You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Da Sun.
Thyroid cancer is the most common endocrine cancer, and its prevalence has been increasing for decades. Approx. 95% of differentiated thyroid carcinomas are treated using 131iodine (131I), a radionuclide with a half-life of 8 days, to achieve optimal thyroid residual ablation following thyroidectomy. However, while 131I is highly enriched in eliminating thyroid tissue, it can also retain and damage other body parts (salivary glands, liver, etc.) without selectivity, and even trigger salivary gland dysfunction, secondary cancer, and other side effects.
- thyroid cancer
- 131I
- oxidative stress
- antioxidant
1. Introduction
Thyroid cancer is a malignant tumor of the endocrine gland that arises from the follicular or parafollicular epithelium of the thyroid gland. As a result of an increased use of diagnostic imaging and surveillance, the incidence of thyroid cancer has been steadily increasing worldwide, with more than 62,000 new cases diagnosed each year [1,2,3][1][2][3]. The most frequent kind of thyroid cancer is differentiated thyroid carcinoma (DTC), which accounts for more than 95% of cases [4,5][4][5]. Thyroidectomy, lymph node dissection, and 131I therapy are the primary therapeutic options [6]. In clinical practice, 131iodine (131I), a γ/β radiation radionuclide with a half-life of 8 days, can accumulate in thyroid tissue. As shown in Figure 1, it is commonly used to ablate residual thyroid tissue after surgery (known as thyroid remnant ablation) to reduce the likelihood of local recurrence, treat metastatic disease, and clear hidden thyroid cancer cells [7,8,9][7][8][9]. Iodine-131 is also used as a means of addressing persistent disease as reflected by the thyroid globulin levels [10], with a typical dosage range of 1110 MBq (30 mCi) to 3700 MBq (100 mCi) [11].
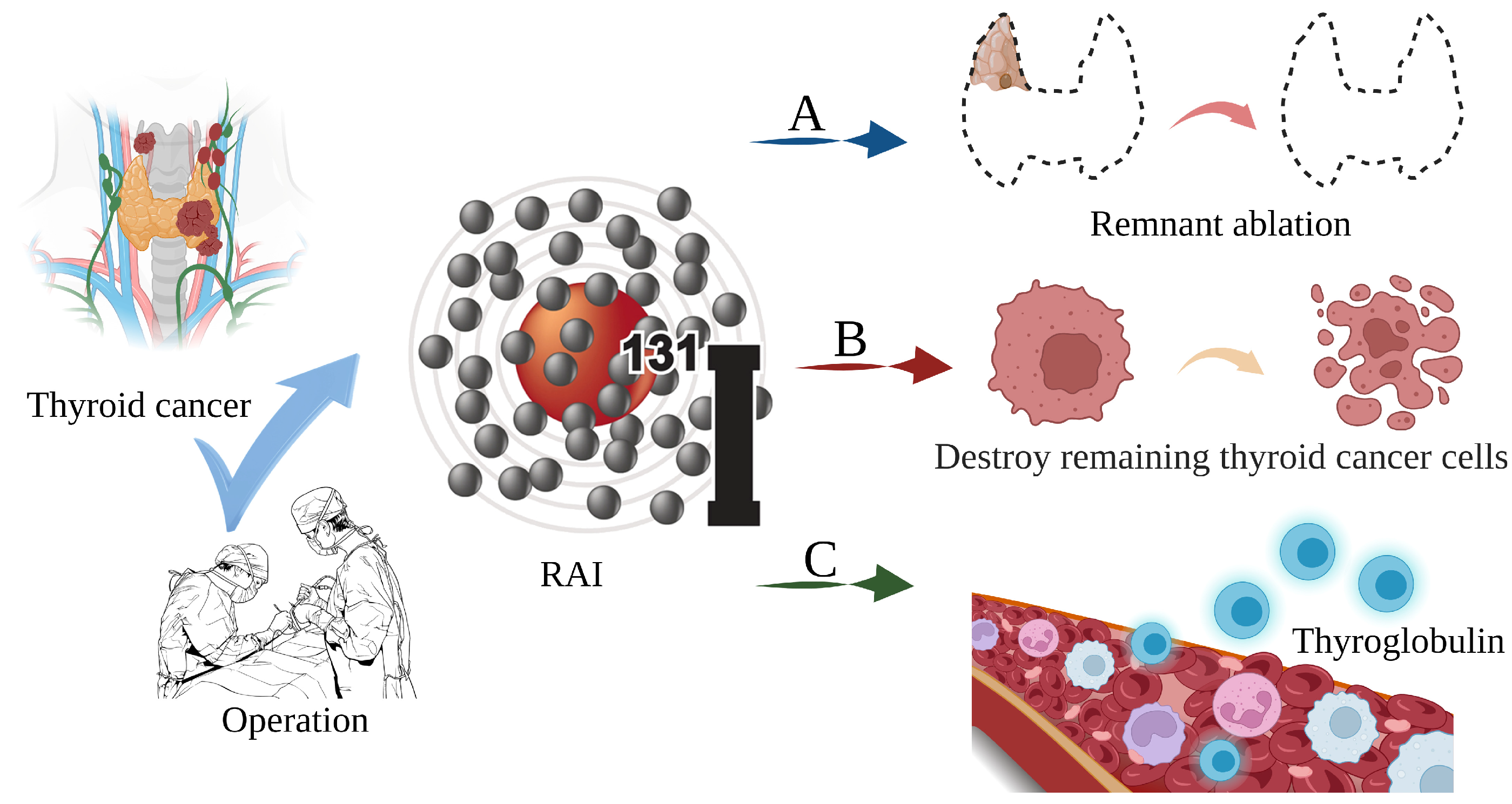
Figure 1. The main role of 131I in the treatment of thyroid cancer. (A) Thyroid remnant ablation for reducing the likelihood of local recurrence; (B) Treating metastatic disease and clearing hidden thyroid cancer cells; (C) As a means of addressing persistent disease as reflected by thyroid globulin levels.
However, there is evidence that 131I γ/β radiation interferes with the REDOX cell signaling pathways, causing an imbalance between cellular oxidants and antioxidants, resulting in systemic oxidative stress, cell and tissue damage, and an increase in the risk of genetic DNA damage and secondary cancer [12,13,14,15][12][13][14][15]. Furthermore, it can cause side effects, including salivary gland dysfunction, gastrointestinal reactions, dry eye, pulmonary fibrosis, gonad damage, nasolacrimal duct obstruction, secondary cancer, permanent myelosuppression, and genetic effects [16,17][16][17]. To achieve optimal effectiveness and minimize discomfort in thyroid cancer patients, adjuvant medication combinations that reduce the adverse effects of 131I are required.
Antioxidants are chemicals that bind free radicals and drastically decrease or prevent substrate oxidation [18,19][18][19]. They limit free radical damage by blocking free radicals from damaging lipids, protein amino acids, polyunsaturated fatty acids, and the double bonds of DNA bases [20,21,22][20][21][22]. Notably, substances such as β-carotene and vitamin E have been proven to dramatically minimize the negative effects of 131I [23,24][23][24].
3. Oxidative Stress Dominates 131I Side Effects
RAI is the standard and effective treatment for DTC. The thyroid gland can accumulate iodine at up to 40 times the concentration of plasma under physiological conditions. This relies on the NIS located in the basolateral membrane of thyrocytes using the electrochemical gradient generated by the Na,K-ATPase as the driving forces that coordinate with the KCNQ1-KCNE2 K+ channels located in the basolateral membrane These promote the potassium efflux, thus facilitating iodine transport into the intracellular compartments, and thereby increasing the oxidative stress and cytotoxic efficacy from the radioactivity [39,48,49,50][25][26][27][28]. Oxidative stress is the result of increased free radical production and/or a decreased antioxidant defense system physiological activity [51,52][29][30]. Each cell in a living organism maintains a reductive environment. The reducing environment is maintained by enzymes, which provide constant metabolic energy input to maintain the reducing state [53,54][31][32]. This disruption of the normal reduction oxidation (REDOX) state can be mediated by the generation of peroxide-reactive radicals (hydrogen peroxide (H2O2), superoxide (O2−), singlet oxygen (1/2O2), ROS, and the hydroxyl radical (∙OH). The abnormal expression of these substances may result in the destruction of all the components of the cell, resulting in toxic effects [55,56,57][33][34][35]. Severe cases can lead to cell death (Figure 32A). The damage can involve multiple parts throughout the body (Figure 32B).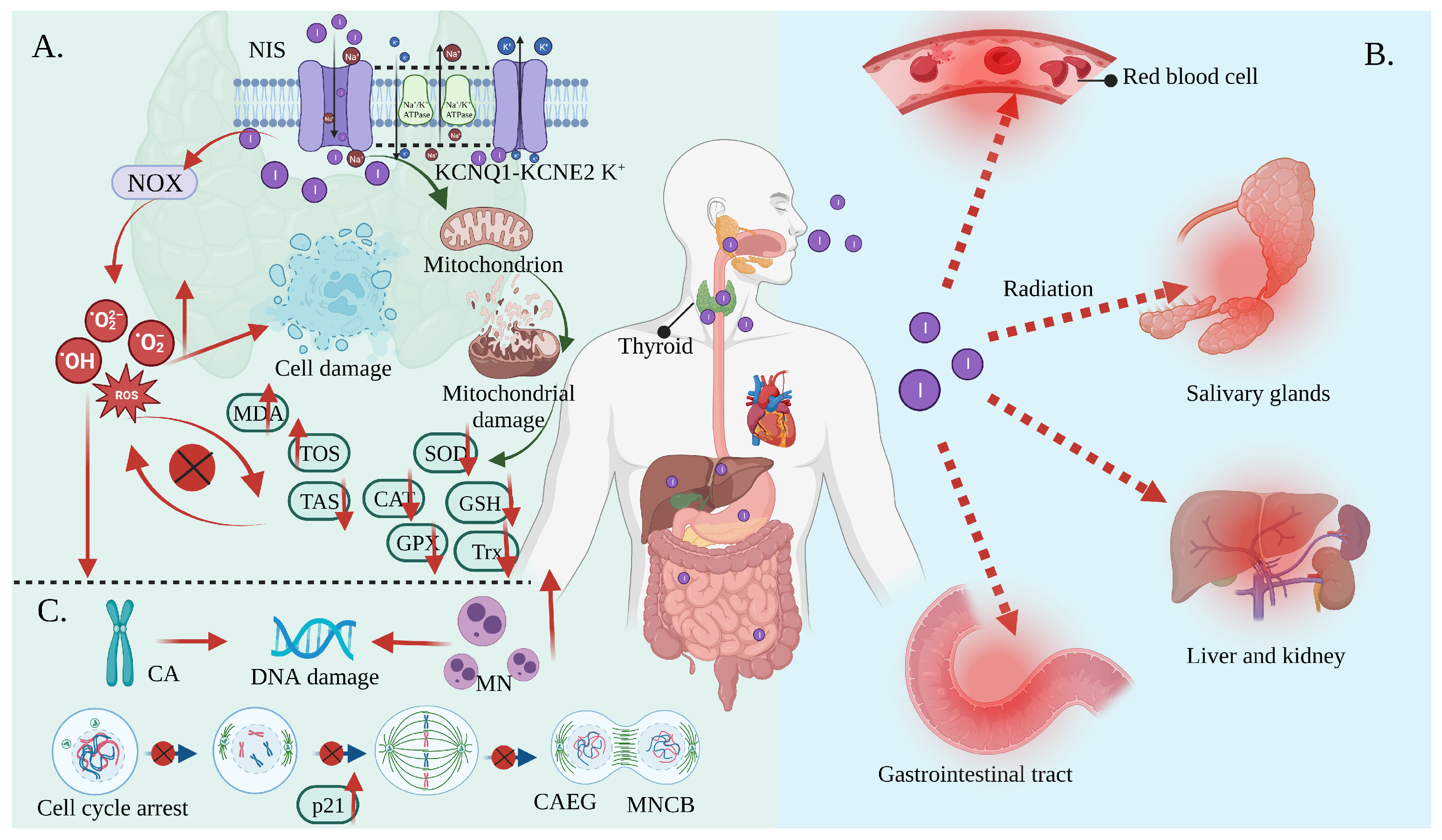
Figure 32. Oxidative stress mediates the side effects of 131I. (A) Iodine-131 enters the cells through the synergistic transport of the NIS and KCNQ1-KCNE2 K+ transporter, and thus increases the expression of NOX1 and changes the ultrastructure of the mitochondria through β/γ radiation, resulting in a reduced antioxidant capacity and the production of numerous ROS. As a result, the activities of CAT and SOD are decreased; the levels of GSH, GPx, Trx, and TAS are decreased; and the levels of MDA and the total oxidative stress (TOS) are increased, leading to systemic oxidative stress. (B) Oxidative stress induces erythrocyte membrane damage and vascular permeability changes, salivary gland dysfunction, and gastrointestinal tract and liver and kidney injury. (C) Oxidative stress induces a CA and MN increase and mediates a significant increase in the frequency of MNCB, CAEG, and bicentric chromosomes.
3. Antioxidants Reduce 131I Side Effects
In general, it can be observed that oxidative stress mediates the pathological process of almost all the 131I side effects. Herein, the antioxidants showed a robust effectiveness against their side effects. The antioxidants that have been proven to alleviate the side effects of 131I are shown in Figure 43 and the drug type, drug treatment, subject, dose, side effects, and drug efficacy are summarized in Table 21.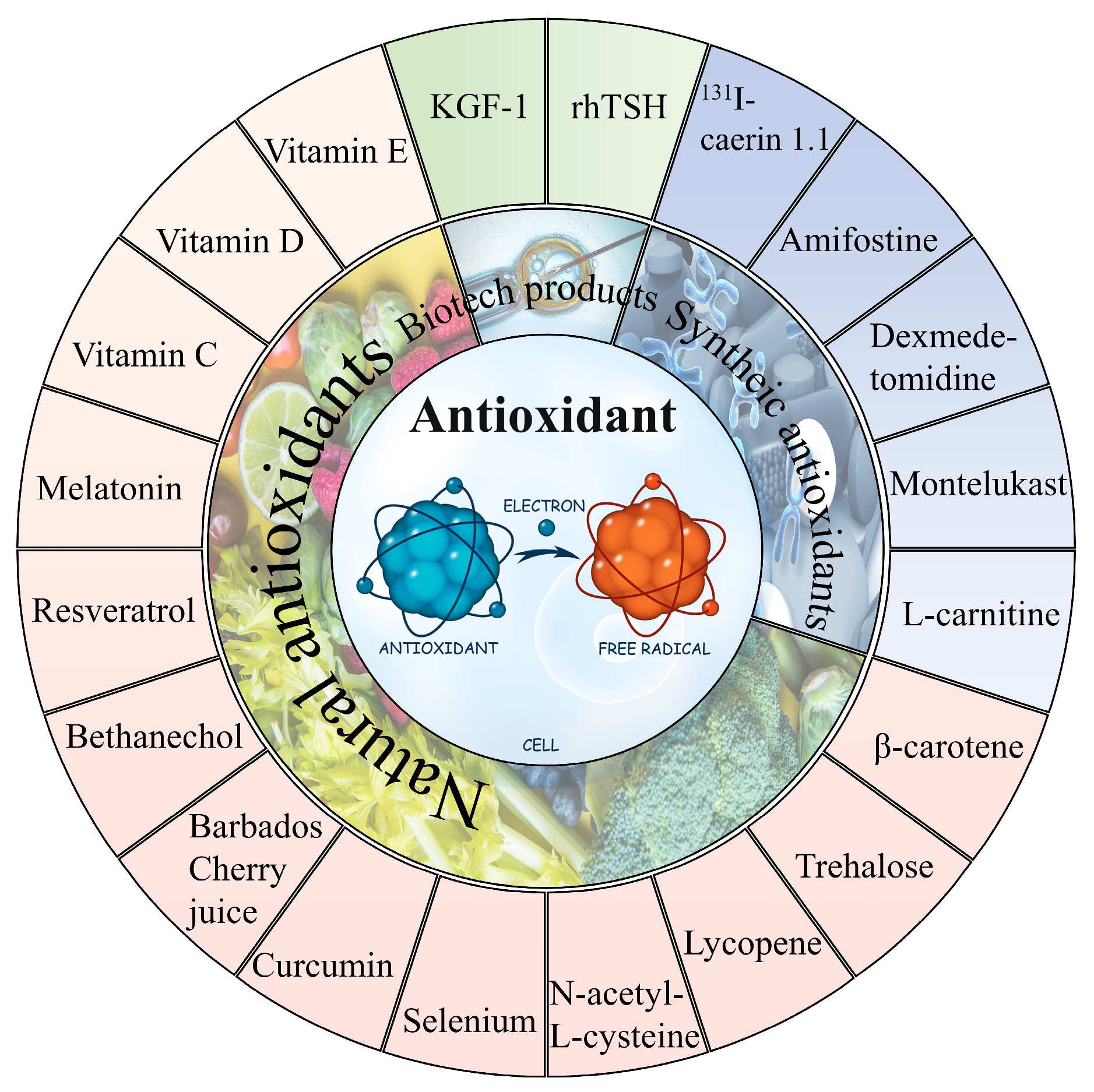
Figure 43.
The natural and synthetic antioxidants applied to combat
131
I side effects.
Table 21. The applications of various antioxidants to alleviate the side effects of 131I. (8-Epi-prostaglandin F2alpha (8-epi-PGF2α); uptake fraction (UF); uptake index (UI); excretion fraction (EF); excretion ratio (ER); first-minute uptake ratio (FUR); maximum uptake ratio (MUR); hypoxia inducible factor-1α (HIF-1α)).
| Drug Type | Drug Treatment | Subject | Dose of 131I | Side Effects of 131I | Drug Efficacy | Ref. |
|---|---|---|---|---|---|---|
| Natural antioxidant | Daily supplementation consisting of 2000 mg vitamin C and 1000 mg vitamin E and 400 µg selenium for 21 days before 131I | Forty patients with thyroid cancer submitted for thyroidectomy (n = 20) | 3.7 GBq | 8-epi-PGF2α↑ | 8-epi-PGF2α↓ | [86][54] |
| 1500 mg vitamin C daily 2 days after (group 2), 2 days before to 2 days after (group 3), and 2 days before RAI (group 4) | Fifty-eight DTC patients ablated with 131I | 5550 MBq | MDA, CAT↑; GSH↓ | MDA↓ (group 2,3,4); GSH↑ (group 3,4); CAT↓ (group 3,4) | [22] | |
| Groups A, B, and C received vitamin E 100, 200, and 300 mg/day orally, respectively, for a duration of 1 week before to 4 weeks after I therapy | Eighty-two DTC patients with 131I | 100 mCi | UF, UI, EF, and ER↓ | UI, EF, UF, ER↑ | [87][55] | |
| Vitamin D (200 ng/kg/day) | Wistar albino rats (n = 12) | 111 MBq/kg | TOS, TNF-α, IL-6↑; IL-10, TAS↓ | TOS, TNF-α, IL-6↓; IL-10, TAS ↑ | [88][56] | |
| Vitamin E (800 IU/day for one week before and four weeks after RAI therapy) | Thirty-six DTC patients with RAI (n = 18) | 3700–5550 MBq | FUR, MUR, MSP, and EF↓ | FUR, MUR, MSP, and EF↑ | [43][57] | |
| Bethanechol (2 mg orally twice a day) for one month after 131I | Fifty DTC patients with RAI (n = 25) | 97.2 to 213.4 mCi | MUR, MSP, ΔMS, EF↓ | Serum amylase↓ | ||
| Selenium 300 mcg orally for ten days (from three days before until six days after RAI therapy) | Sixteen DTC patients with RAI (n = 8) | 3.7 GBq | Xerostomia, sialadenitis symptoms↑ | Xerostomia, sialadenitis symptoms↓ | ||
| KGF-1 (100 ug/1 mL PBS) | Eighteen C57BL/six mice (n = 6) | 0.01 mCi/g | HIF-1α↑; mucin stained acini, amylase↓; periductal fibrosis↑ | HIF-1α↓; mucin stained acini, amylase↑; periductal fibrosis↓ | [89][58] | |
| 50 μg curcumin per mL of blood and 5.738 mg trehalose per mL of blood | Blood of five humans | 20 μCi | DSB increased to 102.9% | DSBs decreased by 42% (curcumin) and 38% (trehalose) | [84][59] | |
| 0.0167 mg melatonin per mL of blood and 0.025 mg Se NPs per mL of blood | Blood of five humans | 20 μCi | DSB increased to 102.9% | DSBs decreased by 38% (melatonin) and 30% (selenium nanoparticles) | [90][60] | |
| 0.0666 mg vitamin E per mL of blood and 0.0167 mg vitamin C per mL of blood | Blood of five humans | 20 μCi | DSB increased to 102.9% | DSBs decreased by 21.5% (vitamin E) and 36.4% (vitamin C) | [23] | |
| Barbados Cherry juice (5 mg)/100 g | Wistar rats (n = 6) | 25 μCi/100 g | 1,1-diphenyl-2-picrylhydrazyl↑; chromosomal and cellular aberrations↑ | 1,1-diphenyl-2-picrylhydrazyl↑; chromosomal and cellular aberrations↑ | [91][61] | |
| 20 mmol N-acetyl-L-cysteine | Normal differentiated rat thyroid cell line PCCL3 | 10 μCi/mL | ROS, DBS, MN↑ | ROS, DBS, MN↓ | [92][62] | |
| 8 mg β-carotene/mL corn oil (0.2 mL/100 g) | Wistar rats (n = 6) | 25 μCi /100 g body weight |
CA, MN, water consumption↑ | CA, MN, water consumption↓ | [12] | |
| 20 mg/kg/day resveratrol | Thirty Wistar albino rats (n = 10) | 3 mCi/kg | Caspase-3, TUNEL, TNF-α, IL-6, nuclear factor-kappa-B (NF-кB), TOS↑; IL-10, TAS↓ | Caspase-3, TUNEL, TNF-α, IL-6, NF-кB, TOS↑; TAS↓ | [93][63] | |
| 1 mL lycopene (5 mg/kg body weight) |
Twenty Wistar albino rats (n = 10) | 3 mCi | Duodenal and ileal lamina propria edema, duodenal ulcer, gastric mucosal erosion, and gastric and colon mucosal degeneration↑ | Duodenal and ileal lamina propria edema, duodenal ulcer, gastric mucosal erosion, gastric and colon mucosal degeneration↓ | [94][64] | |
| Synthetic antioxidants | 200 mg/kg amifostine or L-carnitine | Forty adult guinea pigs | 555–660 MBq | Body weight and thyroid hormone↓ | Body weight and thyroid hormone↑ | [34][65] |
| 200 mg/kg amifostine to three rabbits/500 mg/m2 amifostine before 131I to eight patients | Five rabbits/17 patients | 1 GBq to rabbits/6 GBq to patients | Reduced parenchymal function in parotid and submandibular glands; xerostomia; lipomatosis | None of the parenchymal function in parotid and submandibular glands reduce, xerostomia and lipomatosis occurred | [95][66] | |
| rhTSH (1 mg/2 d and 1 mg/1 d before 131I) | Sixty-two patients prepared with rhTSH or by thyroid hormone withdrawal | 1850 MBq | CA, MN, ROS↑ | CA, MN, ROS↓ | [13] | |
| 8 μg of F1 peptide labeled with 200 μCi 131I every 3 days for a total of three times | Nude mice with human anaplastic thyroid cancer | 200 μCi | Weight loss and 131I enter the internal circulation | Constant weight | [96][67] | |
| Dexmedetomidine (3 μg/kg) | Thirty-six Wistar albino female rats (n = 12) |
111 MBq | MDA, advanced oxidized protein products↑, total sulfur group, CAT↓ | MDA, advanced oxidized protein products↓; total sulfur group, CAT↑; liver protection | [97][68] | |
| Montelukast (10 mg/kg/day) | Fifty female Wistar albino rats (n = 10) | 111 MBq/kg | Inflammation and pulmonary fibrosis | Reduced the degree of inflammation and pulmonary fibrosis | [45][69] |
3.1. Natural Antioxidant
Natural antioxidants, sourced mostly from plants, counteract radiation by neutralizing the free radicals produced in the body when it is exposed to the radiation [98,99][70][71]. The mechanism of action generally involves scavenging free radicals and preventing them from damaging cells, tissues, and DNA. As a result, they are capable of shielding the organism cells from damage and aiding in the prevention of cancer and other health problems associated with exposure to radiation [100][72]. One of the advantages of natural antioxidants is that they are safer than synthetic antioxidants and have been utilized in conventional medicine for centuries. Furthermore, natural antioxidants are metabolized by the body into harmless compounds, most of which are excreted through normal metabolic processes and are more easily tolerated [101,102][73][74]. Vitamin C as ascorbic acid regulates the activity of the glutamate receptors, lowering the level of free radicals produced by the glutamate release, and has been proven to reduce the frequency of chromosomal aberrations by approximately 30%, significantly reduces the number of DNA breaks, and has a repairing effect on DNA [103,104][75][76].Vitamin C reacts directly with alkoxyl, hydroxyl, and lipid peroxyl radicals or neutralizes them and converts them into water, alcohols, and hydroperoxylated lipids, respectively. Importantly, studies have indicated that vitamin C has a radioprotective effect against oxidative stress, regardless of the timing of administration before and after RAI treatment [43][57]. Vitamin C in plasma leads to an increased resistance to lipid peroxidation and a decrease in DNA, lipid, and protein oxidation. In addition, vitamin C leads to the neutralization of free radicals of other antioxidants in the form of glutathione and vitamin E, as well as their regeneration. Approx. 2 days after RAI (5550 MBq), the MDA levels and CAT activity declined and the GSH levels decreased, while the daily administration of 1500 mg vitamin C starting two days before significantly reduced the MDA levels and not only prevented the reduction in GSH, but also significantly increased its levels after RAI treatment [22]. Additionally, vitamin E is the collective term for four tocopherols (α-, β-, γ-, and δ-tocopherols) and four tocotrienols (α-, β-, γ-, and δ-tocotrienols) found in food, and is a lipid-soluble antioxidant that protects polyunsaturated fatty acids in the membranes from oxidation, regulates the production of reactive oxygen species and reactive nitrogen species, and modulates the signal transduction [73][51]. The significant protective effect of vitamin E on the parotid and submandibular glands after 131I (23 mCi) treatment with DTC has been published [87[55][77],105], which was comparable to the results of Filiz Aydoğan et al. [106][78]. RAI (111 MBq/kg) resulted in a significant increase in the tissue TOS, TNF-α, IL-6 levels and a significant decrease in the IL-10 and TAS levels, while vitamin D (200 ng/kg/day) dramatically reversed all these parameters [88][56]. Meanwhile, sialogogues such as lemon candy, vitamin E, lemon juice, and lemon slices as well as parotic gland massages may all minimize injury to the salivary glands [10]. Parotid massages, aromatherapy, vitamin E, selenium, and bethanechol showed a significant reduction in the salivary gland dysfunction induced from the 131I treatment (2960–7890 MBq) [43][57]. Additionally, keratinocyte growth factor-1 (KGF-1) (100 μg/1 mL PBS) restored saliva homeostasis and reduced the 131I-induced (0.01 mCi/g) cell apoptosis in the mice [90][60]. A marker of lipid peroxidation, 8-Epi-PGF2α, is the outcome of free radical-mediated arachidonic acid peroxidation, and the effect of high-activity treatment (2960 or 7400 MBq) is significantly higher and longer in length than that of low-activity treatment (185 or 740 MBq), with a dose-dependent oxidative damage in vivo [107][79]. In the research of Rosário et al., the 8-epi-PGF2α concentrations were significantly higher in thyroid cancer patients 2 days before and 7 days after the 131I injection, and the increase (percentage) was significantly larger (mean 112.3% vs. 56.3% compared to the intervention group). Iodine-131 (3.7 GBq) after 2 days of plasma 8-epi-PGF2α significantly increased, while the daily intake of 2000 mg of vitamin C, 1000 mg of vitamin E, and 400 µg of selenium for 21 days before RAI treatment significantly reduced 8-epi-PGF2α and inhibited oxidative stress [86][54]. In terms of the protection against DNA damage, the use of curcumin and alginate as antioxidants reduced the number of DSBs caused by 131I. At the same time, the radiation protection effect of curcumin exceeded that of trehalose [84][59]. Melatonin and Se NPs (as radioprotective agents) reduced the 131I-induced DSBs levels in peripheral lymphocytes [90][60]. Vitamins E and C were capable of reducing the DSBs levels by 21.5% and 36.4%, respectively [23]. The positive results of the Barbados cherry fruit radiation protection may be due in part to its rich content of antioxidant compounds, including vitamins A, B1, B2 and C; carotenoids; anthocyanins; phenols; and flavonoids. The 131I (25 μCi) treatment of Wistar rats with an increased thyroid function and associated vitamins and sugars from the Barbados cherry fruit stimulated a significant increase in the mitotic index in the normal cells of the rat bone marrow. In particular, the Barbados cherry juice (5 mg) may act as an effective scavenger of the reactive oxygen species in acute radiation protection treatment, protecting the cells by neutralizing free radicals before and during treatment. Meanwhile, it may play a role in the healing process of ionizing radiation-induced damage after treatment. Barbados cherry sub-chronic treatment has higher radioprotective activity in terms of trapping free radicals or preventing their formation [91][61]. N-acetyl-L-cysteine has also been demonstrated to guard against an increase in ROS and eventual DNA damage in thyroid cells caused by 131I in vivo [92][62]. Before, during, and after 131I treatment, β-carotene exerts a significant anti-mutagenic/radioprotective activity, stimulates the DNA repair systems, and minimizes chromosomal aberrations and genetic material damage [12]. Apart from this, resveratrol had anticancer and antioxidant effects, protected the histopathological pattern of the lacrimal gland from damage, reduced inflammation in the histopathological assessment, and decreased the histocytokine levels, apoptosis, and DNA fragmentation on the lacrimal gland after RAI [93][63]. Iodine-131 caused an edema of the duodenum and ileum lamina propria, duodenal ulceration, gastric mucosal erosion, and gastric and colonic mucosal degeneration in the rats, whereas lycopene resulted in a statistically corresponding reduction in the inflammation present [94][64].3.2. Synthetic Antioxidants
Synthetic antioxidants have advantages in radiation protection due to their greater potency, consistency, stability, and application flexibility. Despite the fact that natural substances have been used in traditional medicine for centuries, their variability, lack of specificity, and instability require modifications to their properties [108,109][80][81]. Accordingly, synthetic substances offer a reliable and effective way to protect against the harmful effects of radiation. Thus, further research and development is required to create more effective radiation protection, safer synthetic substances for human consumption, and to determine the safe limits for their applications [110,111,112][82][83][84]. However, it is important to note that synthetic antioxidants can frequently cause adverse health effects when used in high doses [113][85]. Iodine-131 (555–660 MBq) treatment with 200 mg/kg L-carnitine or amifostine for 10 days can provide radiation protection and reduce salivary gland injury [34][65]. Amifostine is an organic thiophosphate, which is dephosphorylated to the active metabolite WR-1065 in normal tissues. Once activated in the cells, WR-1065 acts as a free radical scavenger. Additionally, many studies have reported the radiation-proof effect on 131I treatment [35,114][86][87]. Iodine-131 causes transient unstable DNA damage composed of reactive oxygen-induced SSBs, and increased chromosome damage in hypothyroidism patients (mutations in enzymes deputed to DNA repair (DNA-1) or in the enzymes involved in the scavenging of free oxygen radicals (DNA-2)). The rhTSH administration reduced radiation exposure by 27% over 120 h and decreased the genomic instability by maintaining hyperthyroidism and normal renal clearance (Epi-GFR and creatinine values). It significantly induced a reduction in the reactive oxygen metabolites-derived compounds. The patients had less radiation-induced chromosome damage, even though several enzyme mutations were present [13]. Lin et al. prepared a drug delivery system with 131I-labeled caerin 1.1 peptide (F1) (200 μCi 131I and 8 μg caerin 1.1 peptide). The MTT results showed that 5 μg F1 had an inhibitory effect on the CAL-62 cells cultured in vitro. Interestingly, studies identified weight loss over time in the 131I treatment group in vivo, but not in the 131I-F1 or F1 groups. It is possible that 131I-F1 or F1 was confined to the tumor after injection, while 131I may have entered the microcirculation through the blood vessels within the tumor and then entered the internal circulation. In view of the fact that radiation entering the human body can cause acute injury, the occurrence of acute radiation sickness or syndrome characterized by weight loss suggests that 131I-F1 is safer with fewer side effects [96][67]. Additionally, synthetic drugs have been studied for the treatment of other side effects. Treatment with dexmedetomidine (3 μg/kg) significantly decreased the levels of MDA, advanced the oxidized protein products induced by RAI (2 MBq), significantly increased the levels of the total sulfur group and CAT, and reduced histopathological abnormalities, which could be applied as a post-131I liver protection regimen [97][68]. In the case of RAI, a high absorbed dose may be produced in the lung parenchyma, thus causing lung damage [115][88]. Montelukast (10 mg/kg/day) significantly reduced the degree of inflammation and pulmonary fibrosis in the Wistar rats treated with 131I (111 MBq/kg). The authors attributed this protective effect in part to the antioxidant effect of montelukast [45][69].3.3. Antioxidant Deficiency
In summary, the application of the above antioxidants will hopefully play an important role in alleviating the side effects of 131I. It is important to highlight that even when the use of antioxidants has been shown to ameliorate the side effects of 131I therapy, there are also reports on the drawbacks of using them. Some antioxidants induce oxidative stress at high concentrations (e.g., β-carotene) [24]. Meanwhile, it has been reported that an excessive vitamin E intake can affect the absorption and function of other fat-soluble vitamins [116][89]. Furthermore, synthetic antioxidants have been reported to cause potential health hazards, including liver damage and cancer [117,118,119][90][91][92]. Therefore, further investigation is needed at a pre-clinical level to standardize the use of antioxidants as adjuvants for 131I treatment.References
- Buczyńska, A.; Sidorkiewicz, I.; Rogucki, M.; Siewko, K.; Adamska, A.; Kościuszko, M.; Maliszewska, K.; Kozłowska, G.; Szumowski, P.; Myśliwiec, J.; et al. Oxidative stress and radioiodine treatment of differentiated thyroid cancer. Sci. Rep. 2021, 11, 17126.
- Tuttle, R.M. Controversial Issues in Thyroid Cancer Management. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 1187–1194.
- Grimm, D. Recent Advances in Thyroid Cancer Research. Int. J. Mol. Sci. 2022, 23, 4631.
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795.
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid cancer. Lancet 2023, 401, 1531–1544.
- Shao, C.; Li, Z.; Zhang, C.; Zhang, W.; He, R.; Xu, J.; Cai, Y. Optical diagnostic imaging and therapy for thyroid cancer. Mater. Today Bio 2022, 17, 100441.
- Ma, C.; Xie, J.; Liu, W.; Wang, G.; Zuo, S.; Wang, X.; Wu, F. Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer. Cochrane Database Syst. Rev. 2010, 2010, CD008302.
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2.
- Jin, Y.; Ruan, M.; Cheng, L.; Fu, H.; Liu, M.; Sheng, S.; Chen, L. Radioiodine Uptake and Thyroglobulin-Guided Radioiodine Remnant Ablation in Patients with Differentiated Thyroid Cancer: A Prospective, Randomized, Open-Label, Controlled Trial. Thyroid Off. J. Am. Thyroid Assoc. 2019, 29, 101–110.
- Christou, A.; Papastavrou, E.; Merkouris, A.; Frangos, S.; Tamana, P.; Charalambous, A. Clinical Studies of Nonpharmacological Methods to Minimize Salivary Gland Damage after Radioiodine Therapy of Differentiated Thyroid Carcinoma: Systematic Review. Evid. Based Complement. Altern. Med. ECAM 2016, 2016, 6795076.
- Monteiro Gil, O.; Oliveira, N.G.; Rodrigues, A.S.; Laires, A.; Ferreira, T.C.; Limbert, E.; Léonard, A.; Gerber, G.; Rueff, J. Cytogenetic alterations and oxidative stress in thyroid cancer patients after iodine-131 therapy. Mutagenesis 2000, 15, 69–75.
- Berti, A.P.; Düsman, E.; Mariucci, R.G.; Lopes, N.B.; Vicentini, V.E.P. Antimutagenic and radioprotective activities of beta-carotene against the biological effects of iodine-131 radiopharmaceutical in Wistar rats. Genet. Mol. Res. GMR 2014, 13, 2248–2258.
- Signore, A.; Campagna, G.; Marinaccio, J.; de Vitis, M.; Lauri, C.; Berardinelli, F.; Tofani, A.; Chianelli, M.; Borro, M.; Gentile, G.; et al. Analysis of Short-Term and Stable DNA Damage in Patients with Differentiated Thyroid Cancer Treated with 131I in Hypothyroidism or with Recombinant Human Thyroid-Stimulating Hormone for Remnant Ablation. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1515–1522.
- Qian, R.; Wang, K.; Guo, Y.; Li, H.; Zhu, Z.; Huang, X.; Gong, C.; Gao, Y.; Guo, R.; Yang, B.; et al. Minimizing adverse effects of Cerenkov radiation induced photodynamic therapy with transformable photosensitizer-loaded nanovesicles. J. Nanobiotechnol. 2022, 20, 203.
- Efanov, A.A.; Brenner, A.V.; Bogdanova, T.I.; Kelly, L.M.; Liu, P.; Little, M.P.; Wald, A.I.; Hatch, M.; Zurnadzy, L.Y.; Nikiforova, M.N.; et al. Investigation of the Relationship Between Radiation Dose and Gene Mutations and Fusions in Post-Chernobyl Thyroid Cancer. JNCI J. Natl. Cancer Inst. 2017, 110, 371–378.
- Ish-Shalom, S.; Durleshter, L.; Segal, E.; Nagler, R.M. Sialochemical and oxidative analyses in radioactive I131-treated patients with thyroid carcinoma. Eur. J. Endocrinol. 2008, 158, 677–681.
- Fard-Esfahani, A.; Emami-Ardekani, A.; Fallahi, B.; Fard-Esfahani, P.; Beiki, D.; Hassanzadeh-Rad, A.; Eftekhari, M. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl. Med. Commun. 2014, 35, 808–817.
- Zeng, C.; Feng, S. The Antioxidant Capacity In Vitro and In Vivo of Polysaccharides From Bergenia emeiensis. Int. J. Mol. Sci. 2020, 21, 7456.
- Carsono, N.; Tumilaar, S.G.; Kurnia, D.; Latipudin, D.; Satari, M.H. A Review of Bioactive Compounds and Antioxidant Activity Properties of Piper Species. Molecules 2022, 27, 6774.
- Skała, E.; Sitarek, P.; Różalski, M.; Krajewska, U.; Szemraj, J.; Wysokińska, H.; Śliwiński, T. Antioxidant and DNA Repair Stimulating Effect of Extracts from Transformed and Normal Roots of Rhaponticum carthamoides against Induced Oxidative Stress and DNA Damage in CHO Cells. Oxid. Med. Cell. Longev. 2016, 2016, 5753139.
- Coskun, M.; Kayis, T.; Gulsu, E.; ALP, E. Effects of Selenium and Vitamin E on Enzymatic, Biochemical, and Immunological Biomarkers in Galleria mellonella L. Sci. Rep. 2020, 10, 9953.
- Jafari, E.; Alavi, M.; Zal, F. The evaluation of protective and mitigating effects of vitamin C against side effects induced by radioiodine therapy. Radiat. Environ. Biophys. 2018, 57, 233–240.
- Safaei, M.; Jafarpour, S.M.; Mohseni, M.; Salimian, M.; Akbari, H.; Karami, F.; Aliasgharzadeh, A.; Farhood, B. Vitamins E and C Prevent DNA Double-strand Breaks in Peripheral Lymphocytes Exposed to Radiations from Iodine-131. Indian J. Nucl. Med. IJNM Off. J. Soc. Nucl. Med. India 2018, 33, 20–24.
- Almeida, I.V.; Düsman, E.; Heck, M.C.; Pamphile, J.A.; Lopes, N.B.; Tonin, L.T.D.; Vicentini, V.E.P. Cytotoxic and mutagenic effects of iodine-131 and radioprotection of acerola (Malpighia glabra L.) and beta-carotene in vitro. Genet. Mol. Res. GMR 2013, 12, 6402–6413.
- Filetti, S.; Bidart, J.M.; Arturi, F.; Caillou, B.; Russo, D.; Schlumberger, M. Sodium/iodide symporter: A key transport system in thyroid cancer cell metabolism. Eur. J. Endocrinol. 1999, 141, 443–457.
- Cazarin, J.; Dupuy, C.; Pires de Carvalho, D. Redox Homeostasis in Thyroid Cancer: Implications in Na+/I− Symporter (NIS) Regulation. Int. J. Mol. Sci. 2022, 23, 6129.
- Purtell, K.; Paroder-Belenitsky, M.; Reyna-Neyra, A.; Nicola, J.P.; Koba, W.; Fine, E.; Carrasco, N.; Abbott, G.W. The KCNQ1-KCNE2 K+ channel is required for adequate thyroid I− uptake. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 3252–3259.
- Pesce, L.; Kopp, P. Iodide transport: Implications for health and disease. Int. J. Pediatr. Endocrinol. 2014, 2014, 8.
- Dong, Y.; Hou, Q.; Sun, M.; Sun, J.; Zhang, B. Targeted Isolation of Antioxidant Constituents from Plantago asiatica L. and In Vitro Activity Assay. Molecules 2020, 25, 1825.
- Kang, J.S.; Han, M.H.; Kim, G.-Y.; Kim, C.M.; Kim, B.W.; Hwang, H.J.; Choi, Y.H. Nrf2-Mediated HO-1 Induction Contributes to Antioxidant Capacity of a Schisandrae Fructus Ethanol Extract in C2C12 Myoblasts. Nutrients 2014, 6, 5667–5678.
- Ji, H.; Peng, R.; Jin, L.; Ma, J.; Yang, Q.; Sun, D.; Wu, W. Recent Advances in ROS-Sensitive Nano-Formulations for Atherosclerosis Applications. Pharmaceutics 2021, 13, 1452.
- Sangsefidi, Z.S.; Yaghoubi, F.; Hajiahmadi, S.; Hosseinzadeh, M. The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 2020, 8, 1766–1776.
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792.
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435.
- Lei, P.; Chen, H.; Ma, J.; Fang, Y.; Qu, L.; Yang, Q.; Peng, B.; Zhang, X.; Jin, L.; Sun, D. Research progress on extraction technology and biomedical function of natural sugar substitutes. Front. Nutr. 2022, 9, 952147.
- Yang, N.; Guan, Q.-W.; Chen, F.-H.; Xia, Q.-X.; Yin, X.-X.; Zhou, H.-H.; Mao, X.-Y. Antioxidants Targeting Mitochondrial Oxidative Stress: Promising Neuroprotectants for Epilepsy. Oxid. Med. Cell. Longev. 2020, 2020, 6687185.
- Norberg, L.E.; Lundquist, P.G. An ultrastructural study of salivary gland radiosensitivity after alpha-adrenergic stimulation. Auris. Nasus. Larynx 1988, 15, 1–17.
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 532–553.
- Fang, Y.; Ma, J.; Lei, P.; Wang, L.; Qu, J.; Zhao, J.; Liu, F.; Yan, X.; Wu, W.; Jin, L.; et al. Konjac Glucomannan: An Emerging Specialty Medical Food to Aid in the Treatment of Type 2 Diabetes Mellitus. Foods 2023, 12, 363.
- Shi, J.; Wu, P.; Sheng, L.; Sun, W.; Zhang, H. Ferroptosis-related gene signature predicts the prognosis of papillary thyroid carcinoma. Cancer Cell Int. 2021, 21, 669.
- Yuan, Z.; Liu, T.; Huo, X.; Wang, H.; Wang, J.; Xue, L. Glutamine Transporter SLC1A5 Regulates Ionizing Radiation-Derived Oxidative Damage and Ferroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 3403009.
- Lin, R.; Fogarty, C.E.; Ma, B.; Li, H.; Ni, G.; Liu, X.; Yuan, J.; Wang, T. Identification of ferroptosis genes in immune infiltration and prognosis in thyroid papillary carcinoma using network analysis. BMC Genom. 2021, 22, 576.
- Kaur, V.; Goyal, A.K.; Ghosh, G.; Chandra Si, S.; Rath, G. Development and characterization of pellets for targeted delivery of 5-fluorouracil and phytic acid for treatment of colon cancer in Wistar rat. Heliyon 2020, 6, e03125.
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110.
- Díaz-Cubilla, M.; Letón, P.; Luna-Vázquez, C.; Marrón-Romera, M.; Boltes, K. Effect of Carbamazepine, Ibuprofen, Triclosan and Sulfamethoxazole on Anaerobic Bioreactor Performance: Combining Cell Damage, Ecotoxicity and Chemical Information. Toxics 2022, 10, 42.
- Ma, J.; Yong, L.; Lei, P.; Li, H.; Fang, Y.; Wang, L.; Chen, H.; Zhou, Q.; Wu, W.; Jin, L.; et al. Advances in microRNA from adipose-derived mesenchymal stem cell-derived exosome: Focusing on wound healing. J. Mater. Chem. B 2022, 10, 9565–9577.
- Ma, J.; Lei, P.; Chen, H.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Jin, L.; Sun, D. Advances in lncRNAs from stem cell-derived exosome for the treatment of cardiovascular diseases. Front. Pharmacol. 2022, 13, 986683.
- Chen, H.; Lei, P.; Ji, H.; Yang, Q.; Peng, B.; Ma, J.; Fang, Y.; Qu, L.; Li, H.; Wu, W.; et al. Advances in Escherichia coli Nissle 1917 as a customizable drug delivery system for disease treatment and diagnosis strategies. Mater. Today Bio 2023, 18, 100543.
- Wang, L.; Ma, J.; Wu, W.; Fang, Y.; Liu, F.; Yang, Q.; Hu, X.; Gu, X.; He, Z.; Sun, D.; et al. Effect of aerobic exercise as a treatment on type 2 diabetes mellitus with depression-like behavior zebrafish. Life Sci. 2022, 300, 120578.
- El-Benhawy, S.A.; Fahmy, E.I.; Mahdy, S.M.; Khedr, G.H.; Sarhan, A.S.; Nafady, M.H.; Yousef Selim, Y.A.; Salem, T.M.; Abu-Samra, N.; El Khadry, H.A. Assessment of thyroid gland hormones and ultrasonographic abnormalities in medical staff occupationally exposed to ionizing radiation. BMC Endocr. Disord. 2022, 22, 287.
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614.
- Konukoğlu, D.; Hatemi, H.H.; Arikan, S.; Demir, M.; Akçay, T. Radioiodine treatment and oxidative stress in thyroidectomised patients for differentiated thyroid cancers. Pharmacol. Res. 1998, 38, 311–315.
- Baugnet-Mahieu, L.; Lemaire, M.; Léonard, E.D.; Léonard, A.; Gerber, G.B. Chromosome aberrations after treatment with radioactive iodine for thyroid cancer. Radiat. Res. 1994, 140, 429–431.
- Rosário, P.W.; Batista, K.C.S.; Calsolari, M.R. Radioiodine-induced oxidative stress in patients with differentiated thyroid carcinoma and effect of supplementation with vitamins C and E and selenium (antioxidants). Arch. Endocrinol. Metab. 2016, 60, 328–332.
- Upadhyaya, A.; Zhou, P.; Meng, Z.; Wang, P.; Zhang, G.; Jia, Q.; Tan, J.; Li, X.; Hu, T.; Liu, N.; et al. Radioprotective effect of vitamin E on salivary glands after radioiodine therapy for differentiated thyroid cancer: A randomized-controlled trial. Nucl. Med. Commun. 2017, 38, 891–903.
- Eksioglu, U.; Atilgan, H.I.; Yakin, M.; Yazihan, N.; Altiparmak, U.E.; Yumusak, N.; Korkmaz, M.; Demir, A.; Ornek, F.; Aribal Ayral, P.; et al. Antioxidant Effects of Vitamin D on Lacrimal Glands against High Dose Radioiodine-Associated Damage in an Animal Model. Cutan. Ocul. Toxicol. 2019, 38, 18–24.
- Auttara-atthakorn, A.; Sungmala, J.; Anothaisintawee, T.; Reutrakul, S.; Sriphrapradang, C. Prevention of salivary gland dysfunction in patients treated with radioiodine for differentiated thyroid cancer: A systematic review of randomized controlled trials. Front. Endocrinol. 2022, 13, 960265.
- Kim, J.M.; Choi, M.E.; Kim, S.-K.; Kim, J.W.; Kim, Y.-M.; Choi, J.-S. Keratinocyte Growth Factor-1 Protects Radioiodine-Induced Salivary Gland Dysfunction in Mice. Int. J. Environ Res. Public Health 2020, 17, 6322.
- Jafarpour, S.M.; Safaei, M.; Mohseni, M.; Salimian, M.; Aliasgharzadeh, A.; Farhood, B. The Radioprotective Effects of Curcumin and Trehalose Against Genetic Damage Caused By I-131. Indian J. Nucl. Med. IJNM Off. J. Soc. Nucl. Med. India 2018, 33, 99–104.
- Jafarpour, S.M.; Shekarchi, B.; Bagheri, H.; Farhood, B. The Radioprotective Effects of Melatonin and Nanoselenium on DNA Double-Strand Breaks in Peripheral Lymphocytes Caused by I-131. Indian J. Nucl. Med. 2021, 36, 134–139.
- Düsman, E.; Berti, A.P.; Mariucci, R.G.; Lopes, N.B.; Tonin, L.T.D.; Vicentini, V.E.P. Radioprotective effect of the Barbados Cherry (Malpighia glabra L.) against radiopharmaceutical iodine-131 in Wistar rats in vivo. BMC Complement. Altern. Med. 2014, 14, 41.
- Kurashige, T.; Shimamura, M.; Nagayama, Y. N-Acetyl-L-cysteine protects thyroid cells against DNA damage induced by external and internal irradiation. Radiat. Environ. Biophys. 2017, 56, 405–412.
- Koca, G.; Singar, E.; Akbulut, A.; Yazihan, N.; Yumuşak, N.; Demir, A.; Korkmaz, M. The Effect of Resveratrol on Radioiodine Therapy-Associated Lacrimal Gland Damage. Curr. Eye Res. 2021, 46, 398–407.
- Sadic, M.; Aydinbelge, F.N.; Yumusak, N.; Karakok, E.; Akbulut, A.; Koca, G.; Korkmaz, M. Radioprotective effect of lycopene on the gastrointestinal tract after high-dose radioiodine administration in rat models. Nucl. Med. Commun. 2017, 38, 1041–1046.
- Torun, N.; Muratli, A.; Serim, B.D.; Ergulen, A.; Altun, G.D. Radioprotective Effects of Amifostine, L-Carnitine and Vitamin E in Preventing Early Salivary Gland Injury due to Radioactive Iodine Treatment. Curr. Med. Imaging Rev. 2019, 15, 395–404.
- Bohuslavizki, K.H.; Brenner, W.; Klutmann, S.; Hübner, R.H.; Lassmann, S.; Feyerabend, B.; Lüttges, J.; Tinnemeyer, S.; Clausen, M.; Henze, E. Radioprotection of Salivary Glands by Amifostine in High-Dose Radioiodine Therapy. J. Nucl. Med. 1998, 39, 1237–1242.
- Lin, R.; Ma, B.; Liu, N.; Zhang, L.; He, T.; Liu, X.; Chen, T.; Liu, W.; Liang, Y.; Wang, T.; et al. Targeted radioimmunotherapy with the iodine-131-labeled caerin 1.1 peptide for human anaplastic thyroid cancer in nude mice. Ann. Nucl. Med. 2021, 35, 811–822.
- Kismet, K.; Sadic, M.; Bag, Y.M.; Atilgan, H.I.; Koca, G.; Onalan, A.K.; Senes, M.; Peker, S.A.; Yumusak, N.; Korkmaz, M. Hepatoprotective Effect of Dexmedetomidine Against Radioiodine Toxicity in Rats: Evaluation of Oxidative Status and Histopathological Changes. Int. Surg. 2016, 101, 176–184.
- Tokat, A.O.; Akbulut, A.; Billur, D.; Koca, G.; Bayram, P.; Kuru, S.; Karasu, S.; Aydogmus, S.; Cakmak, H.; Ozmert, S.; et al. Montelukast attenuates radioactive I131-induced pulmonary damage on rats. Int. J. Radiat. Biol. 2018, 94, 542–550.
- Çakmak, Y.S.; Aktumsek, A.; Duran, A. Studies on antioxidant activity, volatile compound and fatty acid composition of different parts of Glycyrrhiza echinata L. EXCLI J. 2012, 11, 178–187.
- Jiang, Y.W.; Sun, Z.H.; Tong, W.W.; Yang, K.; Guo, K.Q.; Liu, G.; Pan, A. Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 1723–1733.
- Gao, P.; Zhang, H.; Dinavahi, R.; Li, F.; Xiang, Y.; Raman, V.; Bhujwalla, Z.M.; Felsher, D.W.; Cheng, L.; Pevsner, J.; et al. HIF-dependent Anti-tumorigenic Effect of Anti-oxidants In Vivo. Cancer Cell 2007, 12, 230–238.
- Amara, F.; Berbenni, M.; Fragni, M.; Leoni, G.; Viggiani, S.; Ippolito, V.M.; Larocca, M.; Rossano, R.; Alberghina, L.; Riccio, P.; et al. Neuroprotection by Cocktails of Dietary Antioxidants under Conditions of Nerve Growth Factor Deprivation. Oxid. Med. Cell. Longev. 2015, 2015, 217258.
- Choi, I.S.; Ko, S.H.; Lee, M.E.; Kim, H.M.; Yang, J.E.; Jeong, S.-G.; Lee, K.H.; Chang, J.Y.; Kim, J.-C.; Park, H.W. Production, Characterization, and Antioxidant Activities of an Exopolysaccharide Extracted from Spent Media Wastewater after Leuconostoc mesenteroides WiKim32 Fermentation. ACS Omega 2021, 6, 8171.
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501.
- Patananan, A.N.; Budenholzer, L.M.; Pedraza, M.E.; Torres, E.R.; Adler, L.N.; Clarke, S.G. The invertebrate Caenorhabditis elegans biosynthesizes ascorbate. Arch. Biochem. Biophys. 2015, 569, 32–44.
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131.
- Aydoğan, F.; Atılgan, H.I.; Koca, G.; Yumuşak, N.; Aydın, E.; Sadıç, M.; Korkmaz, M.; Tuncal, S.; Samim, E.E. An evaluation of the radioprotective effect of vitamin E on the salivary glands of radioactive iodine in rats. Kulak Burun Bogaz Ihtis. Derg. 2014, 24, 21–29.
- Wolfram, R.M.; Budinsky, A.C.; Palumbo, B.; Palumbo, R.; Sinzinger, H. Radioiodine therapy induces dose-dependent in vivo oxidation injury: Evidence by increased isoprostane 8-epi-PGF(2 alpha). J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2002, 43, 1254–1258.
- Song, F.-L.; Gan, R.-Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.-B. Total Phenolic Contents and Antioxidant Capacities of Selected Chinese Medicinal Plants. Int. J. Mol. Sci. 2010, 11, 2362–2372.
- Valdes, F.; Brown, N.; Morales-Bayuelo, A.; Prent-Peñaloza, L.; Gutierrez, M. Adenosine Derivates as Antioxidant Agents: Synthesis, Characterization, in Vitro Activity, and Theoretical Insights. Antioxidants 2019, 8, 468.
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants 2021, 10, 531.
- Jameel, S.; Hameed, A.; Shah, T.M. Biochemical Profiling for Antioxidant and Therapeutic Potential of Pakistani Chickpea (Cicer arietinum L.) Genetic Resource. Front. Plant Sci. 2021, 12, 663623.
- Gledovic, A.; Janosevic-Lezaic, A.; Tamburic, S.; Savic, S. Red Raspberry Seed Oil Low Energy Nanoemulsions: Influence of Surfactants, Antioxidants, and Temperature on Oxidative Stability. Antioxidants 2022, 11, 1898.
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215.
- Ma, C.; Xie, J.; Chen, Q.; Wang, G.; Zuo, S. Amifostine for salivary glands in high-dose radioactive iodine treated differentiated thyroid cancer. Cochrane Database Syst. Rev. 2009, 2009, CD007956.
- Ma, C.; Xie, J.; Jiang, Z.; Wang, G.; Zuo, S. Does Amifostine Have Radioprotective Effects on Salivary Glands in High-Dose Radioactive Iodine-Treated Differentiated Thyroid Cancer. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1778–1785.
- Li, Z.; Wang, J.; Ma, Y. Montelukast attenuates interleukin IL-1β-induced oxidative stress and apoptosis in chondrocytes by inhibiting CYSLTR1 (Cysteinyl Leukotriene Receptor 1) and activating KLF2 (Kruppel Like Factor 2). Bioengineered 2021, 12, 8476–8484.
- Chen, H.; Qian, N.; Yan, L.; Jiang, H. Role of serum vitamin A and E in pregnancy. Exp. Ther. Med. 2018, 16, 5185–5189.
- Hu, W.; Zhou, J.; Shen, T.; Wang, X. Target-Guided Isolation of Three Main Antioxidants from Mahonia bealei (Fort.) Carr. Leaves Using HSCCC. Molecules 2019, 24, 1907.
- Ding, Y.; Ko, S.-C.; Moon, S.-H.; Lee, S.-H. Protective Effects of Novel Antioxidant Peptide Purified from Alcalase Hydrolysate of Velvet Antler Against Oxidative Stress in Chang Liver Cells In Vitro and in a Zebrafish Model In Vivo. Int. J. Mol. Sci. 2019, 20, 5187.
- Sharafi, S.M.; Rasooli, I.; Owlia, P.; Taghizadeh, M.; Astaneh, S.D.A. Protective effects of bioactive phytochemicals from Mentha piperita with multiple health potentials. Pharmacogn. Mag. 2010, 6, 147–153.
More
