Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Laura Madalina Cursaru.
Core–shell nanoparticles are functional materials with tailored properties, able to improve the requirements of various applications. Both core and shell components can be inorganic or organic, and there are numerous studies in this field regarding their synthesis methods, properties, and applications.
- iron oxide
- core–shell nanostructures
- magnetic properties
1. Different Types of Core–Shell Structures with Fe3O4 Core for Biomedical Applications
Core–shell nanostructures are defined as heterogeneous nanoparticles composed of two or more nanomaterials that can be identified and are separated by distinct boundaries. Both core and shell components can be inorganic (metals, metal oxides) or organic (polymers, biomolecules) [48,49,50][1][2][3]. Core/shell composite nanostructures (NSs) have attracted much attention in recent years due to their diverse and unique material properties not shown by the core or shell materials alone, such as good mechanical, thermal, and optical properties [48,51][1][4]. These properties are significantly enhanced compared to pure compounds [51][4]. The interaction between the core and the shell of a nanostructure can lead to new properties and functions [45][5].
There are numerous core–shell materials with various applications and much literature about their classification and detailed descriptions of the preparation method.
Fe3O4 can be coated with different types of shells, such as metals (Ag, Au) [52[6][7][8][9][10],53,54,55,56], metal–organic frameworks (Cu–MOF), metal oxides (SiO2, TiO2, ZnO), and organic polymers (polyethyleneimine: PEI, polyacrylic acid: PAA, etc.), to obtain core–shell nanostructures with desired properties [3][11].
Core–shell nanostructures with Fe3O4 as a core have been a popular research topic over the last decade, with more than 700 articles published in the field, as shown in Figure 1a. As can be seen from Figure 1b, most of the papers published on this topic were research articles (>700 papers) and short communications (>40 papers). The data presented in Figure 1 were obtained using the ScienceDirect database (https://www.sciencedirect.com/) and searching for “Fe3O4 core–shell nanoparticles for biomedical applications”. The results were refined by year (selecting from 2012 to 2023) in Figure 1a and by article type in Figure 1b. These data were collected in May 2023.
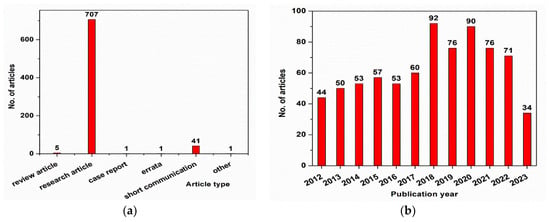
Figure 1.
(
a
) Evolution of the published articles in the field of Fe
3
O
4
core–shell nanoparticles; (
b
) types of papers published in the field of Fe
3
O
4
core–shell nanoparticles.
2. Metal-Coated Fe3O4
Silver-coated Fe3O4 nanohybrids have been used in a broad range of applications, including chemical and biological sensors [48[1][12],57], drug delivery—as successful drug carriers with focused antimicrobial, anticancer properties [48[1][13],58], diagnosis, and cancer therapy [48,59,60][1][14][15].
Different methods were used to synthesize Ag-coated Fe3O4 nanoparticles. Generally, a two-step synthesis procedure is applied: magnetite is prepared by a solvothermal, co-precipitation, or microemulsion route [57[12][16][17],61,62], obtaining spherical-shaped particles, and then Fe3O4 nanoparticles are dispersed in AgNO3 solution in the presence of an organic solvent (ethanol, di-chlorobenzene), a surfactant (oleylamine, cetyltrimethylammonium bromide—CTAB), and a reduction agent for Ag (butylamine, sodium borohydride). Another approach uses combined phyto- and hydrothermal synthesis, preparing the magnetite core in the presence of a plant extract (neem leaf extract, leaf extract of Eryngium planum, Vitis vinifera (grape) stem extract, Euphorbia peplus Linn leaf extract), followed by hydrothermal synthesis of Fe3O4–Ag (silver nitrate was added in the magnetite suspension). Plant extract acts as a reducing agent for silver shells [44,59,63,64][14][18][19][20]. Spherical core–shell structures with 7–80 nm are obtained in these cases [44,57,59,61,62,63,64][12][14][16][17][18][19][20]. Moreover, brick-like Ag-coated Fe3O4 nanoparticles with ~13 nm in width and ~15 nm in length were prepared by single-step thermal decomposition of the magnetite precursors in the presence of AgNO3 salt and 1,2-hexadecane-diol reduction agent [58][13].
It has been discovered that Fe3O4–Ag nanocomposites present a self-sterilizing property that avoids the formation of biofilms, which are the most dangerous source capable of spreading toxic bacteria into the environment [61][16], improving the contrast of magnetic resonance imaging (MRI) in cancer detection [48][1].
Similar synthesis methods as in the case of silver-doped magnetite core–shell structures (coprecipitation, thermal decomposition of Fe3O4), followed by reduction of HAuCl4 or gold acetate with various agents (NaBH4, sodium citrate, 1,2-hexadecane-diol), as well as combined phyto-hydrothermal synthesis (with Juglans regia green husk as reducing and stabilizing agent for HAuCl4), were reported in [65,66,67,68,69,70,71,72][21][22][23][24][25][26][27][28] for gold-coated magnetite nanostructures. In 2023, Danafar et al. [65][21] prepared Fe3O4–Au hybrid nanoparticles coated with bovine serum albumin (BSA) by co-precipitation of magnetite at 60 °C followed by the reduction of HAuCl4 with sodium citrate and NaBH4, resulting in Fe3O4–Au hybrids that were further coated with BSA under magnetic stirring at room temperature. They studied their potential application as a contrast agent in magnetic resonance imaging (cancer diagnosis). Gold nanoparticles represent a good option for Fe3O4 coating due to their good biocompatibility, large specific surface area, “surface plasmon” property, and well-known attraction for thiol groups from organic molecules [66][22]. Fe3O4–Au core–shell nanoparticles can be used in biomedical applications such as magnetic resonance imaging, hyperthermia, biosensors, immunosensors, photothermal therapy, controlled drug delivery, targeted gene delivery, protein separation, DNA detection, and DNA/RNA interaction [67,68,69,70,71][23][24][25][26][27].
3. Metal–Organic Framework (MOF) Coated Fe3O4
Fe3O4 nanoparticle was used as a core for improving the physicochemical properties and the thermal stability of the Cu–MOF compound. Metal–organic frameworks (MOFs) are a class of crystalline, porous materials composed of metal ions surrounded by multi-dented organic molecules. The metal ions form nodes that bind the arms of the organic ligands which act as linkers in the cage-like network structure. MOFs have a high surface area, significant porosity, tunable pore size, and high thermal stability in comparison to other nanostructures. Azizabadi et al. [51][4] prepared Fe3O4–Cu–MOFs by an ultrasonic-assisted reverse micelle synthesis (ultrasonic irradiation time of 10 min, temperature of 25 °C, power of 80 W) and found that this core–shell composite has good antibacterial activities against both Gram-positive and Gram-negative bacteria, which recommends it for advanced biomedical applications.
4. Metal Oxide-Coated Fe3O4
One of the most studied metal oxides as a shell for the Fe3O4 core was SiO2, due to the powerful attraction of magnetic nanoparticles to silica [73][29]. SiO2 particles are non-toxic, highly biocompatible, and abundant in surface hydroxyl groups, which makes them an ideal surface functional coating for magnetic nanoparticles in the medical field [3,74,75,76,77,78,79][11][30][31][32][33][34][35]. Fe3O4 nanoparticles coated with SiO2 shells obtained by Ta et al. through hydrolysis and condensation [75][31] showed increased biocompatible properties and provided new ideas for future bioconjugation studies [3][11]. Moreover, the Fe3O4–SiO2 core–shell structure prepared by Lu et al. using an ultrasound-assisted method [80][36] has good opportunities in the field of biomedicine [3][11].
TiO2 is another metal oxide with interesting properties such as biocompatibility, chemical inertness, high stability, and resistance to body fluids that lead to its use in cosmetics, pharmaceutics, and malignant tumor therapy [43,81,82][37][38][39]. The coating of magnetite nanoparticles with a TiO2 shell protects the core from environmental damage and improves biocompatible properties [43][37]. Fe3O4–TiO2 core–shell structures with various Fe3O4:TiO2 molar ratios were synthesized by a modified sol–gel method [83][40] or hydrothermal process [84][41]. The obtained Fe3O4–TiO2 core–shell nanorods are superparamagnetic and could be further used for magnetic hyperthermia applications [43][37].
Fe3O4–ZnO core–shell nanoparticles represent some of the most studied materials for magnetic hyperthermia and bio-imaging applications [33,85,86,87,88,89][42][43][44][45][46][47]. ZnO is well known for its anti-bacterial and biocompatible properties and possesses unique physical and chemical characteristics due to its wide bandgap and elevated exciton binding energy (piezoelectricity, photoluminescence, chemical stability) [90,91,92][48][49][50]. It has been demonstrated that ZnO–Fe3O4 composites combine the magnetic properties of Fe3O4 with the antibacterial activity of ZnO, resulting in a material with improved biocompatibility and enhanced antibacterial activity. ZnO–Fe3O4 composites inhibit microorganisms’ biofilm formation due to their synergetic activity of ion lixiviation (Fe3+, Zn2+) and oxidative activity. The material’s magnetic properties play a major role in reducing the ability of microorganisms to attach to different surfaces, inhibiting biofilm formation [85][43]. It is very important to hinder the formation of biofilm because its existence makes microorganisms more resistant to antibiotics. ZnO/Fe3O4 composites have shown enhanced antibacterial ability under visible light irradiation compared to single ZnO [93][51]. In 2021, Gupta et al. [33][42] reported the hydrothermal synthesis of Fe3O4–ZnO core–shell nanoparticles. The obtained material preserved the photoluminescence capacity of ZnO and the superparamagnetic properties of Fe3O4, demonstrating its potential use for hyperthermia therapy and fluorescent-based cellular imaging. Fe3O4–ZnO nanoparticles significantly reduced the viability of human cervical cancer cells (HeLa) under the applied AC magnetic field. However, in 2018, Madhubala et al. [87][45] found that only the lowest concentrations of Fe3O4–ZnO core–shell nanoparticles are non-toxic for cells and could be used for cancer treatment using magnetic hyperthermia therapy (MHT). Moreover, the authors concluded that Fe3O4–ZnO with a molar ratio of 1:20 has a small particle size and high crystallinity, and Fe3O4 is completely encapsulated in the ZnO nanoparticles [87][45].
5. Polymer-Coated Fe3O4
Magnetite surface coating with natural or synthetic polymers has been widely investigated [3,32,94,95,96,97,98,99,100][11][52][53][54][55][56][57][58][59] due to their good biocompatibility, biodegradability, non-toxicity, stability, and ability to modify physical-chemical surface properties. Covering magnetite with polymers improves the antibacterial and anticancer properties of core–shell nanoparticles. Different polymers such as polyethylene glycol (PEG), chitosan, poly-N-vinylpyrrolidone (PVP), hydroxyl ethylene cellulose (HEC), nanocrystalline cellulose (NCC), heparin-poloxamer (HP), poly(N-isopropyl acrylamide) (PNIPAAm), polyethyleneimine (PEI), and polyacrylic acid (PAA) have been coated on the Fe3O4 surface for tumor-targeted drug delivery. In 2021, Mohammadi et al. [95][54] synthesized magnetic nanoparticles with cross-linked PEG coatings using plasma treatment. The plasma-induced graft polymerization creates a cross-linked network of PEG chains, resulting in a rigid surface that hinders the burst release of the drug. The classical coprecipitation method of magnetite core followed by direct addition of chitosan or PEG shell and heating at 80 °C for 30 min [96][55] leads to an irregular and dendrimer-like surface morphology with small and large grain sizes. Fe3O4 surface functionalized with PEG has significant results at 20 mg/mL against antimicrobial activities. The anticancer activity was tested against HepG2 liver cancer cell lines, and magnetite-polymer nanoparticles are suitable for hyperthermia therapy to treat carcinoma.
When superparamagnetic iron oxide nanoparticles (SPIONs) were coated with heparin-poloxamer (HP) and the core–shell system was tested for anticancer drug delivery, doxorubicin (DOX) was entrapped in the polymer shell, showing a controlled release up to 120 h without any initial burst effect [98][57]. Moradi et al. [32][52] prepared Fe3O4 core–shell nanoparticles as drug nanocarriers, having PNIPAAm grafted with chitosan as a polymer shell. PNIPAAm is a thermo-responsive polymer, while chitosan is a pH-responsive moiety. Therefore, the highest release percentage of methotrexate (MTX) as a negatively charged anticancer drug has been observed at T = 40 °C and pH = 5.5.
References
- Yeneayehu, K.; Senbeta, T.; Mesfin, B. Enhancement of the Optical Response of Fe3O4@Ag Core-Shell Nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2021, 134, 114822.
- Díez, A.G.; Rincón-Iglesias, M.; Lanceros-Méndez, S.; Reguera, J.; Lizundia, E. Multicomponent Magnetic Nanoparticle Engineering: The Role of Structure-Property Relationship in Advanced Applications. Mater. Today Chem. 2022, 26, 101220.
- Khatami, M.; Alijani, H.Q.; Nejad, M.S.; Varma, R.S. Nanoparticles: Greener Synthesis Using Natural Plant Products. Appl. Sci. 2018, 8, 411.
- Azizabadi, O.; Akbarzadeh, F.; Danshina, S.; Chauhan, N.P.S.; Sargazi, G. An Efficient Ultrasonic Assisted Reverse Micelle Synthesis Route for Fe3O4@Cu-MOF/Core-Shell Nanostructures and Its Antibacterial Activities. J. Solid State Chem. 2021, 294, 121897.
- Izadiyan, Z.; Shameli, K.; Teow, S.Y.; Yusefi, M.; Kia, P.; Rasouli, E.; Tareq, M.A. Anticancer Activity of 5-Fluorouracil-Loaded Nanoemulsions Containing Fe3O4/Au Core-Shell Nanoparticles. J. Mol. Struct. 2021, 1245, 131075.
- Sun, Y.; Tian, Y.; He, M.; Zhao, Q.; Chen, C.; Hu, C.; Liu, Y. Controlled Synthesis of Fe3O4/Ag Core-Shell Composite Nanoparticles with High Electrical Conductivity. J. Electron. Mater. 2012, 41, 519–523.
- Amarjargal, A.; Tijing, L.D.; Im, I.T.; Kim, C.S. Simultaneous Preparation of Ag/Fe3O4 Core-Shell Nanocomposites with Enhanced Magnetic Moment and Strong Antibacterial and Catalytic Properties. Chem. Eng. J. 2013, 226, 243–254.
- Iglesias-Silva, E.; Rivas, J.; León Isidro, L.M.; López-Quintela, M.A. Synthesis of Silver-Coated Magnetite Nanoparticles. J. Non-Cryst. Solids 2007, 353, 829–831.
- Mandal, M.; Kundu, S.; Ghosh, S.K.; Panigrahi, S.; Sau, T.K.; Yusuf, S.M.; Pal, T. Magnetite Nanoparticles with Tunable Gold or Silver Shell. J. Colloid Interface Sci. 2005, 286, 187–194.
- Kalska-Szostko, B.; Wykowska, U.; Satuła, D. Magnetic Nanoparticles of Core-Shell Structure. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 527–536.
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, Surface Functionalization and Application of Fe3O4 Magnetic Nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165.
- Ghazanfari, M.; Johar, F.; Yazdani, A. Synthesis and Characterization of Fe3O4 @Ag Core-Shell: Structural, Morphological, and Magnetic Properties. J. Ultrafine Grained Nanostruct. Mater. 2014, 47, 97–103.
- Brollo, M.E.F.; López-Ruiz, R.; Muraca, D.; Figueroa, S.J.A.; Pirota, K.R.; Knobel, M. Compact 3O4 Core-Shell Nanoparticles by Means of Single-Step Thermal Decomposition Reaction. Sci. Rep. 2014, 4, 6839.
- Dehghan, Z.; Ranjbar, M.; Govahi, M.; Khakdan, F. Green Synthesis of Ag/Fe3O4 Nanocomposite Utilizing Eryngium Planum L. Leaf Extract and Its Potential Applications in Medicine. J. Drug Deliv. Sci. Technol. 2022, 67, 102941.
- Ding, Q.; Liu, D.; Guo, D.; Yang, F.; Pang, X.; Che, R.; Zhou, N.; Xie, J.; Sun, J.; Huang, Z.; et al. Shape-Controlled Fabrication of Magnetite Silver Hybrid Nanoparticles with High Performance Magnetic Hyperthermia. Biomaterials 2017, 124, 35–46.
- Nguyen-Tri, P.; Nguyen, V.T.; Nguyen, T.A. Biological Activity and Nanostructuration of Fe3O4-Ag/High Density Polyethylene Nanocomposites. J. Compos. Sci. 2019, 3, 34.
- Singh, P.; Upadhyay, C. Role of Silver Nanoshells on Structural and Magnetic Behavior of Fe3O4 Nanoparticles. J. Magn. Magn. Mater. 2018, 458, 39–47.
- Madhubala, V.; Nagarajan, C.; Baskaran, P.; Raguraman, V.; Kalaivani, T. Influences of Superparamagnetic Fe3O4@Ag Core-Shell Nanoparticles on the Growth Inhibition of Huh-7 Cells. Mater. Today Commun. 2023, 35, 106139.
- Venkateswarlu, S.; Natesh Kumar, B.; Prathima, B.; Anitha, K.; Jyothi, N.V.V. A Novel Green Synthesis of Fe3O4-Ag Core Shell Recyclable Nanoparticles Using Vitis Vinifera Stem Extract and Its Enhanced Antibacterial Performance. Phys. B Condens. Matter 2015, 457, 30–35.
- Sajjadi, M.; Nasrollahzadeh, M.; Mohammad Sajadi, S. Green Synthesis of Ag/Fe3O4 Nanocomposite Using Euphorbia Peplus Linn Leaf Extract and Evaluation of Its Catalytic Activity. J. Colloid Interface Sci. 2017, 497, 1–13.
- Danafar, H.; Baghdadchi, Y.; Barsbay, M.; Ghaffarlou, M.; Mousazadeh, N.; Mohammadi, A. Synthesis of Fe3O4-Gold Hybrid Nanoparticles Coated by Bovine Serum Albumin as a Contrast Agent in MR Imaging. Heliyon 2023, 9, e13874.
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Determination of Calreticulin Using Fe3O4@AuNPs Core-Shell Functionalized with PT(COOH)2 Polymer Modified Electrode: A New Platform for the Impedimetric Biosensing of Cancer Biomarkers. Sens. Actuators B Chem. 2022, 367, 132099.
- Tarhan, T.; Ulu, A.; Sariçam, M.; Çulha, M.; Ates, B. Maltose Functionalized Magnetic Core/Shell Fe3O4@Au Nanoparticles for an Efficient L-Asparaginase Immobilization. Int. J. Biol. Macromol. 2020, 142, 443–451.
- Wang, W.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed Core-Shell Fe3O4@Au Nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601.
- Chatterjee, K.; Sarkar, S.; Jagajjanani Rao, K.; Paria, S. Core/Shell Nanoparticles in Biomedical Applications. Adv. Colloid Interface Sci. 2014, 209, 8–39.
- Salihov, S.V.; Ivanenkov, Y.A.; Krechetov, S.P.; Veselov, M.S.; Sviridenkova, N.V.; Savchenko, A.G.; Klyachko, N.L.; Golovin, Y.I.; Chufarova, N.V.; Beloglazkina, E.K.; et al. Recent Advances in the Synthesis of Fe3O4@AU Core/Shell Nanoparticles. J. Magn. Magn. Mater. 2015, 394, 173–178.
- Rajkumar, S.; Prabaharan, M. Multi-Functional Core-Shell Fe3O4@Au Nanoparticles for Cancer Diagnosis and Therapy. Colloids Surf. B Biointerfaces 2019, 174, 252–259.
- Izadiyan, Z.; Shameli, K.; Miyake, M.; Teow, S.Y.; Peh, S.C.; Mohamad, S.E.; Mohd Taib, S.H. Green Fabrication of Biologically Active Magnetic Core-Shell Fe3O4/Au Nanoparticles and Their Potential Anticancer Effect. Mater. Sci. Eng. C 2019, 96, 51–57.
- Mostafaei, M.; Hosseini, S.N.; Khatami, M.; Javidanbardan, A.; Sepahy, A.A.; Asadi, E. Isolation of Recombinant Hepatitis B Surface Antigen with Antibody-Conjugated Superparamagnetic Fe3O4/SiO2 Core-Shell Nanoparticles. Protein Expr. Purif. 2018, 145, 1–6.
- Shao, H.; Qi, J.; Lin, T.; Zhou, Y. Preparation and Characterization of Fe3O4@SiO2@NMDP Core-Shell Structure Composite Magnetic Nanoparticles. Ceram. Int. 2018, 44, 2255–2260.
- Ta, T.K.H.; Trinh, M.T.; Long, N.V.; Nguyen, T.T.M.; Nguyen, T.L.T.; Thuoc, T.L.; Phan, B.T.; Mott, D.; Maenosono, S.; Tran-Van, H.; et al. Synthesis and Surface Functionalization of Fe3O4-SiO2 Core-Shell Nanoparticles with 3-Glycidoxypropyltrimethoxysilane and 1,1′-Carbonyldiimidazole for Bio-Applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 376–383.
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W. Synthesis and Characterization of Silica-Coated Iron Oxide Nanoparticles in Microemulsion: The Effect of Nonionic Surfactants. Langmuir 2001, 17, 2900–2906.
- Tadyszak, K.; Kertmen, A.; Coy, E.; Andruszkiewicz, R.; Milewski, S.; Kardava, I.; Scheibe, B.; Jurga, S.; Chybczyńska, K. Spectroscopic and Magnetic Studies of Highly Dispersible Superparamagnetic Silica Coated Magnetite Nanoparticles. J. Magn. Magn. Mater. 2017, 433, 254–261.
- Khalid, A.; Ahmed, R.M.; Taha, M.; Soliman, T.S. Fe3O4 Nanoparticles and Fe3O4 @SiO2 Core-Shell: Synthesize, Structural, Morphological, Linear, and Nonlinear Optical Properties. J. Alloys Compd. 2023, 947, 169639.
- Asgari, M.; Miri, T.; Soleymani, M.; Barati, A. A Novel Method for in Situ Encapsulation of Curcumin in Magnetite-Silica Core-Shell Nanocomposites: A Multifunctional Platform for Controlled Drug Delivery and Magnetic Hyperthermia Therapy. J. Mol. Liq. 2021, 324, 114731.
- Lu, C.H.; Chen, G.H.; Yu, B.; Cong, H.L.; Kong, L.M.; Guo, L. Design and Synthesis of Fe3O4@SiO2 Core-Shell Nanomaterials. Integr. Ferroelectr. 2017, 182, 46–52.
- Madhubala, V.; Nagarajan, C.; Baskaran, P.; Raguraman, V.; Kalaivani, T. Formulation of Magnetic Core-Shell Nanostructured Fe3O4@TiO2 for Cytotoxic Activity against Huh-7 Cells. Inorg. Chem. Commun. 2023, 149, 110430.
- Chen, X.; Selloni, A. Introduction: Titanium Dioxide (TiO2) Nanomaterials. Chem. Rev. 2014, 114, 9281–9282.
- Madhubala, V.; Pugazhendhi, A.; Thirunavukarasu, K. Cytotoxic and Immunomodulatory Effects of the Low Concentration of Titanium Dioxide Nanoparticles (TiO2 NPs) on Human Cell Lines—An In Vitro Study. Process Biochem. 2019, 86, 186–195.
- Khashan, S.; Dagher, S.; Tit, N.; Alazzam, A.; Obaidat, I. Novel Method for Synthesis of Fe3O4@TiO2 Core/Shell Nanoparticles. Surf. Coat. Technol. 2017, 322, 92–98.
- Rani, N.; Dehiya, B.S. Influence of Anionic and Non-Ionic Surfactants on the Synthesis of Core-Shell Fe3O4@TiO2 Nanocomposite Synthesized by Hydrothermal Method. Ceram. Int. 2020, 46, 23516–23525.
- Gupta, J.; Hassan, P.A.; Barick, K.C. Core-Shell Fe3O4@ZnO Nanoparticles for Magnetic Hyperthermia and Bio-Imaging Applications. AIP Adv. 2021, 11, 025207.
- Medina-Ramírez, I.E.; Díaz de León-Macias, C.E.; Pedroza-Herrera, G.; Gonzáles-Segovia, R.; Zapien, J.A.; Rodríguez-López, J.L. Evaluation of the Biocompatibility and Growth Inhibition of Bacterial Biofilms by ZnO, Fe3O4 and 3O4 Photocatalytic Magnetic Materials. Ceram. Int. 2020, 46, 8979–8994.
- Liu, H.; Wu, J.; Min, J.H.; Zhang, X.; Kim, Y.K. Tunable Synthesis and Multifunctionalities of Fe3O4-ZnO Hybrid Core-Shell Nanocrystals. Mater. Res. Bull. 2013, 48, 551–558.
- Madhubala, V.; Kalaivani, T. Phyto and Hydrothermal Synthesis of Fe3O4@ZnO Core-Shell Nanoparticles Using Azadirachta Indica and Its Cytotoxicity Studies. Appl. Surf. Sci. 2018, 449, 584–590.
- Manikandan, A.; Yogasundari, M.; Thanrasu, K.; Dinesh, A.; Raja, K.K.; Slimani, Y.; Jaganathan, S.K.; Srinivasan, R.; Baykal, A. Structural, Morphological and Optical Properties of Multifunctional Magnetic-Luminescent 3O4 Nanocomposite. Phys. E Low Dimens. Syst. Nanostruct. 2020, 124, 114291.
- Ahadpour Shal, A.; Jafari, A. Study of Structural and Magnetic Properties of Superparamagnetic Fe3O4-ZnO Core-Shell Nanoparticles. J. Supercond. Nov. Magn. 2014, 27, 1531–1538.
- Aljohar, A.Y.; Muteeb, G.; Zia, Q.; Siddiqui, S.; Aatif, M.; Farhan, M.; Khan, M.F.; Alsultan, A.; Jamal, A.; Alshoaibi, A.; et al. Anticancer Effect of Zinc Oxide Nanoparticles Prepared by Varying Entry Time of Ion Carriers against A431 Skin Cancer Cells In Vitro. Front. Chem. 2022, 10, 1069450.
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481.
- Wang, J.; Yang, J.; Li, X.; Wang, D.; Wei, B.; Song, H.; Li, X.; Fu, S. Preparation and Photocatalytic Properties of Magnetically Reusable Fe3O4@ZnO Core/Shell Nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2016, 75, 66–71.
- Sin, J.C.; Tan, S.Q.; Quek, J.A.; Lam, S.M.; Mohamed, A.R. Facile Fabrication of Hierarchical Porous ZnO/Fe3O4 Composites with Enhanced Magnetic, Photocatalytic and Antibacterial Properties. Mater. Lett. 2018, 228, 207–211.
- Moradi, S.; Najjar, R.; Hamishehkar, H.; Lotfi, A. Triple-Responsive Drug Nanocarrier: Magnetic Core-Shell Nanoparticles of Fe3O4@poly(N-Isopropylacrylamide)-Grafted-Chitosan, Synthesis and In Vitro Cytotoxicity Evaluation against Human Lung and Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 72, 103426.
- Tang, S.; Lan, Q.; Liang, J.; Chen, S.; Liu, C.; Zhao, J.; Cheng, Q.; Cao, Y.C.; Liu, J. Facile Synthesis of Fe3O4@PPy Core-Shell Magnetic Nanoparticles and Their Enhanced Dispersity and Acid Stability. Mater. Des. 2017, 121, 47–50.
- Mohammadi, M.A.; Asghari, S.; Aslibeiki, B. Surface Modified Fe3O4 Nanoparticles: A Cross-Linked Polyethylene Glycol Coating Using Plasma Treatment. Surf. Interfaces 2021, 25, 101271.
- Munir, T.; Mahmood, A.; Rasul, A.; Imran, M.; Fakhar-e-Alam, M. Biocompatible Polymer Functionalized Magnetic Nanoparticles for Antimicrobial and Anticancer Activities. Mater. Chem. Phys. 2023, 301, 127677.
- Bekaroğlu, M.G.; Alemdar, A.; İşçi, S. Comparison of Ionic Polymers in the Targeted Drug Delivery Applications as the Coating Materials on the Fe3O4 Nanoparticles. Mater. Sci. Eng. C 2019, 103, 109838.
- Thi, T.T.H.; Tran, D.H.N.; Bach, L.G.; Quang, H.V.; Nguyen, D.C.; Park, K.D.; Nguyen, D.H. Functional Magnetic Core-Shell System-Based Iron Oxide Nanoparticle Coated with Biocompatible Copolymer for Anticancer Drug Delivery. Pharmaceutics 2019, 11, 120.
- Ding, Y.; Shen, S.Z.; Sun, H.; Sun, K.; Liu, F.; Qi, Y.; Yan, J. Design and Construction of Polymerized-Chitosan Coated Fe3O4 Magnetic Nanoparticles and Its Application for Hydrophobic Drug Delivery. Mater. Sci. Eng. C 2015, 48, 487–498.
- Yeamsuksawat, T.; Zhao, H.; Liang, J. Characterization and Antimicrobial Performance of Magnetic Fe3O4@ Nanoparticles Synthesized via Suspension Technique. Mater. Today Commun. 2021, 28, 102481.
More
