Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Da Sun and Version 2 by Catherine Yang.
Thyroid cancer is the most common endocrine cancer, and its prevalence has been increasing for decades. Approx. 95% of differentiated thyroid carcinomas are treated using 131iodine (131I), a radionuclide with a half-life of 8 days, to achieve optimal thyroid residual ablation following thyroidectomy. However, while 131I is highly enriched in eliminating thyroid tissue, it can also retain and damage other body parts (salivary glands, liver, etc.) without selectivity, and even trigger salivary gland dysfunction, secondary cancer, and other side effects.
- thyroid cancer
- 131I
- oxidative stress
- antioxidant
1. Introduction
Thyroid cancer is a malignant tumor of the endocrine gland that arises from the follicular or parafollicular epithelium of the thyroid gland. As a result of an increased use of diagnostic imaging and surveillance, the incidence of thyroid cancer has been steadily increasing worldwide, with more than 62,000 new cases diagnosed each year [1][2][3][1,2,3]. The most frequent kind of thyroid cancer is differentiated thyroid carcinoma (DTC), which accounts for more than 95% of cases [4][5][4,5]. Thyroidectomy, lymph node dissection, and 131I therapy are the primary therapeutic options [6]. In clinical practice, 131iodine (131I), a γ/β radiation radionuclide with a half-life of 8 days, can accumulate in thyroid tissue. As shown in Figure 1, it is commonly used to ablate residual thyroid tissue after surgery (known as thyroid remnant ablation) to reduce the likelihood of local recurrence, treat metastatic disease, and clear hidden thyroid cancer cells [7][8][9][7,8,9]. Iodine-131 is also used as a means of addressing persistent disease as reflected by the thyroid globulin levels [10], with a typical dosage range of 1110 MBq (30 mCi) to 3700 MBq (100 mCi) [11].
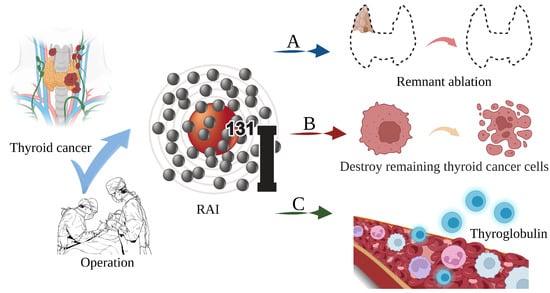
Figure 1. The main role of 131I in the treatment of thyroid cancer. (A) Thyroid remnant ablation for reducing the likelihood of local recurrence; (B) Treating metastatic disease and clearing hidden thyroid cancer cells; (C) As a means of addressing persistent disease as reflected by thyroid globulin levels.
However, there is evidence that 131I γ/β radiation interferes with the REDOX cell signaling pathways, causing an imbalance between cellular oxidants and antioxidants, resulting in systemic oxidative stress, cell and tissue damage, and an increase in the risk of genetic DNA damage and secondary cancer [12][13][14][15][12,13,14,15]. Furthermore, it can cause side effects, including salivary gland dysfunction, gastrointestinal reactions, dry eye, pulmonary fibrosis, gonad damage, nasolacrimal duct obstruction, secondary cancer, permanent myelosuppression, and genetic effects [16][17][16,17]. To achieve optimal effectiveness and minimize discomfort in thyroid cancer patients, adjuvant medication combinations that reduce the adverse effects of 131I are required.
Antioxidants are chemicals that bind free radicals and drastically decrease or prevent substrate oxidation [18][19][18,19]. They limit free radical damage by blocking free radicals from damaging lipids, protein amino acids, polyunsaturated fatty acids, and the double bonds of DNA bases [20][21][22][20,21,22]. Notably, substances such as β-carotene and vitamin E have been proven to dramatically minimize the negative effects of 131I [23][24][23,24].
3. Oxidative Stress Dominates 131I Side Effects
RAI is the standard and effective treatment for DTC. The thyroid gland can accumulate iodine at up to 40 times the concentration of plasma under physiological conditions. This relies on the NIS located in the basolateral membrane of thyrocytes using the electrochemical gradient generated by the Na,K-ATPase as the driving forces that coordinate with the KCNQ1-KCNE2 K+ channels located in the basolateral membrane These promote the potassium efflux, thus facilitating iodine transport into the intracellular compartments, and thereby increasing the oxidative stress and cytotoxic efficacy from the radioactivity [25][26][27][28][39,48,49,50]. Oxidative stress is the result of increased free radical production and/or a decreased antioxidant defense system physiological activity [29][30][51,52]. Each cell in a living organism maintains a reductive environment. The reducing environment is maintained by enzymes, which provide constant metabolic energy input to maintain the reducing state [31][32][53,54]. This disruption of the normal reduction oxidation (REDOX) state can be mediated by the generation of peroxide-reactive radicals (hydrogen peroxide (H2O2), superoxide (O2−), singlet oxygen (1/2O2), ROS, and the hydroxyl radical (∙OH). The abnormal expression of these substances may result in the destruction of all the components of the cell, resulting in toxic effects [33][34][35][55,56,57]. Severe cases can lead to cell death (Figure 23A). The damage can involve multiple parts throughout the body (Figure 23B).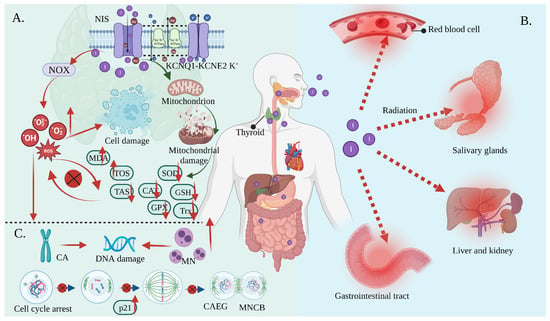
Figure 23. Oxidative stress mediates the side effects of 131I. (A) Iodine-131 enters the cells through the synergistic transport of the NIS and KCNQ1-KCNE2 K+ transporter, and thus increases the expression of NOX1 and changes the ultrastructure of the mitochondria through β/γ radiation, resulting in a reduced antioxidant capacity and the production of numerous ROS. As a result, the activities of CAT and SOD are decreased; the levels of GSH, GPx, Trx, and TAS are decreased; and the levels of MDA and the total oxidative stress (TOS) are increased, leading to systemic oxidative stress. (B) Oxidative stress induces erythrocyte membrane damage and vascular permeability changes, salivary gland dysfunction, and gastrointestinal tract and liver and kidney injury. (C) Oxidative stress induces a CA and MN increase and mediates a significant increase in the frequency of MNCB, CAEG, and bicentric chromosomes.
3. Antioxidants Reduce 131I Side Effects
In general, it can be observed that oxidative stress mediates the pathological process of almost all the 131I side effects. Herein, the antioxidants showed a robust effectiveness against their side effects. The antioxidants that have been proven to alleviate the side effects of 131I are shown in Figure 34 and the drug type, drug treatment, subject, dose, side effects, and drug efficacy are summarized in Table 12.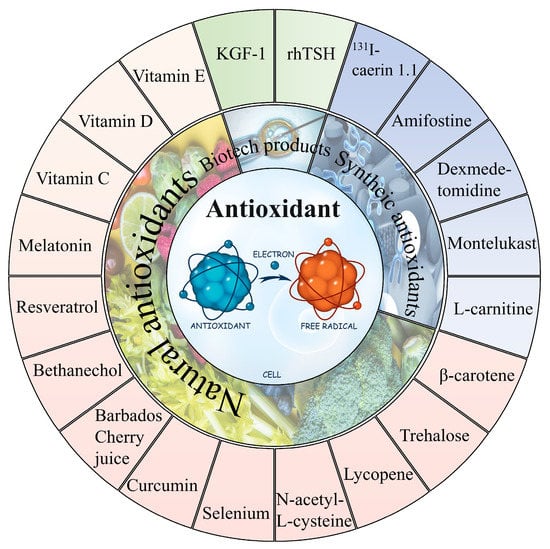
Figure 34.
The natural and synthetic antioxidants applied to combat
131
I side effects.
Table 12. The applications of various antioxidants to alleviate the side effects of 131I. (8-Epi-prostaglandin F2alpha (8-epi-PGF2α); uptake fraction (UF); uptake index (UI); excretion fraction (EF); excretion ratio (ER); first-minute uptake ratio (FUR); maximum uptake ratio (MUR); hypoxia inducible factor-1α (HIF-1α)).
| Drug Type | Drug Treatment | Subject | Dose of 131I | Side Effects of 131I | Drug Efficacy | Ref. |
|---|---|---|---|---|---|---|
| Natural antioxidant | Daily supplementation consisting of 2000 mg vitamin C and 1000 mg vitamin E and 400 µg selenium for 21 days before 131I | Forty patients with thyroid cancer submitted for thyroidectomy (n = 20) | 3.7 GBq | 8-epi-PGF2α↑ | 8-epi-PGF2α↓ | [54][86] |
| 1500 mg vitamin C daily 2 days after (group 2), 2 days before to 2 days after (group 3), and 2 days before RAI (group 4) | Fifty-eight DTC patients ablated with 131I | 5550 MBq | MDA, CAT↑; GSH↓ | MDA↓ (group 2,3,4); GSH↑ (group 3,4); CAT↓ (group 3,4) | [22] | |
| Groups A, B, and C received vitamin E 100, 200, and 300 mg/day orally, respectively, for a duration of 1 week before to 4 weeks after I therapy | Eighty-two DTC patients with 131I | 100 mCi | UF, UI, EF, and ER↓ | UI, EF, UF, ER↑ | [55][87] | |
| Vitamin D (200 ng/kg/day) | Wistar albino rats (n = 12) | 111 MBq/kg | TOS, TNF-α, IL-6↑; IL-10, TAS↓ | TOS, TNF-α, IL-6↓; IL-10, TAS ↑ | [56][88] | |
| Vitamin E (800 IU/day for one week before and four weeks after RAI therapy) | Thirty-six DTC patients with RAI (n = 18) | 3700–5550 MBq | FUR, MUR, MSP, and EF↓ | FUR, MUR, MSP, and EF↑ | [57][43] | |
| Bethanechol (2 mg orally twice a day) for one month after 131I | Fifty DTC patients with RAI (n = 25) | 97.2 to 213.4 mCi | MUR, MSP, ΔMS, EF↓ | Serum amylase↓ | ||
| Selenium 300 mcg orally for ten days (from three days before until six days after RAI therapy) | Sixteen DTC patients with RAI (n = 8) | 3.7 GBq | Xerostomia, sialadenitis symptoms↑ | Xerostomia, sialadenitis symptoms↓ | ||
| KGF-1 (100 ug/1 mL PBS) | Eighteen C57BL/six mice (n = 6) | 0.01 mCi/g | HIF-1α↑; mucin stained acini, amylase↓; periductal fibrosis↑ | HIF-1α↓; mucin stained acini, amylase↑; periductal fibrosis↓ | [58][89] | |
| 50 μg curcumin per mL of blood and 5.738 mg trehalose per mL of blood | Blood of five humans | 20 μCi | DSB increased to 102.9% | DSBs decreased by 42% (curcumin) and 38% (trehalose) | [59][84] | |
| 0.0167 mg melatonin per mL of blood and 0.025 mg Se NPs per mL of blood | Blood of five humans | 20 μCi | DSB increased to 102.9% | DSBs decreased by 38% (melatonin) and 30% (selenium nanoparticles) | [60][90] | |
| 0.0666 mg vitamin E per mL of blood and 0.0167 mg vitamin C per mL of blood | Blood of five humans | 20 μCi | DSB increased to 102.9% | DSBs decreased by 21.5% (vitamin E) and 36.4% (vitamin C) | [23] | |
| Barbados Cherry juice (5 mg)/100 g | Wistar rats (n = 6) | 25 μCi/100 g | 1,1-diphenyl-2-picrylhydrazyl↑; chromosomal and cellular aberrations↑ | 1,1-diphenyl-2-picrylhydrazyl↑; chromosomal and cellular aberrations↑ | [61][91] | |
| 20 mmol N-acetyl-L-cysteine | Normal differentiated rat thyroid cell line PCCL3 | 10 μCi/mL | ROS, DBS, MN↑ | ROS, DBS, MN↓ | [62][92] | |
| 8 mg β-carotene/mL corn oil (0.2 mL/100 g) | Wistar rats (n = 6) | 25 μCi /100 g body weight |
CA, MN, water consumption↑ | CA, MN, water consumption↓ | [12] | |
| 20 mg/kg/day resveratrol | Thirty Wistar albino rats (n = 10) | 3 mCi/kg | Caspase-3, TUNEL, TNF-α, IL-6, nuclear factor-kappa-B (NF-кB), TOS↑; IL-10, TAS↓ | Caspase-3, TUNEL, TNF-α, IL-6, NF-кB, TOS↑; TAS↓ | [63][93] | |
| 1 mL lycopene (5 mg/kg body weight) |
Twenty Wistar albino rats (n = 10) | 3 mCi | Duodenal and ileal lamina propria edema, duodenal ulcer, gastric mucosal erosion, and gastric and colon mucosal degeneration↑ | Duodenal and ileal lamina propria edema, duodenal ulcer, gastric mucosal erosion, gastric and colon mucosal degeneration↓ | [64][94] | |
| Synthetic antioxidants | 200 mg/kg amifostine or L-carnitine | Forty adult guinea pigs | 555–660 MBq | Body weight and thyroid hormone↓ | Body weight and thyroid hormone↑ | [65][34] |
| 200 mg/kg amifostine to three rabbits/500 mg/m2 amifostine before 131I to eight patients | Five rabbits/17 patients | 1 GBq to rabbits/6 GBq to patients | Reduced parenchymal function in parotid and submandibular glands; xerostomia; lipomatosis | None of the parenchymal function in parotid and submandibular glands reduce, xerostomia and lipomatosis occurred | [66][95] | |
| rhTSH (1 mg/2 d and 1 mg/1 d before 131I) | Sixty-two patients prepared with rhTSH or by thyroid hormone withdrawal | 1850 MBq | CA, MN, ROS↑ | CA, MN, ROS↓ | [13] | |
| 8 μg of F1 peptide labeled with 200 μCi 131I every 3 days for a total of three times | Nude mice with human anaplastic thyroid cancer | 200 μCi | Weight loss and 131I enter the internal circulation | Constant weight | [67][96] | |
| Dexmedetomidine (3 μg/kg) | Thirty-six Wistar albino female rats (n = 12) |
111 MBq | MDA, advanced oxidized protein products↑, total sulfur group, CAT↓ | MDA, advanced oxidized protein products↓; total sulfur group, CAT↑; liver protection | [68][97] | |
| Montelukast (10 mg/kg/day) | Fifty female Wistar albino rats (n = 10) | 111 MBq/kg | Inflammation and pulmonary fibrosis | Reduced the degree of inflammation and pulmonary fibrosis | [69][45] |
