opioids [49]. Therefore, 6-O-sulfate esters of morphine and codeine were synthesized, such as morphine-6-O-sulfate (M6SU) [50][51], codeine-6-O-sulfate (C6SU) [50][51][52], 14-methoxymorphine-6-O-sulfate (14-O-MeM6SU) [53], 14-methoxycodeine-6-O-sulfate (14-O-MeC6SU) [53] and others [51][54][55]. For the synthesis of sulfate esters, a number of procedures have been reported and reviewed [56][57]. The most common methods are the reaction of phenol or alcohol with chlorosulfonic acid in pyridine, and with trimethylamine-SO3 complex or pyridine-SO3 complex in DMF, 1,4-dioxane or pyridine. The early synthesis of M6SU and C6SU was based on employing chlorosulfonic acid as a sulfonating reagent [50]. Other synthetical procedures of sulfate esters using pyridine-SO3 complex were reported for morphine derivatives [51][55]. For example, preparation of 6-O-sulfate esters of morphine and codeine, M6SU and C6SU, respectively, was achieved by means of the sulfation of the C-6 hydroxyl function with pyridine-SO3 complex (Scheme 5) [51].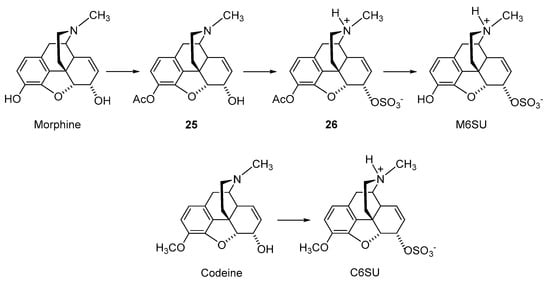
| Compound | Opioid Receptor Binding, Ki (nM) a | |||
|---|---|---|---|---|
| MOR | DOR | KOR | Ki Ratio MOR/DOR/KOR |
|
| Morphine | 4.37 | 2951 | 113 | |
| Compound | MVD Bioassay | [35S]GTPγS Binding Assay | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EC50 (nM) | Tissue | EC50 (nM) | Emax (%) | ||||||
| 1/675/26 | |||||||||
| Morphine | 347 | Rat brain Guinea-pig brain |
250 462 |
129 | |||||
| Codeine | 737 | - b | - | - | |||||
| 119 | |||||||||
| Codeine | >1000 | Rat brain Guinea-pig brain |
- a - |
110 104 |
M6SU | 11.5 | 525 | 275 | 1/46/24 |
| M6SU | 103 | Rat brain Guinea-pig brain |
105 - |
133 - |
C6SU | 96.9 | 968 | ||
| C6SU | b | - | 1/10/- | ||||||
| >1000 | Rat brain | Guinea-pig brain |
>10,000 - |
121 102 |
14-O-MeM6SU | 1.12 | 10.2 | ||
| 14-O-MeM6SU | 4.38 | Rat brain | 295 | 1/9/263 | |||||
| Guinea-pig brain | 19.1 | - |
201 - |
14-O-MeC6SU | 3.37 | 346 | 246 | 1/103/73 | |
| 14- | ||||
| O | ||||
| -MeC6SU | ||||
| 238 | Rat brain | Guinea-pig brain |
301 >1000 |
128 130 |
| formalin-induced inflammatory pain (rat) | ||||
| Phase I: 3506 and 1012 nmol/kg, s.c. | ||||
| a | ||||
| Phase II: 253, 506 and 1012 nmol/kg, s.c. | ||||
| a | ||||
| [ | ||||
| 65 | ||||
| ] | ||||
| CFA-induced inflammatory pain—paw pressure test (rat) | ||||
| 45 nmol/kg, s.c. | ||||
| [ | ||||
| 62 | ||||
| ] | ||||
| STZ-induced diabetic neuropathic pain—paw withdrawal test (rat) | 253, 506 and 1012 nmol/kg, s.c. a | [66] | ||
| 14-O-MeC6SU | radiant heat tail-flick test (rat) | 5.34 µmol/kg, s.c. | 0.017 µmol/animal, i.c.v. | [53] |
| CFA-induced inflammatory pain—paw pressure test (rat) | 6.1 and 12.2 µmol/kg, s.c. a | [53] |
| 25.1 | ||||||||||||
| 1/2.8/70 | ||||||||||||
| 0.51 | ||||||||||||
| 93 | ||||||||||||
| 2.52 | ||||||||||||
| 102 | ||||||||||||
| 70.1 | ||||||||||||
| 61 | ||||||||||||
| −1.03 | ||||||||||||
| 9b | β-L-Trp | 0.65 | 1.19 | 8.66 | 1/1.8/13 | 1.64 | 101 | 2.18 | 96 | 181 | 87 | −1.03 |
| 10a | α-L-Asn | 1.17 | 3.37 | 74.0 | 1/2.9/63 | 0.83 | 99 | 9.78 | 106 | 81.7 | 67 | −4.29 |
| 10b | β-L-Asn | 1.26 | 2.25 | 103 | 1/1.8/82 | 2.04 | 96 | 3.18 | 88 | 923 | 71 | −4.29 |
| 11a | α-L-Gln | 3.24 | 5.13 | 351 | 1/1.6/108 | 2.27 | 90 | 7.80 | 104 | 185 | 70 | −4.04 |
| 11b | β-L-Gln | 2.48 | 4.87 | 290 | 1/2.0/117 | 9.54 | 98 | 3.96 | 103 | 1410 | 63 | −4.04 |
| 12a | α-L-Asp | 1.36 | 14.6 | 50.2 | 1/11/37 | 4.10 | 90 | 10.1 | 97 | 2991 | 83 | −5.64 |
| 12b | β-L-Asp | 3.42 | 22.6 | 351 | 1/6.6/103 | 1.45 | 74 | 11.8 | 101 | 753 | 49 | −5.64 |
| 13a | α-L-Glu | 1.45 | 9.03 | 87.2 | 1/6.2/60 | 3.11 | 105 | 10.8 | 98 | 1167 | 68 | −5.39 |
| 13b | β-L-Glu | 11.6 | 7.64 | 1252 | 1/0.7/108 | 12.7 | 98 | 4.60 | 101 | 2233 | 76 | −5.39 |
| 14a | α-D-Ala | 0.69 | 10.4 | 71.5 | 1/15/104 | 1.44 | 100 | 24.3 | 106 | 254 | 67 | −2.81 |
| 14b | β-D-Ala | 1.48 | 11.3 | 142 | 1/7.6/96 | 15.4 | 102 | 5.46 | 106 | 1001 | 86 | −2.81 |
| 15a | α-D-Val | 1.70 | 1.93 | 202 | 1/1.1/119 | 4.51 | 105 | 1.12 | 93 | 2218 | 96 | −1.94 |
| 15b | β-D-Val | 1.02 | 1.68 | 159 | 1/1.6/156 | 2.38 | 101 | 1.30 | 99 | 1278 | 98 | −1.94 |
| 16a | α-D-Phe | 0.61 | ||||||||||
| 1/16/59 | ||||||||||||
| 2.88 | ||||||||||||
| 103 | ||||||||||||
| 34.4 | 103 | 2034 | 104 | −2.93 | ||||||||
| 20b | β-GABA | 1.41 | 6.61 | 147 | 1/4.7/104 | 12.3 | 86 | 6.06 | 103 | 3396 | 74 | −2.93 |
| 21a | α-L-Val-L-Tyr | 0.82 | 1.19 | 69.0 | 1/1.5/84 | 0.89 | 84 | 1.16 |
| Compound | Amino Acid Substitution at Position 6 |
Radiant Heat Tail-Flick Test (Rat) ED50 (nmol/kg, s.c.) |
Writhing Assay (Mouse) ED50 (µg/kg, s.c.) |
Formalin Test (Rat) ED50 (nmol/kg, s.c.) |
|
|---|---|---|---|---|---|
| Phase I | Phase II | ||||
| 18a | |||||
| α-L-Abu | |||||
| 17.5 | |||||
| 3.69 | ||||||||||||
| 76.4 | ||||||||||||
| 1/6.0/125 | ||||||||||||
| 0.77 | ||||||||||||
| 96 | ||||||||||||
| 8.36 | ||||||||||||
| 95 | ||||||||||||
| 215 | ||||||||||||
| 80 | ||||||||||||
| −1.13 | ||||||||||||
| 16b | ||||||||||||
| β-D-Phe | ||||||||||||
| 1.28 | ||||||||||||
| 1.19 | ||||||||||||
| 139 | ||||||||||||
| 1/0.9/109 | 0.68 | 78 | 1.71 | 96 | 611 | 92 | −1.13 | |||||
| 17a | α-L-Chg | 1.23 | 14.3 | 177 | 1/12/144 | 2.88 | 86 | 20.5 | 101 | 250 | 52 | −1.26 |
| 17b | β-L-Chg | 1.66 | 1.30 | 118 | 1/0.8/71 | 5.33 | 86 | 3.57 | 96 | 282 | 59 | −1.26 |
| 18a | α-L-Abu | 0.76 | 37.5 | 144 | 1/49/189 | 5.12 | 88 | 98.2 | 104 | 942 | 61 | −2.34 |
| 18b | β-L-Abu | 1.83 | 1.30 | 201 | 1/0.7/110 | 5.47 | 83 | 2.22 | 100 | 572 | 72 | −2.34 |
| 19a | α-β-Ala | 1.30 | 60.0 | 182 | 1/46/140 | 3.52 | 99 | 96.4 | 97 | 186 | 78 | −3.18 |
| 19b | β-β-Ala | 1.04 | 13.9 | 71.4 | 1/13/69 | 5.74 | 78 | 20.1 | 99 | 622 | 67 | −3.18 |
| 20a | α-GABA | 0.77 | 12.5 | |||||||||
| 88 | 330 | 50 | −1.02 | |||||||||
| 21b | β-L-Val-L-Tyr | 0.44 | 1.38 | 390 | 1/3.1/886 | 0.16 | 73 | 1.56 | 89 | 1884 | 63 | −1.02 |
| 22a | β-Gly-Gly | 4.62 | 7.52 | 203 | 1/1.6/44 | 4.39 | 85 | 2.84 | 102 | 885 | 75 | −4.27 |
2.2.3. Morphine-6-glucuronide
Peripheral restriction can also be achieved by O-glucuronidation at position 6 in the morphinan skeleton. A prominent example is morphine-6-glucuronide (M6G) (Figure 4), the active metabolite of morphine and a potent agonist to the MOR [47][71]. Approximately 10% of morphine is metabolized to M6G [72]. M6G contributes to the clinical analgesic effect of morphine, showing equivalent analgesia but with an improved side effect profile compared to that of morphine [73][74][75].
2.3. Nanocarrier-Based Approaches of Drug Delivery
A promising strategy to alter the pharmacokinetic profile and improve therapeutic effects of drugs is nanotechnology, that is, the use of biocompatible nanocarriers, including nanoparticles, liposomes, nanocapsules, micelles, dendrimers and nanotubes that may carry different therapeutic agents (for reviews, see [83][84][85][86][87][88][89]). The advantage of such nanocarriers is the direct delivery of drugs to the region or cells of interest, improved efficacy and decreased risk of negative side effects. Furthermore, carriers should be biologically stable, should protect the drug from degradation and the host body from toxic side effects, and should be able to deliver the loaded drug specifically to the target cell population in vivo (for reviews, see [83][84][85][86][87][88][89]). Nanotechnology has been extensively examined for tumor-directed delivery of chemotherapeutics to reduce their off-target toxicity [87], and has also been proposed for pain management [88][89]. Recently, a nanocarrier-based approach was developed that uses hyperbranched, dendritic polyglycerols (PG) to selectively deliver morphine to peripheral inflamed tissue [90]. Morphine was covalently bound to PG, via a cleavable ester linker sensitive to esterases and low pH. The rationale was that due to its high molecular weight and hydrophilicity, the i.v. injection of PG-morphine will not cross the BBB, but will selectively extravasate from leaky blood vessels characteristic of inflamed tissue. The local low pH and leukocyte esterases will then trigger the release of morphine from PG-morphine to reduce pain behavior [90]. In radioligand binding studies using human embryonic kidney (HEK) 293 cells stably expressing the rat MOR, PG-morphine was 10,000 times less effective than morphine (IC50 of 18.2 µM vs. 0.002 µM, respectively), indicating that PG-M does not bind to the MOR [90]. Different to morphine, dosages containing equivalent amounts of morphine-free PG-morphine exclusively activated peripheral opioid receptors following systemic i.v. administration in rats with unilateral hind paw inflammation. PG-M selectively induced antinociception in the inflamed paw in a naloxone-methiodide-dependent manner. Free morphine was only detected in inflamed paw tissue, but not in the contralateral, non-inflamed paw tissue, blood and brain of rats [90]. Furthermore, PG-M was reported not to cause sedation and constipation at antinociceptive doses after i.v. injection in rats with inflammatory pain [90]. However, the organ toxicity and broader side effect profile, including abuse potential and effects on respiration of PG-M were not reported yet to strengthen the clinical applicability of this strategy, which is able to deliver morphine exclusively in injured tissue, precluding not only CNS side effects but also constipation. Further, morphine-loaded hydrogels have been reported. Preclinical studies demonstrated that peptide-based hydrogels loaded with morphine as new controlled-drug delivery systems produced effective, sustained antinociceptive effects in mice after s.c. administration, and no sedative effects were observed [91][92].3. Conclusions
In the current context of the ‘opioid crisis’, the development of new opioid analgesics with improved pharmacology (i.e., efficacy in various pain conditions and reduced capability of inducing unwanted side effects) is of crucial clinical and public health attention. Diverse opioid analgesic discovery efforts are therefore made towards identifying effective and well-tolerated opioids that have an improved benefit/risk ratio compared with currently available drugs.
The basis for drug discovery targeting peripheral opioid receptors is supported by the knowledge that opioid receptors are expressed in the CNS, PNS and peripheral tissues. Furthermore, activating opioid receptors in the periphery leads to an effective analgesic response, and the most serious opioid-related adverse effects (i.e., apnea, sedation, physical dependence and addiction) are due to the activation of opioid receptors in the CNS. Thus, peripherally restricted opioids are viewed as viable targets to avoid many of the lethal side effects associated with opioids targeting the CNS.
In this entry, different peripheralization strategies applied to opioids are discussed. Emphasis was placed on the morphinan class of opioid ligands represented by morphine and its structurally related analogues that are used extensively not only clinically but also as experimental tools and that are important as scaffolds for the design of new ligands. Broad chemical and pharmacological work was performed on modifications of the morphinan scaffold to reduce the ability to cross the BBB, and substantial achievements have been made in the field, increasing the feasibility for clinical application. Researchers discussed chemical variations on the morphinan skeleton to increase the hydrophilicity, such as quaternization of the morphinan nitrogen (N17), the introduction of polar/ionizable substituents at C-6 position (i.e., amino acid, sulfation and glucuronidation), and nanocarrier-based approaches to selectively deliver morphine to peripheral tissue. Although the available preclinical and clinical data are favorable to the potential use of these compounds, their clinical impact, and the extent to which they will replace existing opioids, needs further investigations.
References
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci. 2018, 41, 453–473.
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid analgesia and opioid-induced adverse effects: A review. Pharmaceuticals 2021, 14, 1091.
- Volkow, N.; Benveniste, H.; McLellan, T.A. Use and misuse of opioids in chronic pain. Annu. Rev. Med. 2018, 69, 451–465.
- Sobczak, Ł.; Goryński, K. Pharmacological aspects of over-the-counter opioid drugs misuse. Molecules 2020, 25, 3905.
- National Institute on Drug Abuse (NIDA). Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed on 28 April 2023).
- Spetea, M. Opioid receptors and their ligands in the musculoskeletal system and relevance for pain control. Curr. Pharm. Des. 2013, 19, 7382–7390.
- Sein, C. Opioid receptors. Annu. Rev. Med. 2016, 67, 433–451.
- Che, T.; Roth, B.L. Structural insights accelerate the discovery of opioid alternatives. Annu. Rev. Biochem. 2021, 90, 739–761.
- Spetea, M.; Asim, M.F.; Wolber, G.; Schmidhammer, H. The opioid receptor and ligands acting at the µ opioid receptor, as therapeutics and potential therapeutics. Curr. Pharm. Des. 2013, 19, 7415–7434.
- Busserolles, J.; Lolignier, S.; Kerckhove, N.; Bertin, C.; Authier, N.; Eschalier, A. Replacement of current opioid drugs focusing on MOR-related strategies. Pharmacol. Ther. 2020, 210, 107519.
- Darcq, E.; Kieffer, B.L. Opioid receptors: Drivers to addiction? Nat. Rev. Neurosci. 2018, 19, 499–514.
- Imam, M.Z.; Kuo, A.; Ghassabian, S.; Smith, M.T. Progress in understanding mechanisms of opioid-induced gastrointestinal adverse effects and respiratory depression. Neuropharmacology 2018, 131, 238–255.
- Fürst, S.; Hosztafi, S. The chemical and pharmacological importance of morphine analogues. Acta Physiol. Hung. 2008, 95, 3–44.
- Pasternak, G.W.; Pan, Y.X. Mu opioids and their receptors: Evolution of a concept. Pharmacol. Rev. 2013, 65, 1257–1317.
- Spetea, M.; Schmidhammer, H. Recent advances in the development of 14-alkoxy substituted morphinans as potent and safer opioid analgesics. Curr. Med. Chem. 2012, 19, 2442–2457.
- Lewis, J.W.; Husbands, S.M. 14-Amino-4,5-epoxymorphinan derivatives and their pharmacological actions. Top. Curr. Chem. 2011, 299, 93–119.
- Stavitskaya, L.; Coop, A. Most recent developments and modifications of 14-alkylamino and 14-alkoxy-4,5-epoxymorphinan derivatives. Mini. Rev. Med. Chem. 2011, 11, 1002–1008.
- Schmidhammer, H.; Spetea, M.; Windisch, P.; Schütz, J.; Riba, P.; Al-Khrasani, M.; Fürst, S. Functionalization of the carbonyl group in position 6 of morphinan-6-ones. Development of novel 6-amino and 6-guanidino substituted 14-alkoxymorphinans. Curr. Pharm. Des. 2013, 19, 7391–7399.
- Devereaux, A.L.; Mercer, S.L.; Cunningham, C.W. DARK classics in chemical neuroscience: Morphine. ACS Chem. Neurosci. 2018, 9, 2395–2407.
- Spetea, M.; Schmidhammer, H. Recent chemical and pharmacological developments on 14-oxygenated-N-methylmorphinan-6-ones. Molecules 2021, 26, 5677.
- Kalso, E.; Smith, L.; McQuay, H.J.; Moore, R.A. No pain, no gain: Clinical excellence and scientific rigour—Lessons learned from IA morphine. Pain 2002, 98, 269–275.
- Stein, C.; Schäfer, M.; Machelska, H. Attaching pain at its source: New perspectives on opioids. Nat. Med. 2003, 9, 1003–1008.
- Kapitzke, D.; Vetter, I.; Cabot, P.J. Endogenous opioid analgesia in peripheral tissues and the clinical implications for pain control. Ther. Clin. Risk Manag. 2005, 1, 279–297.
- Martinez, V.; Abalo, R. Peripherally acting opioid analgesics and peripherally-induced analgesia. Behav. Pharmacol. 2020, 31, 136–158.
- Stein, C.; Comisel, K.; Haimaeri, E.; Yassouridis, A.; Lehrberger, K. Analgesic effects of intraarticular morphine after arthroscopic knee surgery. N. Engl. J. Med. 1991, 325, 1123–1126.
- Likar, R.; Kapral, S.; Steinkellner, H.; Stein, C.; Schäfer, M. Dose-dependency of intra-articular morphine analgesia. Br. J. Anaesth. 1999, 83, 241–244.
- Iorio, M.A.; Frigeni, V. Narcotic agonist/antagonist properties of quaternary diastereoisomers derived from oxymorphone and naloxone. Eur. J. Med. Chem. 1984, 19, 301–303.
- Brown, D.R.; Goldberg, L.I. The use of quaternary narcotic antagonists in opiate research. Neuropharmacology 1985, 24, 181–191.
- Foster, R.S.; Jenden, D.J.; Lomax, P. A comparison of the pharmacologic effects of morphine and N-methyl morphine. J. Pharmacol. Exp. Ther. 1967, 157, 185–195.
- Smith, T.W.; Buchan, P.; Parsons, D.N.; Wilkinson, S. Peripheral antinociceptive effects of N-methyl morphine. Life Sci. 1982, 31, 1205–1208.
- Oluyomi, A.O.; Hart, S.L.; Smith, T.W. Differential antinociceptive effects of morphine and methylmorphine in the formalin test. Pain 1992, 49, 415–418.
- Kosterlitz, H.W.; Waterfield, A.A. In vitro models in the study of structure-activity relationships of narcotic analgesics. Annu. Rev. Pharmacol. 1975, 15, 29–47.
- Botros, S.; Lipkowski, A.; Larson, D.; Stark, P.; Takemori, A.; Portoghese, P.S. Opioid agonist and antagonist activities of peripherally selective derivatives of naltrexamine and oxymorphamine. J. Med. Chem. 1989, 32, 2068–2071.
- Larson, D.L.; Hua, M.; Takemori, A.K.; Portoghese, P.S. Possible contribution of a glutathione conjugate to the long-duration action of funaltrexamine. J. Med. Chem. 1993, 36, 3669–3673.
- Schütz, J.; Brandt, W.; Spetea, M.; Wurst, K.; Wunder, G.; Schmidhammer, H. Synthesis of 6-amino acid substituted derivatives of the highly potent analgesic 14-O-methyloxymorphone. Helv. Chim. Acta 2003, 86, 2142–2148.
- Spetea, M.; Rief, S.B.; Haddou, T.B.; Fink, M.; Kristeva, E.; Mittendorfer, H.; Haas, S.; Hummer, N.; Follia, V.; Guerrieri, E.; et al. Synthesis, biological, and structural explorations of new zwitterionic derivatives of 14-O-methyloxymorphone, as potent μ/δ opioid agonists and peripherally selective antinociceptives. J. Med. Chem. 2019, 62, 641–653.
- Spetea, M.; Windisch, P.; Guo, Y.; Bileviciute-Ljungar, I.; Schütz, J.; Asim, M.F.; Berzetei-Gurske, I.P.; Riba, P.; Kiraly, K.; Fürst, S.; et al. Synthesis and pharmacological activities of 6-glycine substituted 14-phenylpropoxymorphinans, a novel class of opioids with high opioid receptor affinities and antinociceptive potencies. J. Med. Chem. 2011, 54, 980–988.
- Spetea, M.; Friedmann, T.; Riba, P.; Schütz, J.; Wunder, G.; Langer, T.; Schmidhammer, H.; Fürst, S. In vitro opioid activity profiles of 6-amino acid substituted derivatives of 14-O-methyloxymorphone. Eur. J. Pharmacol. 2004, 483, 301–308.
- Lattanzi, R.; Rief, S.; Schmidhammer, H.; Negri, L.; Spetea, M. In vitro and in vivo pharmacological activities of 14-O-phenylpropyloxymorphone, a potent mixed mu/delta/kappa-opioid receptor agonist with reduced constipation in mice. Front. Pharmacol. 2018, 9, 1002.
- Fürst, S.; Riba, P.; Friedmann, T.; Tímar, J.; Al-Khrasani, M.; Obara, I.; Makuch, W.; Spetea, M.; Schütz, J.; Przewlocki, R.; et al. Peripheral versus central antinociceptive actions of 6-amino acid-substituted derivatives of 14-O-methyloxymorphone in acute and inflammatory pain in the rat. J. Pharmacol. Exp. Ther. 2005, 312, 609–618.
- Bileviciute-Ljungar, I.; Spetea, M.; Guo, Y.; Schütz, J.; Windisch, P.; Schmidhammer, H. Peripherally mediated antinociception of the mu-opioid receptor agonist 2-(4,5alpha-epoxy-3-hydroxy-14beta-methoxy-17-methylmorphinan-6beta-yl)aminoacetic acid (HS-731) after subcutaneous and oral administration in rats with carrageenan-induced hindpaw inflammation. J. Pharmacol. Exp. Ther. 2006, 317, 220–227.
- Al-Khrasani, M.; Spetea, M.; Friedmann, T.; Riba, P.; Király, K.; Schmidhammer, H.; Fürst, S. DAMGO and 6β-glycine substituted 14-O-methyloxymorphone but not morphine show peripheral, preemptive antinociception after systemic administration in a mouse visceral pain model and high intrinsic efficacy in the isolated rat vas deferens. Brain Res. Bull. 2007, 74, 369–375.
- Baillie, L.D.; Schmidhammer, H.; Mulligan, S.J. Peripheral μ-opioid receptor mediated inhibition of calcium signaling and action potential-evoked calcium fluorescent transients in primary afferent CGRP nociceptive terminals. Neuropharmacology 2015, 93, 267–273.
- Obara, I.; Makuch, W.; Spetea, M.; Schütz, J.; Schmidhammer, H.; Przewlocki, R.; Przewlocka, B. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur. J. Pharmacol. 2007, 558, 60–67.
- Puls, K.; Schmidhammer, H.; Wolber, G.; Spetea, M. Mechanistic characterization of the pharmacological profile of HS-731, a peripherally acting opioid analgesic, at the µ-, δ-, κ-opioid and nociceptin receptors. Molecules 2022, 27, 919.
- Fürst, S.; Zádori, Z.S.; Zádor, F.; Király, K.; Balogh, M.; László, S.B.; Hutka, B.; Mohammadzadeh, A.; Calabrese, C.; Galambos, A.R.; et al. On the role of peripheral sensory and gut mu opioid receptors: Peripheral analgesia and tolerance. Molecules 2020, 25, 2473.
- Herz, A.; Teschemacher, H.J. Activities and sites of antinociceptive action of morphine-like analgesics and kinetics of distribution following intravenous, intracerebral and intraventricular application. In Advances in Drug Research; Harper, N., Simmonds, A.B., Eds.; Academic Press Limited: London, UK, 1971; Volume 6, pp. 79–118.
- Frances, B.; Gout, R.; Monsarrat, B.; Cros, J.; Zajac, J.M. Further evidence that morphine-6β-glucuronide is a more potent opioid agonist than morphine. J. Pharmacol. Exp. Ther. 1992, 262, 2531.
- Tukey, R.H.; Strassburg, C.P. Human UDP-glucuronosyltransferases: Metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581–616.
- Mori, M.; Oguri, K.; Yoshimura, H.; Shimomura, K.; Kamata, O.; Ueki, S. Chemical synthesis and analgesic effect of morphine ethereal sulfates. Life Sci. 1972, 11, 525–533.
- Váradi, A.; Gergely, A.; Béni, S.; Jankovics, P.; Noszál, B.; Hosztafi, S. Sulfate esters of morphine derivatives: Synthesis and characterization. Eur. J. Pharm. Sci. 2011, 42, 65–72.
- Zuckerman, A.; Bolan, E.; de Paulis, T.; Schmidt, D.; Spector, S.; Pasternak, G.W. Pharmacological characterization of morphine-6-sulfate and codeine-6-sulfate. Brain Res. 1999, 842, 1–5.
- Zádor, F.; Mohammadzadeh, A.; Balogh, M.; Zádori, Z.S.; Király, K.; Barsi, S.; Galambos, A.R.; László, S.B.; Hutka, B.; Váradi, A.; et al. Comparisons of in vivo and in vitro opioid effects of newly synthesized 14-methoxycodeine-6-O-sulfate and codeine-6-O-sulfate. Molecules 2020, 25, 1370.
- Preechagoon, D.; Brereton, I.; Staatz, C.; Prankerd, R. Ester prodrugs of a potent analgesic, morphine-6-sulfate: Syntheses, spectroscopic and physicochemical properties. Int. J. Pharm. 1998, 163, 177–190.
- Crooks, P.A.; Kottayil, S.G.; Al-Ghananeem, A.M.; Byrn, S.R.; Butterfield, A.D. Opiate receptor binding properties of morphine-, dihydromorphine-, and codeine 6-O-sulfate ester congeners. Bioorg. Med. Chem. Lett. 2006, 16, 4291–4295.
- Kaspersen, F.M.; Van Boeckel, C.A.A. A review of the methods of chemical synthesis of sulphate and glucuronide conjugates. Xenobiotica 1987, 17, 1451–1471.
- Al-Horani, R.A.; Desai, U.R. Chemical sulfation of small molecules—Advances and challenges. Tetrahedron 2010, 66, 2907–2918.
- Lacko, E.; Varadi, A.; Rapavi, R.; Zador, F.; Riba, P.; Benyhe, S.; Borsodi, A.; Hosztafi, S.; Timar, J.; Noszal, B.; et al. A novel µ-opioid receptor ligand with high in vitro and in vivo agonist efficacy. Curr. Med. Chem. 2012, 54, 4699–4707.
- Brown, C.E.; Roerig, S.C.; Burger, V.T. Analgesic potencies of morphine 3- and 6-sulfates, after intracerebroventricular administration in mice: Relationship to structural characteristics defined by mass spectrometry and nuclear magnetic resonance. J. Pharm. Sci. 1985, 74, 821–824.
- Holtman, J.R.; Crooks, P.A.; Johnson-Hardy, J.; Wala, E.P. Antinociceptive effects and toxicity of morphine-6-O-sulfate sodium salt in rat models of pain. Eur. J. Pharmacol. 2010, 648, 87–94.
- Al-Khrasani, M.; Lackó, E.; Riba, P.; Király, K.; Sobor, M.; Timár, J.; Mousa, S.; Schäfer, M.; Fürst, S. The central versus peripheral antinociceptive effects of µ-opioid receptor agonists in the new model of rat visceral pain. Brain Res. Bull. 2012, 87, 238–243.
- Lackó, E.; Riba, P.; Giricz, Z.; Váradi, A.; Cornic, L.; Balogh, M.; Király, K.; Cseko, K.; Mousa, S.A.; Hosztafi, S.; et al. New morphine analogs produce peripheral antinociception within a certain dose range of their systemic administration. J. Pharmacol. Exp. Ther. 2016, 359, 171–181.
- Yadlapalli, J.S.K.; Ford, B.M.; Ketkar, A.; Wan, A.; Penthala, N.R.; Eoff, R.L.; Prather, P.L.; Dobretsov, M.; Crooks, P.A. Antinociceptive effects of the 6-O-sulfate ester of morphine in normal and diabetic rats: Comparative role of mu- and delta-opioid receptors. Pharmacol. Res. 2016, 13, 335–347.
- Yadlapalli, J.S.K.; Jai Shankar, K.; Dogra, N.; Walbaum, A.W.; Wessinger, W.D.; Prather, P.L.; Crooks, P.A.; Dobretsov, M. Evaluation of analgesia, tolerance, and the mechanism of action of morphine-6-O-sulfate across multiple pain modalities in Sprague-Dawley Rats. Anesth. Analg. 2017, 125, 1021–1031.
- Balogh, M.; Zádori, Z.S.; Lázár, B.; Karádi, D.; László, S.; Mousa, S.A.; Hosztafi, S.; Zádor, F.; Riba, P.; Schäfer, M.; et al. The peripheral versus central antinociception of a novel opioid agonist: Acute inflammatory pain in rats. Neurochem. Res. 2018, 43, 1250–1257.
- Balogh, M.; Zádor, F.; Zádori, Z.S.; Shaqura, M.; Király, K.; Mohammadzadeh, A.; Varga, B.; Lázár, B.; Mousa, S.A.; Hosztafi, S.; et al. Efficacy-based perspective to overcome reduced opioid analgesia of advanced painful diabetic neuropathy in rats. Front. Pharmacol. 2019, 10, 347.
- Khalefa, B.I.; Mousa, S.A.; Shaqura, M.; Lackó, E.; Hosztafi, S.; Riba, P.; Schäfer, M.; Ferdinandy, P.; Fürst, S.; Al-Khrasani, M. Peripheral antinociceptive efficacy and potency of a novel opioid compound 14-O-MeM6SU in comparison to known peptide and non-peptide opioid agonists in a rat model of inflammatory pain. Eur.J. Pharmacol. 2013, 713, 54–57.
- Rosow, C.E. Anesthetic drug interaction: An overview. J. Clin. Anesthn. 1997, 9, 27S–32S.
- Yadlapalli, J.S.K.; Albayati, Z.A.F.; Penthala, N.R.; Hendrickson, H.P.; Crooks, H.A. Stability studies of potent opioid analgesic, morphine-6-O-sulfate in various buffers and biological matrices by HPLC-DAD analysis. Biomed. Chromatogr. 2017, 31, e3957.
- Kiraly, K.; Caputi, F.F.; Hanuska, A.; Kató, E.; Balogh, M.; Köles, L.; Palmisano, M.; Riba, P.; Hosztafi, S.; Romualdi, P.; et al. A new potent analgesic agent with reduced liability to produce morphine tolerance. Brain Res. Bull. 2015, 117, 32–38.
- Paul, D.; Standifer, K.M.; Inturrisi, C.E.; Pasternak, G.W. Pharmacological characterization of morphine-6 beta-glucuronide, a very potent morphine metabolite. J. Pharmacol. Exp. Ther. 1989, 251, 477–483.
- Osborne, R.; Joel, S.; Trew, D.; Slevin, M. Morphine and metabolite behavior after different routes of morphine administration: Demonstration of the importance of the active metabolite morphine-6-glucuronide. Clin. Pharmacol. Ther. 1990, 47, 12–19.
- Tegeder, I.; Meier, S.; Burian, M.; Schmidt, H.; Geisslinger, G.; Lötsch, J. Peripheral opioid analgesia in experimental human pain models. Brain 2003, 126, 1092–1102.
- Hanna, M.H.; Elliott, K.M.; Fung, M. Randomized, double-blind study of the analgesic efficacy of morphine-6-glucuronide versus morphine sulfate for postoperative pain in major surgery. Anesthesiology 2005, 102, 815–821.
- Binning, A.R.; Przesmycki, K.; Sowinski, P.; Morrison, L.M.M.; Smith, T.W.; Marcus, P.; Lees, J.P.; Dahan, A. A randomised controlled trial on the efficacy and side-effect profile (nausea/vomiting/sedation) of morphine-6-glucuronide versus morphine for post-operative pain relief after major abdominal surgery. Eur. J. Pain 2011, 15, 402–408.
- Sverrisdóttir, E.; Lund, T.M.; Olesen, A.E.; Drewes, A.M.; Christrup, L.L.; Kreilgaard, M. A review of morphine and morphine-6-glucuronide’s pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur. J. Pharm. Sci. 2015, 74, 45–62.
- Kilpatrick, G.J.; Smith, T.W. Morphine-6-glucuronide: Actions and mechanisms. Med. Res. Rev. 2005, 25, 521–544.
- Lötsch, J.; Geisslinger, G. Morphine-6-glucuronide: An analgesic of the future? Clin. Pharmacokinet. 2001, 40, 485–499.
- Bickel, U.; Schumacher, O.P.; Kang, Y.S.; Voigt, K. Poor permeability of morphine 3-glucuronide and morphine 6-glucuronide through the blood barrier in the rat. J. Pharmacol. Exp. Ther. 1996, 278, 107–113.
- Huwyler, J.; Drewe, J.; Klusemann, C.; Fricker, G. Evidence for P-glycoprotein-modulated penetration of morphine-6-glucuronide into brain capillary endothelium. Br. J. Pharmacol. 1996, 118, 1879–1885.
- Sattari, M.; Routledge, P.; Mashayekhi, S. The influence of active transport systems on morphine -6-glucuronide transport in MDCKII and MDCK-PGP cells. Daru 2011, 19, 412–416.
- Bourasset, F.; Cisternino, S.; Temsamani, J.; Scherrmann, J.-M. Evidence for an active transport of morphine-6-β-d-glucuronide but not P-glycoprotein-mediated at the blood–brain barrier. J. Neurochem. 2003, 86, 1564–1567.
- Koning, G.A.; Schiffelers, R.M.; Storm, G. Endothelial cells at inflammatory sites as target for therapeutic intervention. Endothelium 2002, 9, 161–171.
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884.
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52.
- Nehoff, H.; Parayath, N.N.; Domanovitch, L.; Taurin, S.; Greish, K. Nanomedicine for drug targeting: Strategies beyond the enhanced permeability and retention effect. Int. J. Nanomed. 2014, 22, 2539–2555.
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264.
- Moradkhani, M.R.; Karimi, A.; Negahdari, B. Nanotechnology application for pain therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 368–373.
- Chakravarthy, K.V.; Boehm, F.J.; Christo, P.J. Nanotechnology: A promising new paradigm for the control of pain. Pain Med. 2018, 19, 232–243.
- González-Rodríguez, S.; Quadir, M.A.; Gupta, S.; Walker, K.A.; Zhang, X.; Spahn, V. Polyglycerol-opioid conjugate produces analgesia devoid of side effects. Elife 2017, 6, e27081.
- Martin, C.; Oyen, E.; Mangelschots, J.; Bibian, M.; Ben Haddou, T.; Andrade, J.; Gardiner, J.; Van Mele, B.; Madder, A.; Hoogenboom, R.; et al. Injectable peptide hydrogels for controlled-release of opioids. MedChemComm 2016, 7, 542–549.
- Martin, C.; Oyen, E.; Van Wanseele, Y.; Haddou, T.B.; Schmidhammer, H.; Andrade, J.; Waddington, L.; Van Eeckhaut, A.; Van Mele, B.; Gardiner, J.; et al. Injectable peptide-based hydrogel formulations for the extended in vivo release of opioids. Mater. Today Chem. 2017, 3, 49–59.
