You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Eleftheria Hatzimichael.
Classic Hodgkin lymphoma (cHL) is a lymphoid neoplasm composed of rare neoplastic Hodgkin and Reed–Sternberg (HRS) cells surrounded by a reactive tumor microenvironment (TME) with suppressive properties against anti-tumor immunity. TME is mainly composed of T cells (CD4 helper, CD8 cytotoxic and regulatory) and tumor-associated macrophages (TAMs), but the impact of these cells on the natural course of the disease is not absolutely understood.
- Hodgkin lymphoma
- tumor microenvironment
- tumor associated macrophages
- CD169+ macrophages
- immune evasion
- immunosuppression
1. T-Cells
T cells in the tumor microenvironment (TME) of HL have been the object of intense investigation, among all other immune cells, due to their abundance and functional plasticity.
1.1. CD4+ T-Cells
The most abundant cellular population in the TME of HL comprises CD4+ T cells, with a T-helper (Th) phenotype, which tend to gather around HRS cells in formations called rosettes (Figure 1). These cells express T cell exhaustion markers including PD-1, TOX and TOX2 [1]. A high percentage (>75%) of tumor-infiltrating CD4+ T cells was associated with decreased freedom from treatment failure (FFTF) [2]. Initially, it was believed that the TME of cHL is dominated by Th2 CD4+ T cells, with their increased number predicting improved disease-free survival (DFS) and event-free survival (EFS) [3]. Th2 cells are generally involved in type 2 immune response, mediated by IL-4, IL-5, IL-9 and IL-13, which participate in anti-helminthic immunity and tissue regeneration [4]. Nevertheless, more recent research revealed that Th cells in the TME of HL primarily polarized towards the Th1 phenotype [5][6]. Th1 immunity is based on IL-2, interferon (IFN)-γ and tumor necrosis factor (TNF)-β and regulates cell-mediated immune reactions that also protect against tumor cells [7][8].
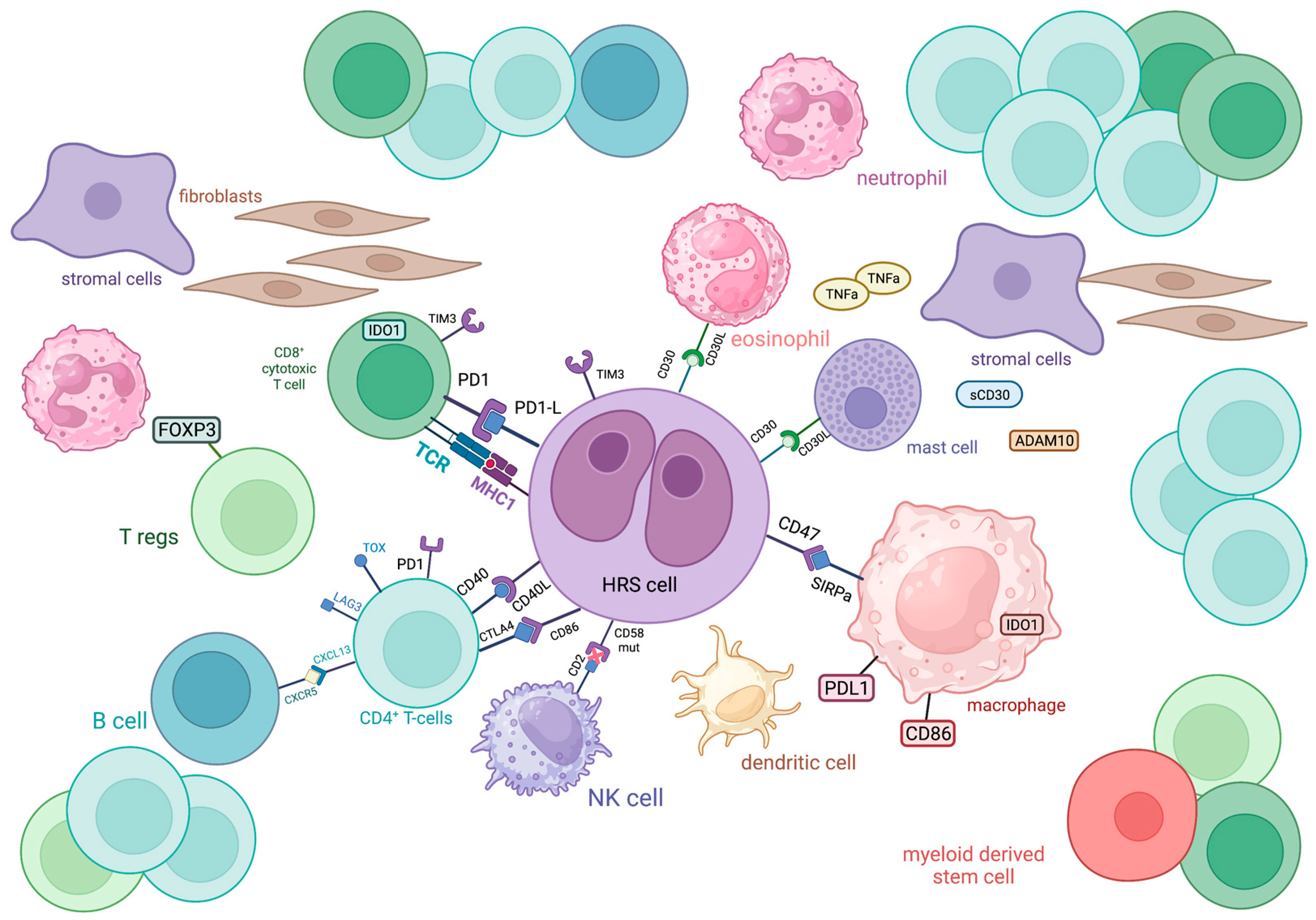
Figure 1.
Schematic representation of the immune cellular interlay within the tumor microenvironment of classic Hodgkin lymphoma.
FOXP3+ regulatory T cells (Tregs) are also present in the TME of cHL. These cells restrict Th-mediated immune responses through the secretion of immunosuppressive cytokines. In this way, they are involved in sustaining self-tolerance but also inhibit antitumor immunity [9]. In cHL, low numbers of FOXP3+ Tregs in TME, in combination with high numbers of cytolytic T cells, were correlated with shortened survival [10]. Additionally, the Tregs/Th17 ratio in cHL patients was found to be positively associated with survival, thus implying that higher Th17 infiltration might characterize a more aggressive disease course [11][12]. However, this reflects peripheral blood values and not the actual TME, present in tissues affected by cHL, where more extensive research is warranted to establish the putative prognostic significance of Tregs in the TME of cHL.
ADAM10: a disintegrin and metalloproteinase domain-containing protein 10; CTLA4: cytotoxic T-lymphocyte-associated protein 4; CXCL13: chemokine (C-X-C motif) ligand 13; CXCR5: C-X-C chemokine receptor type 5; FOXP3: forkhead box P3, IDO1: indoleamine-pyrrole 2,3-dioxygenase 1; LAG3: lymphocyte-activation gene 3; MHC: major histocompatibility complex; PD1: programmed cell death protein 1; PDL1: programmed death-ligand 1; SIRPa: signal regulatory protein α; TCR: T cell receptor; TIM3: T cell immunoglobulin and mucin domain-containing protein 3; TNFa: tumor necrosis factor α; TOX: thymocyte selection-associated high mobility group box
T cells in cHL express a variety of immune checkpoint regulators, including CTLA-4, PD-1 and LAG-3, shaping a unique immunosuppressive TME that enables HRS cells to escape antitumor immunity, as discussed later (Figure 1).
1.2. CD8+ T-Cells
CD8+ T cells are generally known as cytolytic T cells (CTLs) due to their capacity to directly kill infected or neoplastic cells after recognizing antigens bound to MHC (major histocompatibility complex)-I molecules on their surface and are, therefore, considered as important mediators of antitumor immunity, along with other major cytolytic cells, NK cells. In the case of cHL, the CD8+ T cell subpopulation is less abundant than the CD4+ one, with contradictory results regarding its prognostic value. Alonso-Álvarez et al. found that high numbers of CD8+ T cells predict better outcomes in patients treated with ABVD as first-line therapy [2]. In contrast, the presence of activated CTLs (positive for TIA-1 and granzyme B) in the TME of cHL has been correlated with decreased survival in the relapsed/refractory (R/R) setting [10][13]. Regardless of their prognostic role, CD8+ T cells seem to have an important role in shaping the TME of cHL, since they express immune checkpoint molecules such as PD-1, indoleamine 2,3-Dioxygenase 1 (IDO)-1 and TIM-3 more frequently than CD4+ T cells [14] (Figure 1). However, these cells seem to have diverged from their cytotoxic role against neoplastic cells. A subset of CD8+ T cells was identified in cHL TME that shares phenotypic and functional characteristics with T-follicular helper cells. Specifically, they co-express CXCR5 and ICOS, Bcl-6, PD-1 and CD200 and show deficient cytotoxicity and low IFN-γ secretion [15]. The function of CD8+ T cells might be negatively influenced by Galectin-1 produced by HRS cells [16].
2. B-Cells
The presence and prognostic value of non-neoplastic B cells of TME in cHL has been studied by independent research groups, based on their negative impact on several solid malignancies. Interestingly, high proportions of CD20+ background cells in TME were correlated with increased overall survival (OS), while low B cell counts were associated with shortened progression-free survival (PFS) and OS among patients treated with BEACOPP-based regimens; thus, B cells show both a prognostic and predictive value [17][18]. A possible explanation for B cells’ favorable effect might be the competition with neoplastic cells for survival and growth signals, although more research is needed to clarify whether all B cells or specific sub-populations have a favorable predictive impact since the presence of the PAX5+/CD38+ sub-population was shown to correlate with adverse outcomes [19][20].
3. Plasma Cells
Data on the role of plasma cells in the TME of cHL has been scarce so far. Tumor infiltration by CD138+ plasma cells is associated with advanced disease stage, eosinophil infiltration and the presence of B symptoms and a tendency towards inferior OS and EFS [21]. Additionally, elevated polyclonal serum-free light chains in patients with cHL showed a correlation with decreased survival and it has been assumed that these light chains are produced by plasma cells of the TME since HRS cells are considered incapable of secreting immunoglobulins [22].
4. NK-Cells
Although NK cells are innate lymphoid cells with known anti-tumor cytotoxic activity, in the case of HL TME, these cells seem to be numerically and functionally diminished. The inhibition of NK cytotoxic activity is primarily mediated by ligands found in TME which bind to NK-inactivating receptors [23]. Furthermore, in patients with HL, the ratio of CD56dimDNAM-1pos NK cells over CD56dimDNAM-1neg NK cells is reduced, indicating a shift of the NK phenotype towards the less cytotoxic DNAM-1neg population. Even CD56dimDNAM-1pos NK cells were found to show impaired cytotoxic activity in HL patients compared to healthy individuals [24]. One possible explanation for the limited NK population in HL TME might be the induction of apoptosis triggered by the binding of the Fas-L of HRS cells to the Fas receptor of NK cells [12]. Again, these results were obtained from peripheral blood analysis, and their contribution to the understanding of the actual TME of cHL should be analyzed by comparing data from similar biological materials.
5. Myeloid Cells
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells, expressing CD11b and CD33, which exert immunosuppressive roles when infiltrating tumors. Their immunosuppressive effect is mainly towards T cells, since their high expression of Arginase-I (Arg-I) is believed to deprive T cells in TME of L-arginine which is essential for their function [25][26] (Figure 1). High tissue levels of Arg-I-positive myeloid cells were associated with inferior disease outcomes in HL [27]. Similarly, a subset of MDSCs, circulating CD34+ MDSCs, were found to negatively influence the PFS of patients with HL [28]. Interestingly, MDSCs were reduced in patients after treatment with brentuximab vedotin (BV), and baseline serum Arg-I levels emerged as a potential predictive biomarker for BV treatment response [29].
Eosinophils represent one of the most typical cellular populations found in HL biopsies and they are believed to develop a close crosstalk with HRS cells via CD30-CD30L binding, but their prognostic value remains a matter of debate. Although research indicated that eosinophilic tumor infiltration strongly correlates with FFTF, Axdorph et al. did not find any association with clinical outcomes, thus implying the need for further relevant investigation [30][31].
Tumor-associated neutrophils (TANs), similarly to tumor-associated macrophages (TAMs), appear with variable effects in TME, from suppressing anti-tumor immunity to cytotoxicity against neoplastic cells [32]. Due to this and the immunohistochemical overlap with MDSCs, research on the prognostic impact of neutrophils in HL has been limited to their peripheral blood counts rather than tumor infiltration. Indeed, in cHL, a high absolute neutrophil count to a high absolute lymphocyte count ratio is an independent prognostic factor for patients’ reduced OS [33].
6. Mast Cells
Evidence on the prognostic role of mast cells in HL is controversial, although their biological properties have been well-described. Mast cells are the predominant cells of the TME that express CD30L, the ligand for the CD30 receptor of HRS cells, thus indicating a close interaction with the neoplastic population [34] (Figure 1). Additionally, mast cells are believed to promote tumor growth via the induction of neovascularization and fibrosis, functions that can be inhibited by bortezomib, thus providing a potential therapeutic target [35]. The hypothesized negative prognostic impact, in terms of reduced relapse-free survival, of mast cells in the TME of HL was indeed demonstrated by Molin et al. [36], although other researchers did not find a correlation between mast cell infiltration and prognosis [37].
7. Dendritic Cells
Dendritic cells (DCs) have also been studied in the TME of HL with variable results, depending on the specific DC subtype. CD123+ plasmacytoid DCs are the most abundant DC type in cHL, although they do not seem to correlate with disease-specific survival and they produce reduced amounts of IFN-a compared to healthy individuals, implying an immune functional defect [38][39]. As for myeloid DCs, most of them in cHL TME are identified as a mature CD83+ subtype whose number is positively associated with improved disease-specific survival of patients [39]. Finally, the presence of follicular DCs in most subtypes of HL was found to predict a favorable outcome [40]. Patients with cHL were also found to have lower counts of all subtypes of circulating DCs compared to healthy individuals [41].
8. Tumor-Associated Macrophages
In general, macrophages are derived from mononuclear cells and have multiple roles including, but not limited to, phagocytosis, antigen presentation to other immune cells and tissue remodeling. Among all immune cells found in the TME of cHL, macrophages have attracted the most research interest. This is because of the great plasticity of these cells, indicated by their ability to acquire different phenotypes that influence the tumor microenvironment towards an immunosuppressive or inflammatory state. Accordingly, macrophages variably influence disease progression, and this is probably the reason why the association of TAMs with disease outcomes, treatment response and patients’ survival has been so challenging over the years.
TAMs are recruited in TME through GM-CSF, CCL2, CCL5, CCL7 and CXCL1, which are secreted by neoplastic cells. There, TAMs are programmed towards M1 or M2 phenotypes. Although initially considered as distinct subtypes, this view has been criticized as oversimplified, and the current notion is that M1 and M2 phenotypes actually represent the extremities of a continuum spectrum [42][43].
The M1 phenotype is triggered by GM-CSF, IFN-γ and lipopolysaccharides and is characterized by cytotoxic, pro-inflammatory and anti-neoplastic effects mediated by the secretion of TNF-a, NO, CXCL 9, CXCL10, CXCL11, IL-1, IL-6, IL-12, IL-23 and ROS by M1 macrophages. On the other hand, M2-polarized macrophages, driven by M-CSF, TGF-B, IL-4, IL-10 and IL-13, are believed to promote wound healing, angiogenesis and tumor growth by producing tumor growth factor (TGF)-β, IL-10, CCL17, CCL18, CCL22, CD206, CD204 and CD163 [44]. Apparently, the balance between the anti-neoplastic and pro-tumorigenic phenotypes is crucial for determining disease outcomes and a tempting field for therapeutic interventions (Figure 2).
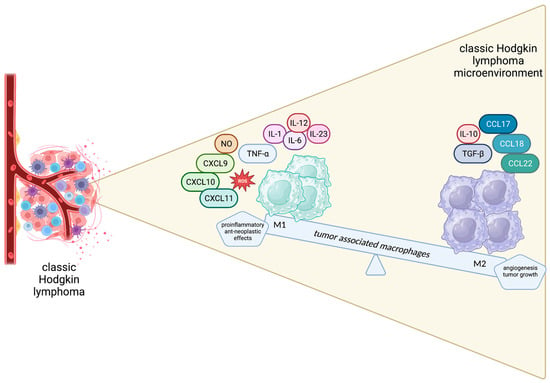
Figure 2.
Schematic representation of the spectrum of macrophages in the Hodgkin lymphoma microenvironment.
In cHL, HRS cells can lead TAMs to polarize towards the tumor-promoting M2 phenotype via the secretion of TGF-β and IL-13. In turn, M2 macrophages support the survival of HRS cells, partially through the activation of the STAT3 signaling pathway [44]. As previously discussed, TAMs actively participate in shaping the protective niche around HRS cells and express several immunosuppressive molecules such as IDO-1 and immune checkpoint proteins such as PD-L1 and CD86, through which they interact with immune cells of the TME, further promoting the suppression of anti-tumor immunity [45][46]. Another mechanism in which TAMs might promote tumor growth is supposed to be the induction of the genetic instability of HRS cells, probably through the release of free radicals which contribute to a mutagenic microenvironment. This was based on the observation that TP53 amplification in neoplastic cells, linked with poorer patients’ survival, was associated with increased infiltration by M2 macrophages [47].
9. CD169+ Macrophages: A New Regulator of Antitumor Immunity
CD169+ macrophages constitute a subpopulation distinct from M1 and M2 phenotypes, as they can simultaneously express markers of both M1 and M2 subtypes. Normally, they are primarily detected in the metallophilic marginal zone of the spleen and in the medulla and the subcapsular sinus of lymph nodes, but they can also be found in the intestine, liver and bone marrow. Based on their localization, CD169+ macrophages (also known as Siglec-1 positive macrophages) basically function as “gatekeepers” of secondary lymphoid organs, since they are the first cell type that captures antigens in lymph nodes and the spleen, present them to other immune cells and, thus, help the activation of T cells and initiate adaptive immune responses. Apart from viral and bacterial inflammatory responses, CD169+ macrophages participate in immune tolerance induced by apoptotic cell clearance [48][49]. More interestingly, it has been demonstrated that CD169+ macrophages phagocytize dead tumor cells transported via lymphatic flow and present tumor-associated peptides to CD8+ T cells, whose cytotoxic activity is augmented, which is considered a crucial step for the induction of antitumor immunity [50][51].
Indeed, the biological and prognostic role of CD169+ macrophages in several human malignancies has gathered research interest. A higher concentration of CD169+ macrophages in the primary tumors or regional lymph nodes has been associated with improved outcomes in patients with melanoma, hepatocellular carcinoma, colorectal, bladder and endometrial cancers, with contradictory results in breast cancer [52][53][54][55][56][57][58] (Figure 3). Moreover, Marmey et al. investigated, using immunohistochemistry on paraffin-embedded tissues, the expression of CD169 in 51 cases of B cell non-Hodgkin lymphomas (including diffuse large B cell lymphomas, B-chronic lymphocytic leukemias, follicular lymphomas, mantle cell lymphomas and splenic marginal zone lymphomas). Only splenic marginal zone lymphomas (15 cases) showed a remarkable increase in CD169+ cells, with preferential distribution in the splenic cords of the red pulp [59]. These CD169+ cells were also positive for CD14 (monocyte/macrophage marker), and it has been hypothesized that the CD169+/CD14+ cells observed in the splenic cords of splenic marginal zone lymphomas might have a dendritic cell differentiation potential [59]. In this latter restudyearch, however, no information about any prognostic implication of the CD169 immunostaining patterns in B cell non-Hodgkin lymphomas was reported [59]. Interestingly, to the best of our knowledge, there are no published studies regarding the potential prognostic impact of the immunohistochemical expression patterns of the CD169+ macrophages in the TME of cHL. This investigation could permit a better understanding of the interactions taking place in the TME of cHL and might indicate a new prognostic biomarker or even a therapeutic target, given the already-discussed role of M1 and M2 subpopulations in cHL.
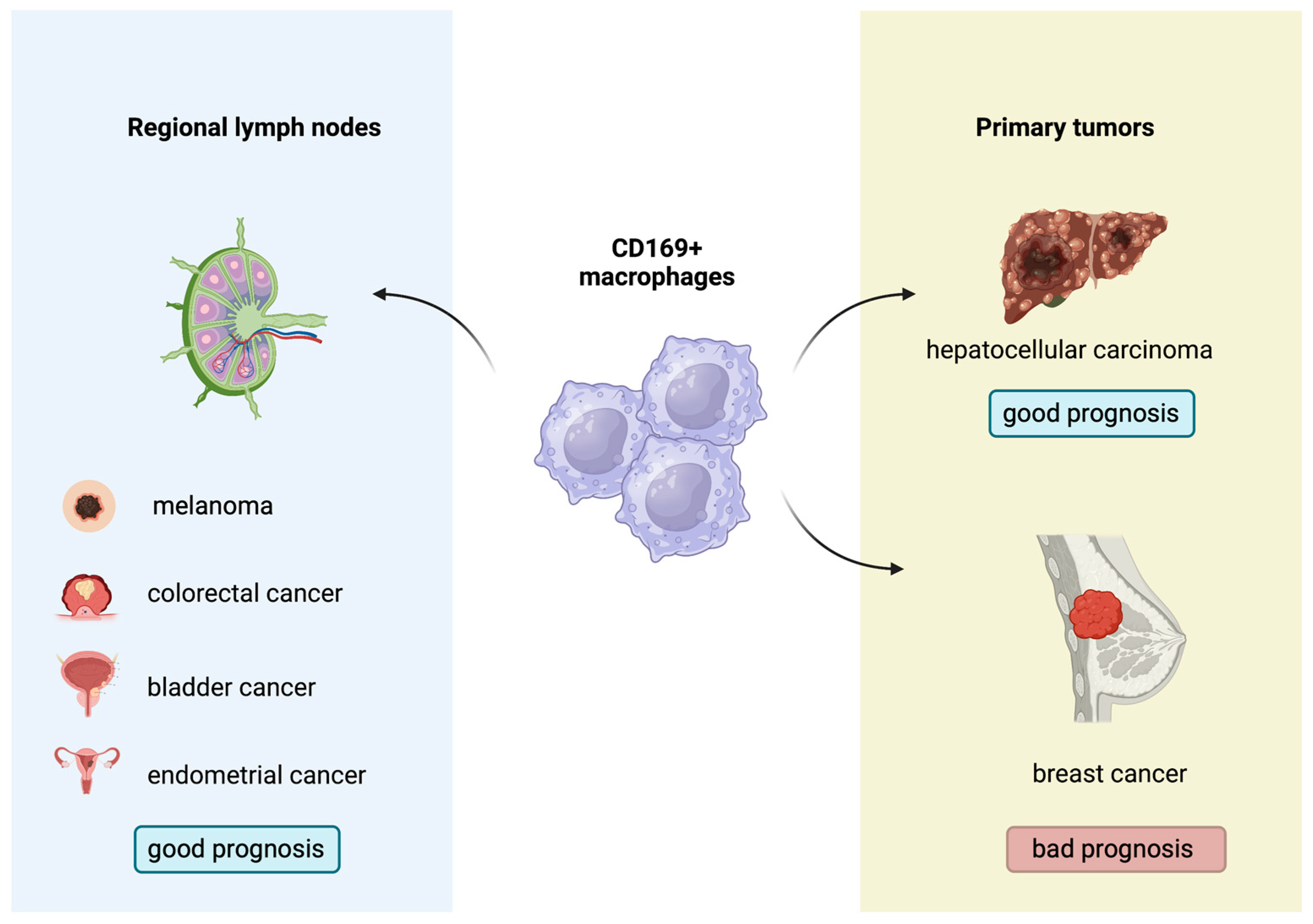
Figure 3.
Prognostic role of CD169
+
macrophages in several solid malignancies.
References
- Takahara, T.; Satou, A.; Tsuzuki, T.; Nakamura, S. Hodgkin Lymphoma: Biology and Differential Diagnostic Problem. Diagnostics 2022, 12, 1507.
- Alonso-Álvarez, S.; Vidriales, M.B.; Caballero, M.D.; Blanco, O.; Puig, N.; Martin, A.; Peñarrubia, M.J.; Zato, E.; Galende, J.; Bárez, A.; et al. The number of tumor infiltrating T-cell subsets in lymph nodes from patients with Hodgkin lymphoma is associated with the outcome after first line ABVD therapy. Leuk. Lymphoma 2017, 58, 1144–1152.
- Schreck, S.; Friebel, D.; Buettner, M.; Distel, L.; Grabenbauer, G.; Young, L.S.; Niedobitek, G. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol. Oncol. 2009, 27, 31–39.
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133.
- Cader, F.Z.; Schackmann, R.C.J.; Hu, X.; Wienand, K.; Redd, R.; Chapuy, B.; Ouyang, J.; Paul, N.; Gjini, E.; Lipschitz, M.; et al. Mass cytometry of Hodgkin lymphoma reveals a CD4+ regulatory T-cell–rich and exhausted T-effector microenvironment. Blood 2018, 132, 825–836.
- Greaves, P.; Clear, A.; Owen, A.; Iqbal, S.; Lee, A.; Matthews, J.; Wilson, A.; Calaminici, M.; Gribben, J.G. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood 2013, 122, 2856–2863.
- Romagnani, S. Th1/Th2 Cells. Inflamm. Bowel Dis. 1999, 5, 285–294.
- Nishimura, T.; Nakui, M.; Sato, M.; Iwakabe, K.; Kitamura, H.; Sekimoto, M.; Ohta, A.; Koda, T.; Nishimura, S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother. Pharmacol. 2000, 46, S52–S61.
- Cretney, E.; Kallies, A.; Nutt, S.L. Differentiation and function of Foxp3+ effector regulatory T cells. Trends Immunol. 2013, 34, 74–80.
- Álvaro, T.; Lejeune, M.; Salvadó, M.T.; Bosch, R.; García, J.F.; Jaén, J.; Banham, A.H.; Roncador, G.; Montalbán, C.; Piris, M.A. Outcome in Hodgkin’s Lymphoma Can Be Predicted from the Presence of Accompanying Cytotoxic and Regulatory T Cells. Clin. Cancer Res. 2005, 11, 1467–1473.
- Dehghani, M.; Kalani, M.; Golmoghaddam, H.; Ramzi, M.; Arandi, N. Aberrant peripheral blood CD4+ CD25+ FOXP3+ regulatory T cells/T helper-17 number is associated with the outcome of patients with lymphoma. Cancer Immunol. Immunother. 2020, 69, 1917–1928.
- Ferrarini, I.; Rigo, A.; Visco, C.; Krampera, M.; Vinante, F. The Evolving Knowledge on T and NK Cells in Classic Hodgkin Lymphoma: Insights into Novel Subsets Populating the Immune Microenvironment. Cancers 2020, 12, 3757.
- Koreishi, A.F.; Saenz, A.J.; Persky, D.O.; Cui, H.; Moskowitz, A.; Moskowitz, C.H.; Teruya-Feldstein, J. The Role of Cytotoxic and Regulatory T cells in Relapsed/Refractory Hodgkin Lymphoma. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 206–211.
- Karihtala, K.; Leivonen, S.-K.; Karjalainen-Lindsberg, M.-L.; Chan, F.C.; Steidl, C.; Pellinen, T.; Leppä, S. Checkpoint protein expression in the tumor microenvironment defines the outcome of classical Hodgkin lymphoma patients. Blood Adv. 2022, 6, 1919–1931.
- Le, K.-S.; Amé-Thomas, P.; Tarte, K.; Gondois-Rey, F.; Granjeaud, S.; Orlanducci, F.; Foucher, E.D.; Broussais, F.; Bouabdallah, R.; Fest, T.; et al. CXCR5 and ICOS expression identifies a CD8 T-cell subset with TFH features in Hodgkin lymphomas. Blood Adv. 2018, 2, 1889–1900.
- Gandhi, M.; Moll, G.; Smith, C.; Dua, U.; Lambley, E.; Ramuz, O.; Gill, D.; Marlton, P.; Seymour, J.F.; Khanna, R. Galectin-1 mediated suppression of Epstein-Barr virus–specific T-cell immunity in classic Hodgkin lymphoma. Blood 2007, 110, 1326–1329.
- Jachimowicz, R.D.; Pieper, L.; Reinke, S.; Gontarewicz, A.; Plütschow, A.; Haverkamp, H.; Frauenfeld, L.; Fend, F.; Overkamp, M.; Jochims, F.; et al. Whole-slide image analysis of the tumor microenvironment identifies low B-cell content as a predictor of adverse outcome in patients with advanced-stage classical Hodgkin lymphoma treated with BEACOPP. Haematologica 2021, 106, 1684–1692.
- Panico, L.; Tenneriello, V.; Ronconi, F.; Lepore, M.; Cantore, N.; Dell’angelo, A.C.; Ferbo, L.; Ferrara, F. High CD20+ background cells predict a favorable outcome in classical Hodgkin lymphoma and antagonize CD68+ macrophages. Leuk. Lymphoma 2015, 56, 1636–1642.
- Calabretta, E.; D’amore, F.; Carlo-Stella, C. Immune and Inflammatory Cells of the Tumor Microenvironment Represent Novel Therapeutic Targets in Classical Hodgkin Lymphoma. Int. J. Mol. Sci. 2019, 20, 5503.
- Tudor, C.S.; Distel, L.V.; Eckhardt, J.; Hartmann, A.; Niedobitek, G.; Buettner, M. B cells in classical Hodgkin lymphoma are important actors rather than bystanders in the local immune reaction. Hum. Pathol. 2013, 44, 2475–2486.
- Gholiha, A.R.; Hollander, P.; Hedstrom, G.; Sundstrom, C.; Molin, D.; Smedby, K.E.; Hjalgrim, H.; Glimelius, I.; Amini, R.; Enblad, G. High tumour plasma cell infiltration reflects an important microenvironmental component in classic Hodgkin lymphoma linked to presence of B-symptoms. Br. J. Haematol. 2019, 184, 192–201.
- Thompson, C.A.; Maurer, M.J.; Cerhan, J.R.; Katzmann, J.A.; Ansell, S.M.; Habermann, T.M.; Macon, W.R.; Weiner, G.J.; Link, B.K.; Witzig, T.E. Elevated serum free light chains are associated with inferior event free and overall survival in Hodgkin lymphoma. Am. J. Hematol. 2011, 86, 998–1000.
- Chiu, J.; Ernst, D.M.; Keating, A. Acquired Natural Killer Cell Dysfunction in the Tumor Microenvironment of Classic Hodgkin Lymphoma. Front. Immunol. 2018, 9, 267.
- Stannard, K.A.; Lemoine, S.; Waterhouse, N.J.; Vari, F.; Chatenoud, L.; Gandhi, M.K.; Martinet, L.; Smyth, M.J.; Guillerey, C. Human peripheral blood DNAM-1neg NK cells are a terminally differentiated subset with limited effector functions. Blood Adv. 2019, 3, 1681–1694.
- Raber, P.; Ochoa, A.C.; Rodríguez, P.C. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: Mechanisms of T cell suppression and therapeutic perspectives. Immunol. Investig. 2012, 41, 614–634.
- Elliott, L.A.; Doherty, G.A.; Sheahan, K.; Ryan, E.J. Human Tumor-Infiltrating Myeloid Cells: Phenotypic and Functional Diversity. Front. Immunol. 2017, 8, 86.
- Bertuzzi, C.; Sabattini, E.; Agostinelli, C. Immune Microenvironment Features and Dynamics in Hodgkin Lymphoma. Cancers 2021, 13, 3634.
- Romano, A.; Parrinello, N.L.; Vetro, C.; Forte, S.; Chiarenza, A.; Figuera, A.; Motta, G.; Palumbo, G.A.; Ippolito, M.; Consoli, U.; et al. Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin Lymphoma patients treated up-front with a risk-adapted strategy. Br. J. Haematol. 2015, 168, 689–700.
- Romano, A.; Parrinello, N.L.; Chiarenza, A.; Motta, G.; Tibullo, D.; Giallongo, C.; La Cava, P.; Camiolo, G.; Puglisi, F.; Palumbo, G.A.; et al. Immune off-target effects of Brentuximab Vedotin in relapsed/refractory Hodgkin Lymphoma. Br. J. Haematol. 2019, 185, 468–479.
- Axdorph, U.; Porwit-MacDonald, A.; Grimfors, G.; Björkholm, M. Tissue Eosinophilia in Relation to Immunopathological and Clinical Characteristics in Hodgkin’s Disease. Leuk. Lymphoma 2001, 42, 1055–1065.
- von Wasielewski, R.; Seth, S.; Franklin, J.; Fischer, R.; Hubner, K.; Hansmann, M.L.; Diehl, V.; Georgii, A. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin’s disease, allowing for known prognostic factors. Blood 2000, 95, 1207–1213.
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146.
- Koh, Y.W.; Kang, H.J.; Park, C.; Yoon, D.H.; Kim, S.; Suh, C.; Kim, J.E.; Kim, C.-W.; Huh, J. Prognostic Significance of the Ratio of Absolute Neutrophil Count to Absolute Lymphocyte Count in Classic Hodgkin Lymphoma. Am. J. Clin. Pathol. 2012, 138, 846–854.
- Molin, D.; Fischer, M.; Xiang, Z.; Larsson, U.; Harvima, I.; Venge, P.; Nilsson, K.; Sundström, C.; Enblad, G.; Nilsson, G. Mast cells express functional CD30 ligand and are the predominant CD30L-positive cells in Hodgkin’s disease. Br. J. Haematol. 2001, 114, 616–623.
- Mizuno, H.; Nakayama, T.; Miyata, Y.; Saito, S.; Nishiwaki, S.; Nakao, N.; Takeshita, K.; Naoe, T. Mast cells promote the growth of Hodgkin’s lymphoma cell tumor by modifying the tumor microenvironment that can be perturbed by bortezomib. Leukemia 2012, 26, 2269–2276.
- Molin, D.; Edström, A.; Glimelius, I.; Glimelius, B.; Nilsson, G.; Sundström, C.; Enblad, G. Mast cell infiltration correlates with poor prognosis in Hodgkin’s lymphoma. Br. J. Haematol. 2002, 119, 122–124.
- Keresztes, K.; Szollosi, Z.; Simon, Z.; Tarkanyi, I.; Nemes, Z.; Illes, A. Retrospective analysis of the prognostic role of tissue eosinophil and mast cells in Hodgkin’s lymphoma. Pathol. Oncol. Res. 2007, 13, 237–242.
- Shodell, M.; Kempin, S.; Siegal, F.P. Plasmacytoid dendritic cell and CD4+ T cell deficiencies in untreated Hodgkin disease: Implications for susceptibility to opportunistic infections. Leuk. Lymphoma 2014, 55, 2656–2657.
- Tudor, C.S.; Bruns, H.; Daniel, C.; Distel, L.V.; Hartmann, A.; Gerbitz, A.; Buettner, M.J. Macrophages and Dendritic Cells as Actors in the Immune Reaction of Classical Hodgkin Lymphoma. PLoS ONE 2014, 9, e114345.
- Alavaikko, M.J.; Blanco, G.; Aine, R.; Lehtinen, T.; Fellbaum, C.; Taskinen, P.J.; Sarpola, M.A.; Hansmann, M.-L. Follicular Dendritic Cells Have Prognostic Relevance in Hodgkin’s Disease. Am. J. Clin. Pathol. 1994, 101, 761–767.
- Galati, D.; Zanotta, S.; Corazzelli, G.; Bruzzese, D.; Capobianco, G.; Morelli, E.; Arcamone, M.; De Filippi, R.; Pinto, A. Circulating dendritic cells deficiencies as a new biomarker in classical Hodgkin lymphoma. Br. J. Haematol. 2018, 184, 594–604.
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365.
- Cencini, E.; Fabbri, A.; Sicuranza, A.; Gozzetti, A.; Bocchia, M. The Role of Tumor-Associated Macrophages in Hematologic Malignancies. Cancers 2021, 13, 3597.
- Xie, Y.; Yang, H.; Yang, C.; He, L.; Zhang, X.; Peng, L.; Zhu, H.; Gao, L. Role and Mechanisms of Tumor-Associated Macrophages in Hematological Malignancies. Front. Oncol. 2022, 12, 933666.
- Carey, C.D.; Gusenleitner, D.; Lipschitz, M.; Roemer, M.G.M.; Stack, E.C.; Gjini, E.; Hu, X.; Redd, R.; Freeman, G.J.; Neuberg, D.; et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 2017, 130, 2420–2430.
- Patel, S.S.; Weirather, J.L.; Lipschitz, M.; Lako, A.; Chen, P.-H.; Griffin, G.K.; Armand, P.; Shipp, M.A.; Rodig, S.J. The microenvironmental niche in classic Hodgkin lymphoma is enriched for CTLA-4-positive T cells that are PD-1-negative. Blood 2019, 134, 2059–2069.
- Hančić, S.; Gršković, P.; Gašparov, S.; Kolonić, S.O.; Dominis, M.; Korać, P. Macrophage Infiltration Correlates with Genomic Instability in Classic Hodgkin Lymphoma. Biomedicines 2022, 10, 579.
- Liu, Y.; Xia, Y.; Qiu, C.-H. Functions of CD169 positive macrophages in human diseases (Review). Biomed. Rep. 2021, 14, 26.
- Grabowska, J.; Lopez-Venegas, M.; Affandi, A.J.; Haan, J.D. CD169+ Macrophages Capture and Dendritic Cells Instruct: The Interplay of the Gatekeeper and the General of the Immune System. Front. Immunol. 2018, 9, 2472.
- Affandi, A.J.; Olesek, K.; Grabowska, J.; Twilhaar, M.K.N.; Rodríguez, E.; Saris, A.; Zwart, E.S.; Nossent, E.J.; Kalay, H.; de Kok, M.; et al. CD169 Defines Activated CD14+ Monocytes with Enhanced CD8+ T Cell Activation Capacity. Front. Immunol. 2021, 12, 697840.
- Asano, K.; Nabeyama, A.; Miyake, Y.; Qiu, C.-H.; Kurita, A.; Tomura, M.; Kanagawa, O.; Fujii, S.-I.; Tanaka, M. CD169-Positive Macrophages Dominate Antitumor Immunity by Crosspresenting Dead Cell-Associated Antigens. Immunity 2011, 34, 85–95.
- Komohara, Y.; Ohnishi, K.; Takeya, M. Possible functions of CD169-positive sinus macrophages in lymph nodes in anti-tumor immune responses. Cancer Sci. 2017, 108, 290–295.
- Asano, T.; Ohnishi, K.; Shiota, T.; Motoshima, T.; Sugiyama, Y.; Yatsuda, J.; Kamba, T.; Ishizaka, K.; Komohara, Y. CD 169-positive sinus macrophages in the lymph nodes determine bladder cancer prognosis. Cancer Sci. 2018, 109, 1723–1730.
- Zhang, Y.; Li, J.-Q.; Jiang, Z.-Z.; Li, L.; Wu, Y.; Zheng, L. CD169 identifies an anti-tumour macrophage subpopulation in human hepatocellular carcinoma. J. Pathol. 2016, 239, 231–241.
- Briem, O.; Källberg, E.; Kimbung, S.; Veerla, S.; Stenström, J.; Hatschek, T.; Hagerling, C.; Hedenfalk, I.; Leandersson, K. CD169+ Macrophages in Primary Breast Tumors Associate with Tertiary Lymphoid Structures, Tregs and a Worse Prognosis for Patients with Advanced Breast Cancer. Cancers 2023, 15, 1262.
- Ohnishi, K.; Komohara, Y.; Saito, Y.; Miyamoto, Y.; Watanabe, M.; Baba, H.; Takeya, M. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013, 104, 1237–1244.
- Saito, Y.; Ohnishi, K.; Miyashita, A.; Nakahara, S.; Fujiwara, Y.; Horlad, H.; Motoshima, T.; Fukushima, S.; Jinnin, M.; Ihn, H.; et al. Prognostic Significance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunol. Res. 2015, 3, 1356–1363.
- Ohnishi, K.; Yamaguchi, M.; Erdenebaatar, C.; Saito, F.; Tashiro, H.; Katabuchi, H.; Takeya, M.; Komohara, Y. Prognostic significance of CD 169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. 2016, 107, 846–852.
- Marmey, B.; Boix, C.; Barbaroux, J.-B.; Dieu-Nosjean, M.-C.; Diebold, J.; Audouin, J.; Fridman, W.-H.; Mueller, C.G.; Molina, T.J. CD14 and CD169 expression in human lymph nodes and spleen: Specific expansion of CD14+CD169− monocyte-derived cells in diffuse large B-cell lymphomas. Hum. Pathol. 2006, 37, 68–77.
More
