Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Ghais Kharmanda and Version 4 by Rita Xu.
Additive manufacturing (AM), which is also called rapid prototyping/3D printing/layered manufacturing, can be considered as a rapid conversion between digital and physical models. One of the most used materials in AM is polylactic acid (PLA), which has advantageous material properties such as biocompatibility, biodegradability, and nontoxicity. For many medical applications, it is considered as a leading biomaterial. In dentistry, in addition to its uses in dental models (education, teaching, simulation needs), it can be used for therapeutic objectives and tissue engineering.
- additive manufacturing
- fused filament fabrication
- polylactic acid
- dentistry
1. Introduction
Traditional methods of transplantation of organs have several risks such as limited sources, complications, and secondary injuries. Additive manufacturing (AM) technology potentially helps to solve these issues since it can rapidly fabricate personalized tissue structures such as scaffolds, repair certain tissue defects, and even directly manufacture tissues and organs [1]. It is true that these types of additively manufactured structures cannot perfectly match the patient’s damaged tissue, but they may possess suitable microstructures and cell arrangements for the promotion of cell growth and differentiation. Figure 1 presents the roadmap of the three main axes. The first axis is represented by the most common AM applications that can be found in the following areas: aerospace, spare parts, arts and design, prototyping, and medicine. The medical applications can be divided into orthopedics and orthodontics. Using AM technology, it is possible to build complicated models for surgery preparation. AM models of a patient’s anatomy can be very helpful to better understand the patient’s anatomy prior to surgery instead of using CT scans and MRI. In addition, these models can be used for surgical simulation and training purposes [2][3][4]. Dentistry involves the diagnosis, treatment, and condition counteraction, turmoil, and sicknesses of the teeth, gums, jaws, and mouth. It is frequently viewed as fundamental for complete oral well-being. The human mouth’s oral cavity includes the upper and lower jaws, which are respectively called maxilla and mandible. Each jaw (upper/lower) contains 16 teeth, nerves, blood vessels and muscles [5]. Dentistry was selected to be treated since it can affect the whole human body’s health. The second axis is represented by the most common AM materials: plastics, metals, ceramics, and composites [6]. Plastic materials have two types: thermosets and thermoplastics. Thermoplastic materials are utilized in two kinds of AM techniques: powder bed fusion and material extrusion. Among these kinds, amorphous thermoplastic materials are utilized for material extrusion processes due to their melt properties (high viscous melt). The typical size of the nozzle used for extrusion of these materials is 0.2–0.5 mm [7]. Polylactic acid (PLA) [8][9] and acrylonitrile butadiene styrene (ABS) [10][11] are the two most common examples. Polycarbonate (PC) [12], PC/ABS blend [13], and polyetherimide (PEI) [14] are other examples of amorphous materials that are used in material extrusion. The thirst axis is represented by the most common material-based AM processes. The classifications of these processes are based on the material states: solid, liquid, and powder. They can be respectively labeled as follows: solid-based AM methods, liquid-based AM methods, and powder-based AM methods [15]. Material extrusion, powder bed fusion directed energy deposition material jetting, binder jetting, vat photopolymerization, and sheet lamination are among the seven additive manufacturing techniques classified by ASTM/F2921 [4]. Extrusion-based techniques, being cost-effective, are widely used for solid materials. Several materials such as multi-colored plastics and living cells are manufactured following a material extrusion-based technology [16]. Additionally, this process can be completely based on the functional aspects of the products [17][18]. Among these techniques, fused filament fabrication (FFF), also called fused deposition modeling (FDM), is a desirable AM technique in order to fabricate PLA due to its geometrical flexibility and relatively low cost. An illustration and details of the FFF technique can be found in the previous work [19]. Using the FFF technique, several dental applications can be performed in a simple way. For example, with this technique, it is possible to produce polymer dentures with hollow, semi-hollow, and solid structures [20].
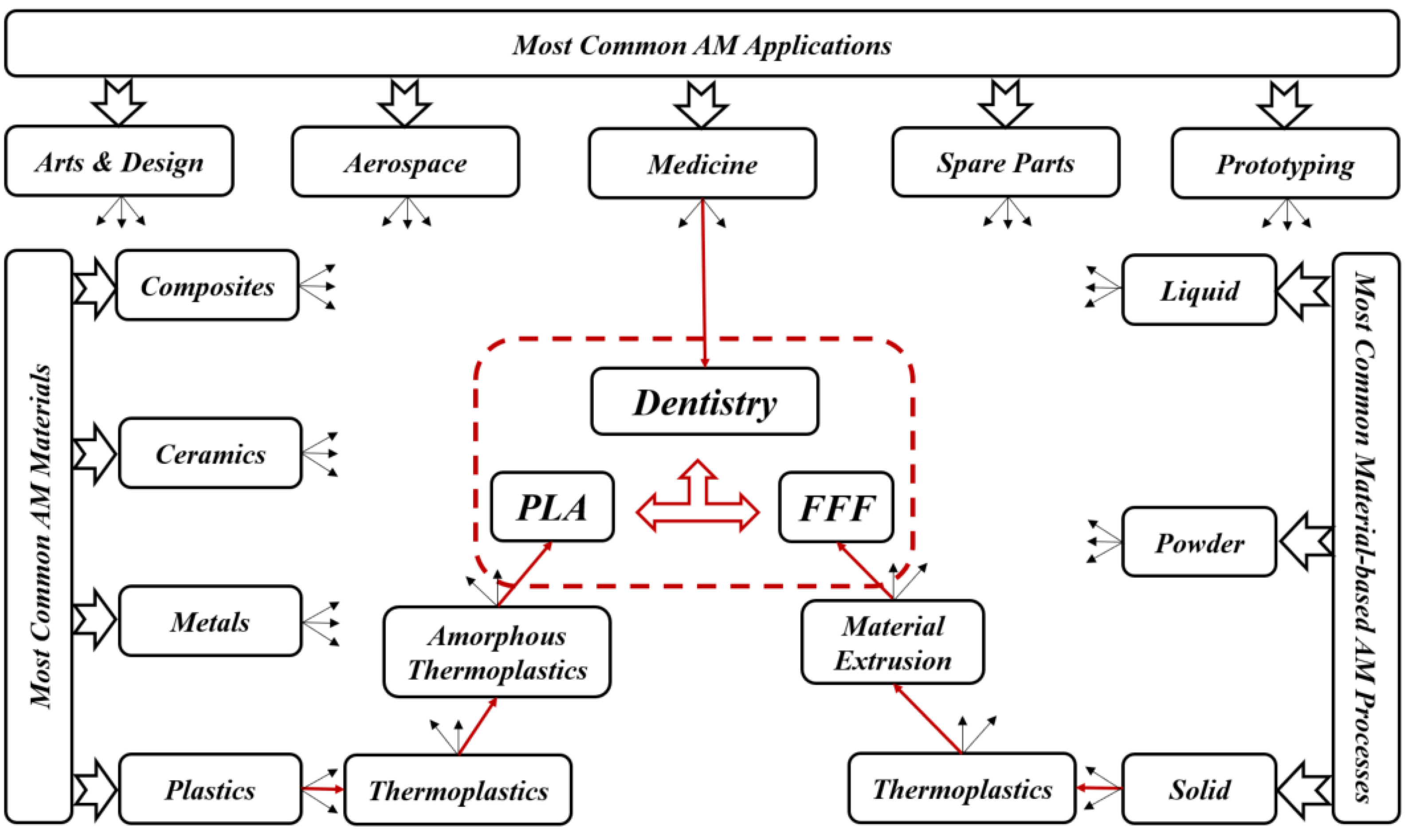
Figure 1. Roadmap of current review topics.
2. AM Techniques Used in Dentistry
Day by day, additive manufacturing technology is expanding, and, because of its frequently used layer-by-layer construction, it has a remarkable perspective for biomedical applications. This technology may directly fabricate specific functional components with the help of scanned data in CT images that give superior visualization of a particular framework. In typical presurgical preparation, it helps medical experts to practically replan surgical events. In addition, it can be considered as a communication tool between medical experts and patients [15][21].
Fields such as dentistry, where the anisotropy is needed as per the requirement, pose as best clients for AM technology. Implant section is one of the most important medical applications for AM. The implants are customized as per the needs and patient’s requirements; AM can then help in fabricating those implants as required [22]. For dental applications, models, splints, and drill guides are developed by AM technology. In addition, the development of artificial tissues and organs has been carried out by using AM techniques [23]. AM techniques are currently widely used for 3D organ models, which are useful to understand complex human anatomy. In a recent review by Rouf et al. [6], several AM techniques for various aspects of medical needs (orthopedics and orthodontics) were presented. Some AM works in dentistry are presented since AM for dentistry has been applied for almost 20 years. As shown in Figure 2, for metallic dental crowns, researchers used several AM techniques such as fused filament fabrication (FFF) [24], selective laser sintering (SLS) [25], stereolithography (SLA) [26], and laminated object manufacturing (LOM) [27]. Dental pieces, bridges and crowns were the dental applications for these AM techniques [28][29]. For non-metallic oral implants, crowns and bridges, models for dental study, and surgical equipment (specifically surgical guides for dental surgery), SLA and FFF are generally used [30]. For maxillofacial implants, the metallic powder using the selective laser melting method (SLM) [31] replaces the patient’s entire jaw. Furthermore, AM technologies have been used for creating complete or partial dentures, where direct laser metal sintering (DLMS) processes have been utilized to create metallic dentures [32][33][34]. The FFF technique has been used to create polymer dentures with solid, hollow, or semi-hollow structures [20]. Research is currently being conducted using AM techniques to develop dentures that possess anti-microbial properties [35]. FFF and SLA have been used to generate bioresorbable polymer dental implants that display odontogenic properties [36][37]. Recently, powder bed fusion technologies became leading AM techniques in dental applications [6]. However, the roughness associated with printed components can represent a real issue. So, there is a need to remedy the roughness in dental implants. On the other hand, FFF technique can be utilized in order to create complex and functional geometries, starting directly from CAD models [38]. This technique can then be employed in order to produce cellular structures with controllable pore shape, pore size, and porosity. These kinds of structures are fundamental in orthopedic scaffolds due to their high compressive strength, low elastic modulus, and adequate cell accommodation spaces. Several developments are needed to extend the application of these advantageous structures to dentistry, especially when a patient suffers from bone loss due to injuries or accidents. Furthermore, other AM applications can be found in maxillofacial surgery. For example, it is possible to develop surgical equipment to correct facial defects. This kind of equipment can reduce the surgery risks and provide aesthetic results [39]. By developing 3D inkjet-printed bones in the jaw area, a group of researchers found a solution for mandibular deformities [40]. These fabricated bones had dimensional compatibility in patients, and there was a link between artificial bones and host ones. That can allow reducing operational time and risk and simplify adjusting the size for bone fixation during treatment. In addition to these applications, splints can be performed by using AM techniques. Sun et al. [41] provided AM splints for maxillofacial surgery that helped to identify the positions of mandible and maxilla as parts of facial bone treatment. These splints showed high accuracy levels and improved mechanical strength. For mandible fractures or defects, AM technology also helped to provide bone implants. As part of surgical treatment for square-shaped or asymmetric faces, titanium implants based on the anatomy of the patient’s mandible have been developed, and part of the mandible has been replaced with that. With this implant invention, it is much easier for surgeons to treat mandible defects and fractures that were very difficult to solve in the early stages [42]. When dealing with neurosurgery, additively manufactured skull models are also useful for skull defects.
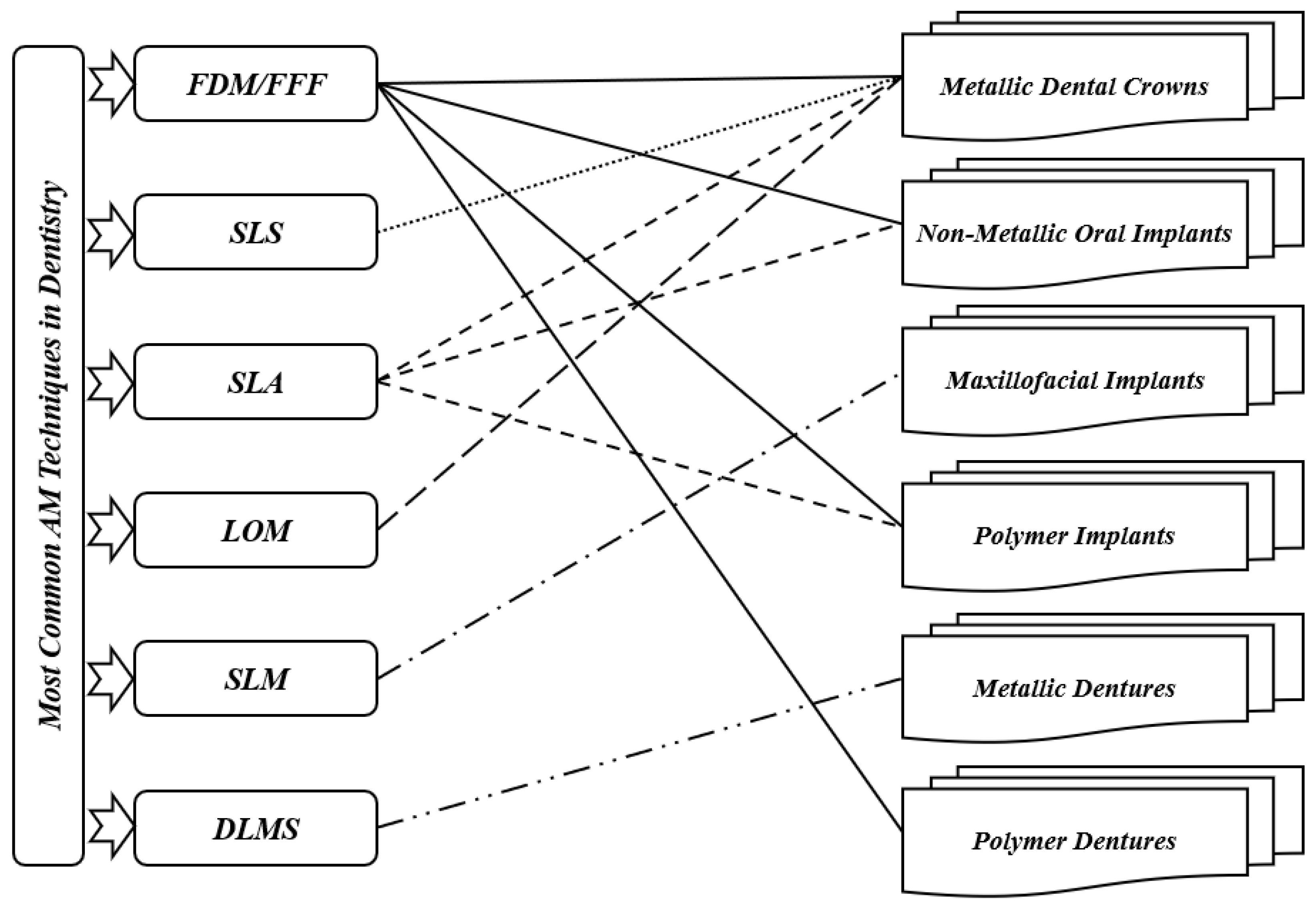
Figure 2. Most common AM techniques in dentistry.
3. AM Materials Used in Dentistry
Ceramics, metals, and polymers are the most common materials utilized for dental applications such as crowns, bridges, implants, splints, etc. It is true that ceramics and metals are generally preferred for many dental applications; however, polymers are used for biodegradable applications [5]. In literature, many materials have been used in dentistry. However, researchers present only some recent studies of AM materials applied to dentistry. Bae et al. [43] used SLM and cold isostatic pressing (CIP) techniques for 3Y-TZP ceramics for fabrication of dental crowns and prostheses and for dental restoration. According to their study, the maximum flexural strength and maximum densification were achieved through sintering at 1500 °C. Their method led to the establishment of SLS/CIP technology for 3Y-TZP ceramics. Next, Muta et al. [44] used the FFF technique for polyvinyl alcohol (PVA) material to fabricate provisional dental crowns. They found that with good accuracy, additively manufactured PVA models can be utilized to fabricate crowns. In the same period, a research paper on the use of alumina ceramics with the FFF technique to fabricate dental crowns was published by Arnesano et al. [45]. At 1150 °C, it was found that the used samples were pre-sintered, and the mechanical properties were like those of pure alumina. Their method was cost-effective and energy-efficient. Revilla-León et al. [46] used SLM and conventional milling (CM) techniques with cobalt chromium alloy (Co-Cr) to fabricate dental prostheses. When fabricating the samples by the two processes, it was found that the shear bond strength had no significant impact, while the roughness was enhanced with the SLM process. In the same period, Baciu et al. [47] published a research paper on using Co–Cr–W alloy with the SLM technique to fabricate dental bridges and inlays. A modification in roughness was found after fabricating specimens using different blasting media. For more information about the different materials used in dentistry, such as titanium and other alloys, the interested reader can refer to [5][6]. Table 1 presents a summary of the different results of recent material studies in dentistry. According to this table, there is no recent work studying AM-PLA for dental applications, while one can find many recent works that aimed to improve the mechanical properties of AM-PLA parts in different areas [19]. The researchers focused on studying the mechanical properties of AM-PLA parts, considering simple specimens (simple geometrical models); however, when dealing with dental applications, complex geometrical models that can largely affect the mechanical properties of the final printed parts should be considered.
Table 1. Recent works in additively manufactured materials for dental applications.
| Authors | Material | AM Technique | Dental Application | Results | |
|---|---|---|---|---|---|
| Bae et al. | [43] | 3Y-TZP ceramics | SLM + CIP | Dental crown, prostheses, restoration. | Foundation of SLS/CIP technology for 3Y-TZP dental ceramics |
| Muta et al. | [44] | PVA | FDM | Provisional dental crown | Good accuracy |
| Arnesano et al. | [45] | Alumina-Ceramic | FDM | Dental crown | Energy efficiency |
| Revilla-León et al. | [46] | Co-Cr alloy | SLM + CM | Dental prostheses | Improved roughness with SLM process |
| Baciu et al. | [47] | Co–Cr–W alloy | SLM | Dental inlays and bridges | Increased hardness |
In general, and according to the best knowledge, there have been no significant developments that use PLA materials in dental applications during the last three decades despite this material possessing many advantageous properties. Researchers only focus on PLA material for dentistry in order to extend its applications considering several criteria such as sustainability, biodegradability, and biocompatibility. To meet this objective, some suggestions will be added, especially when dealing with composite PLA materials to improve their mechanical properties.
4. AM-PLA Material and Its Application to Dentistry
It is known that one of the most utilized AM materials is PLA since it is considered as a biocompatible, biodegradable, and nontoxic material [48][49][50][51]. Several works have been carried out to identify the design and process parameter effects on the quality of the final product [52]. PLA material is considered as a leading biomaterial for many medical applications and may replace conventional petrochemical-based polymers [53][54]. Due to its high potential for applicability in several areas such as medicine, chemistry, and biotechnology, it has been considered as a promising product under the concept of “green plastic”, since most of the polymers currently produced, are petroleum-based and non-renewable raw materials. The availability of pure lactic acid isomers is considered as an essential aspect for producing PLA with more interesting thermal and mechanical properties. In addition to its low environmental effects, it can be recycled in a traditional way [55]. This enhances its use as a promising polymer in medical applications, especially dental ones. Ramot et al. [56] reviewed the inflammatory reaction process that can be expected following PLA implantation, and they highlighted specific cases in which the inflammatory reaction could lead to some safety issues. In addition, some cases from different medical fields have been reported with the objective of demonstrating possible clinical side effects due to its use. Two kinds of biomaterials can be primarily utilized to prepare biodegradable scaffolds and medical models: natural and synthetic biomaterials. Usually, chitosan, fibrin, and collagen are utilized as natural medical polymeric materials that have excellent compatibility levels, stimulate cell adhesion and proliferation, and also maintain cell phenotypes; however, they can lead to poor mechanical strength (can be easily deformed). The degradation time, shape, and relative molecular mass of synthetic polymers such as PLA, PVA, and polycaprolactone (PCL) [57] can be precisely controlled. However, the polymer surfaces lack recognition sites for cell adhesion, which leads to heterogeneous cell distribution and then cell loss. Therefore, the mechanical performance of the polymer, such as fluidity and surface roughness, must be enhanced for it to be used in medical implants. Yue et al. [58] integrated more complex functions into polymers. After preparing antimicrobial composite resins, they found that antibacterial printed implants killed bacteria on contact without damaging human cells and may be eventually used to replace conventional dental fillings. Furthermore, their approach utilized for fabricating antimicrobial polymers can easily be transferred to other, nonmedical applications such as food packaging, water purification, or even toys for children. Despite PLA material having many advantages, it is difficult for the moment to use it in permanent replacements such as inlays and onlays since it may lead to total or partial loss of these replacements. So, for inlays and onlays, it is recommended to continue using ceramic/porcelain, gold, resin, zirconia materials (alloys), and metals (alloys) such as gold, palladium, chromium, nickel, etc., for permanent crowns and bridges. According to the best knowledge, dental PLA models such as replacement teeth are largely used for simulation needs (teaching, training, etc. This material can also be used in plates and tissues [1] for treating maxilla and mandible fractures. In certain periodical (provisional) parts (crowns, bridges, plates, etc.) this material can be used during the treatment period because of its biodegradability properties.
References
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742.
- Vikayavenkataraman, S.; Jerry, Y.H.F.; Wen, F.L. 3D Printing and 3D Bioprinting in Pediatrics. Bioengineering 2017, 4, 1–11.
- Low, Z.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2017, 523, 596–613.
- Jadhav, A.; Jadhav, V.S. A review on 3D printing: An additive manufacturing technology. Mater. Today Proc. 2022, 62, 2094–2099.
- Pradeep, K.; Pal, B. Chapter 24—Selected biomedical applications of additive manufacturing techniques. In Advances in Additive Manufacturing: Artificial Intelligence, Nature-Inspired, and Biomanufacturing; Elsevier: Amsterdam, The Netherlands, 2023.
- Rouf, S.; Malik, A.; Singh, N.; Raina, A.; Naveed, N.; Siddiqui, M.I.H.; Haq, M.I.U. Additive manufacturing technologies: Industrial and medical applications. Sustain. Oper. Comput. 2022, 3, 258–274.
- Bourell, D.; Kruth, J.P.; Leu, M.; Levy, G.; Rosen, D.; Beese, A.M.; Clare, A. Materials for additive manufacturing. CIRP Ann. 2017, 66, 659–681.
- Sin, L.T.; Rahmat, A.R.; Abdul Rahman, W.A.W. Polylactic Acid, A Volume in Plastics Design Library; Elsevier: Oxford, UK, 2013.
- Sin, L.T.; Tueen, B.S. Polylactic Acid, A Practical Guide for the Processing, Manufacturing, and Applications of PLA, A Volume in Plastics Design Library, 2nd ed.; Elsevier: Oxford, UK, 2019.
- Massey, L.K. Chapter 1—Acrylonitrile-Butadiene-Styrene. In Plastics Design Library, The Effects of UV Light and Weather on Plastics and Elastomers, 2nd ed.; Massey, L.K., Ed.; William Andrew Publishing: Norwich, NY, USA, 2007; pp. 13–32. ISBN 9780815515258.
- Peterson, A.M. Review of acrylonitrile butadiene styrene in fused filament fabrication: A plastics engineering-focused perspective. Addit. Manuf. 2019, 27, 363–371.
- Kyriacos, D. Chapter 17—Polycarbonates. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2007; pp. 457–485. ISBN 9780323358248.
- Zhang, B.B.; Chen, Y.; Wang, F.; Hong, R.Y. Surface modification of carbon black for the reinforcement of polycarbonate/acrylonitrile–butadiene–styrene blends. Appl. Surf. Sci. 2015, 351, 280–288.
- Trivedi, P.D. Chapter 3—Polyetherimides (PEI). In Specialty Thermoplastics; Trivedi, P.D., Ed.; Hanser: New York, NY, USA, 2023; pp. 77–114. ISBN 9781569907009.
- Shoeb, M.; Kumar, L.; Haleem, A.; Javaid, M. Chapter 2—Trends in additive manufacturing: An exploratory study. In Additive Manufacturing Materials and Technologies, Advances in Additive Manufacturing; Kumar, A., Mittal, R.K., Haleem, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 15–25. ISBN 9780323918343.
- Müller, A.; Karevska, S. How will 3D printing make your company the strongest link in the value chain, EY’s Global 3D printing Report 2016; EY: London, UK; 72p.
- Yap, Y.L.; Tan, Y.S.E.; Tan, H.K.J.; Peh, Z.K.; Low, X.Y.; Yeong, W.Y.; Tan, C.S.H.; Laude, A. 3D printed bio-models for medical applications. Rapid Prototyp. J. 2017, 23, 227–235.
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37.
- Kharmanda, G. A Review on Uncertainty Cases in Additively Manufactured Polylactic Acid Using Fused Filament Fabrication Technique. Int. J. Addit. Manuf. Struct. 2023, 2, 1.
- Arafa, K.A.O. Comparing the effects of titanium alloy and chrome cobalt in removable partial denture connectors on tooth mobility, bone loss and tissue reaction. Saudi J. Dent. Res. 2016, 7, 112–117.
- Dhakshyani, R.; Nukman, Y.; Osman, A. Preliminary report: Rapid prototyping models for dysplastic hip surgery. Cent. Eur. J. Med. 2011, 6, 266–270.
- Pettersson, A.B.V.; Salmi, M.; Vallittu, P.; Serlo, W.; Tuomi, J.; Mäkitie, A.A. Main clinical use of additive manufacturing (three-dimensional printing) in Finland restricted to the head and neck area in 2016–2017. Scand. J. Surg. 2020, 109, 166–173.
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs; Springer: New York, NY, USA, 2017.
- Singh, S.; Singh, G.; Prakash, C.; Seeram Ramakrishna, S. Current status and future directions of fused filament fabrication. J. Manuf. Process. 2020, 55, 288–306.
- Tikhomirov, E.; Åhlén, M.; Strømme, M.; Lindh, J. In situ thermal image analysis of selective laser sintering for oral dosage form manufacturing. J. Pharm. Biomed. Anal. 2023, 231, 115396.
- Kushwaha, A.K.; Rahman, M.H.; Hart, D.; Hughes, B.; Saldana, D.A.; Zollars, C.; Rajak, D.K.; Menezes, P.L. Chapter 3—Fundamentals of stereolithography: Techniques, properties, and applications. In Elsevier Series on Tribology and Surface Engineering, Tribology of Additively Manufactured Materials; Kumar, P., Misra, M., Menezes, P.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 87–106. ISBN 9780128213285.
- Ahn, D.; Kweon, J.H.; Choi, J.; Lee, S. Quantification of surface roughness of parts processed by laminated object manufacturing. J. Mater. Process. Technol. 2012, 212, 339–346.
- Zaharia, C.; Gabor, A.G.; Gavrilovici, A.; Stan, A.T.; Idorasi, L.; Sinescu, C.; Negruțiu, M.L. Digital dentistry-3D printing applications. J. Interdiscip. Med. 2017, 2, 50–53.
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing Encompassing the facets of dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172.
- Dikova, T.; Dzhendov, D.A.; Ivanov, D.; Bliznakova, K. Dimensional accuracy and surface roughness of polymeric dental bridges produced by different 3D printing processes. Arch. Mater. Sci. Eng. 2018, 94, 65–75.
- Liu, J.; Hwang, H.H.; Wang, P.; Whang, G.; Chen, S. Direct 3D-printing of cell-laden constructs in microfluidic architectures. Lab Chip 2016, 16, 1430–1438.
- Chang, S.L.; Lo, C.H.; Jiang, C.P.; Juan, D.J. The fit consideration of the denture manufactured by 3D printing and sintering. Int. J. Pharma Med. Biol. Sci. 2015, 4, 184–187.
- Carrel, J.P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin. Oral Implant. Res. 2016, 27, 55–62.
- Jasim, H.B.; Farhan, B.A. Practical analysis of direct metal laser sintering process. Mater. Today Proc. 2021, 45 Pt 6, 5469–5475.
- Ratanajanchai, M.; Kanchanavasita, W.; Suputtamongkol, K.; Wonglamsam, A.; Thamapipol, S.; Sae-Khow, O. Heat-cured poly(methyl methacrylate) resin incorporated with different food preservatives as an anti-microbial denture base material. J. Dent. Sci. 2021, 16, 706–712.
- Scheithauer, U.; Schwarzer, E.; Richter, H.J.; Moritz, T. Thermoplastic 3D printing—An additive manufacturing method for producing dense ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, 26–31.
- Poh, P.S.P.; Chhaya, M.P.; Wunner, F.M.; De-Juan-Pardo, E.M.; Schilling, A.F.; Schantz, J.-T.; van Griensven, M.; Hutmacher, D.W. Polylactides in additive biomanufacturing. Adv. Drug Deliv. Rev. 2016, 107, 228–246.
- Myers, D.; Abdel-Wahab, A.; Hafeez, F.; Kovacev, N.; Essa, K. Optimisation of the additive manufacturing parameters of polylactic acid (PLA) cellular structures for biomedical applications. J. Mech. Behav. Biomed. Mater. 2022, 136, 105447.
- Shengwei, H.; Zhiyong, W.; Qingang, H.; Wei, H. Combined use of an anterolateral thigh flap and rapid prototype modeling to reconstruct maxillary oncologic resections and midface defects. J. Craniofac. Surg. 2014, 25, 1147–1149.
- Saijo, H.; Igawa, K.; Kanno, Y.; Mori, Y.; Kondo, K.; Shimizu, K.; Suzuki, S.; Chikazu, D.; Iino, M.; Anzai, M. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J. Artif. Organs 2009, 12, 200–205.
- Sun, Y.; Luebbers, H.T.; Agbaje, J.O.; Schepers, S.; Vrielinck, L.; Lambrichts, I.; Politis, C. Accuracy of upper jaw positioning with intermediate splint fabrication after virtual planning in bimaxillary orthognathic surgery. J. Craniofac. Surg. 2013, 24, 1871–1876.
- Wang, G.; Li, J.; Khadka, A.; Hsu, Y.; Li, W.; Hu, J. CAD/CAM and rapid prototyped titanium for reconstruction of ramus defect and condylar fracture caused by mandibular reduction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 356–361.
- Bae, E.J.; Jeong, I.D.; Kim, W.C.; Kim, J.H. A comparative study of additive and subtractive manufacturing for dental restorations. J. Prosthet. Dent. 2017, 118, 187–193.
- Muta, S.; Ikeda, M.; Nikaido, T.; Sayed, M.; Sadr, A.; Suzuki, T.; Tagami, J. Chairside fabrication of provisional crowns on FDM 3D-printed PVA model. J. Prosthodont. Res. 2020, 64, 401–407.
- Arnesano, A.; Padmanabhan, S.K.; Notarangelo, A.; Montagna, F.; Licciulli, A. Fused deposition modeling shaping of glass infiltrated alumina for dental restoration. Ceram. Int. 2020, 46, 2206–2212.
- Revilla-León, M.; Husain, N.A.H.; Methani, M.M.; Özcan, M. Chemical composition, surface roughness, and ceramic bond strength of additively manufactured cobalt-chromium dental alloys. J. Prosthet. Dent. 2021, 125, 825–831.
- Baciu, E.R.; Cimpoe, R.; Vi, A.; Baciu, C.; Cimpoe, N.; Sodor, A.; Zegan, G.; Murariu, A. Surface analysis of 3D (SLM) CO–CR–W dental metallic materials. Appl. Sci. 2021, 11, 255.
- Avérous, L. Chapter 21—Polylactic Acid: Synthesis, Properties and Applications. In Alessandro Gandini, Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 433–450. ISBN 9780080453163.
- Jin, F.L.; Hu, R.R.; Park, S.J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. Part B Eng. 2019, 164, 287–296.
- Pandey, A.K.; Sirohi, R.; Upadhyay, S.; Mishra, M.; Kumar, V.; Singh, L.K.; Pandey, A. Chapter 12—Production and applications of polylactic acid. In Biomass, Biofuels, Biochemicals; Binod, P., Raveendran, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 309–357. ISBN 9780128218884.
- Mehrpouya, M.; Vahabi, H.; Janbaz, S.; Darafsheh, A.; Mazur, T.R.; Seeram Ramakrishna, S. 4D printing of shape memory polylactic acid (PLA). Polymer 2021, 230, 124080.
- Popescu, D.; Zapciu, A.; Amza, C.; Baciu, F.; Marinescu, R. FDM process parameters influence over the mechanical properties of polymer specimens: A review. Polym. Test. 2018, 69, 157–166.
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392.
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14.
- de Albuquerque, T.L.; Júnior, J.E.M.; de Queiroz, L.P.; Souza Ricardo, A.D.; Ponte Rocha, M.V. Polylactic acid production from biotechnological routes: A review. Int. J. Biol. Macromol. 2021, 186, 933–951.
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162.
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013, 5, 015001.
- Yue, J.; Zhao, P.; Gerasimov, J.Y.; Marieke, V.D.L.; Grotenhuis, A. 3D-printableantimicrobial composite resins. Adv. Funct. Mater. 2015, 25, 6756–6767.
More
