Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Haruaki Kitagawa.
Surface Pre-Reacted Glass-ionomer (S-PRG) filler, which releases strontium (Sr2+), borate (BO33−), fluoride (F−), sodium (Na+), silicate (SiO32−), and aluminum (Al3+) ions at high concentrations, is a unique glass filler that are utilized in dentistry. Because of its multiple-ion releasing characteristics, S-PRG filler exhibits several bioactivities such as tooth strengthening, acid neutralization, promotion of mineralization, inhibition of bacteria and fungi, inhibition of matrix metalloproteinases, and enhancement of cell activity. Therefore, S-PRG filler per se and S-PRG filler-containing materials have the potential to be beneficial for various dental treatments and care.
- dental
- bioactive
- ion release
- glass filler
- prevention
- S-PRG
1. What Is S-PRG Filler
S-PRG filler technology was developed by Shofu Inc. and the resin composites incorporating S-PRG filler were first commercialized in 2000. S-PRG filler is a three-layered fine glass particle composed of a SiO2 coating in the outermost layer followed by a pre-reacted glass-ionomer phase and a glass core (Figure 1A). The pre-reacted glass-ionomer phase is prepared by spraying a polyacrylic acid that penetrates the SiO2 coating layer and causes an acid-base reaction with the fluoro-boro-aluminosilicate core glass. Many products containing S-PRG filler are already available on the market, and a series of those materials are called GIOMER. Three types of S-PRG filler with particle sizes of 3.0, 0.8, and 0.4 μm are utilized for different materials among the GIOMER series. In addition to commercial products, S-PRG filler has been experimentally incorporated into a variety of materials.
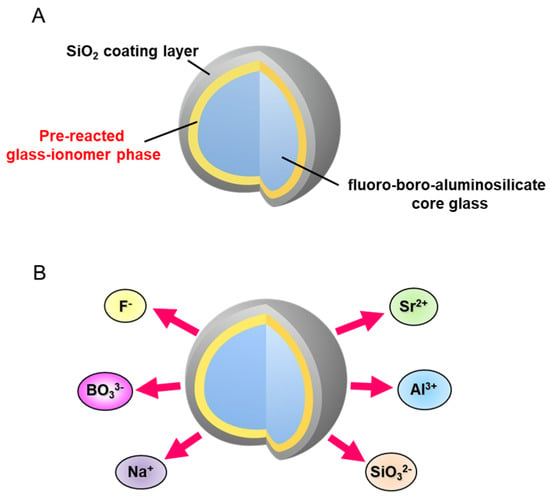
Figure 1. Surface Pre-Reacted Glass-ionomer (S-PRG) filler. (A) S-PRG filler is composed of three layers: outer SiO2 coating layer, pre-reacted glass-ionomer phase, and inner functional glass core. (B) S-PRG filler releases multiple ions: strontium (Sr2+), borate (BO33−), fluoride (F−), sodium (Na+), silicate (SiO32−), and aluminum (Al3+) ions.
The pre-reacted glass-ionomer phase surrounding the glass core allows the S-PRG filler to release fluoride ions (F−). Additionally, because specially fabricated fluoro-boro-aluminosilicate glass is used as the core material, S-PRG filler releases five other ions: strontium (Sr2+), borate (BO33−), sodium (Na+), silicate (SiO32−), and aluminum (Al3+) ions (Figure 1B). The concentrations of the six ions released into water from S-PRG filler, especially BO33−, Sr2+, and F−, are relatively high [8][1], much greater than those liberated from unreacted filler of conventional glass-ionomer cement (Figure 2). Notably, the six ions do not form salts and are found separately in the eluate.
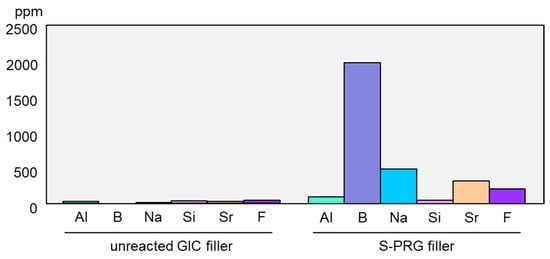
Figure 2. Comparison of ion release from S-PRG filler and conventional glass-ionomer filler. Release of ions from S-PRG filler or unreacted filler of conventional glass-ionomer cement was determined after stirring for 24 h in distilled water (mixing ratio of 1:1). The concentration of fluoride ions in the eluate was measured using a fluoride ion electrode, and those of other ions were measured using an inductively coupled plasma atomic emission spectrometer.
2. Benefits of S-PRG Filler for Dental Treatment and Care
Many products containing S-PRG filler are commercially available, such as resin composites, adhesives, resin cements, coating resins, fissure sealants, polishing pastes, and temporary fillings. Additionally, S-PRG filler has been incorporated experimentally into inorganic cements, root canal sealers, denture bases, tissue conditioners, denture adhesives, toothpastes, varnishes, CAD/CAM composites, and toothbrush filaments. Even when S-PRG filler is incorporated into resin-based materials with silanization, multiple ions are released at high concentrations. The capacity for releasing six ions (i.e., Sr2+, BO33−, F−, Na+, SiO32−, and Al3+) endows the S-PRG filler with various therapeutic effects, which are useful for restorative treatment, caries prevention/management, vital pulp therapy, endodontic treatment, prevention/treatment of periodontal disease, prevention of denture stomatitis, and perforation repair/root end filling. In this regard, S-PRG filler-containing materials differ from conventional ones that only release ions to promote remineralization, such as glass-ionomer cements or fluoride-releasing resin composites.
2.1. Restorative Treatment
To develop restorative treatments with preventive effects, S-PRG filler has been incorporated in resin composites, adhesives, and resin cements.
A ligand exchange mechanism within the glass-ionomer phase offers S-PRG filler the ability to release and recharge fluoride ions. The proprietary resin composites containing S-PRG filler (Beautifil II, Shofu Inc., Kyoto, Japan) release high concentrations of fluoride ions and can be recharged by exposure to 5000 ppm sodium fluoride solution for 5 min [28][2].
Several studies have demonstrated that resin composites containing S-PRG filler exhibit antibacterial effects against oral bacteria [4,5][3][4]. Miki et al. [19][5] examined the inhibitory effects of experimental resin composites containing different ratios of S-PRG filler on S. mutans growth and reported that the specimens containing S-PRG filler at 28.0 vol% or greater inhibited bacterial growth on their surfaces. They further demonstrated that the inhibitory effects were mainly attributed to the release of BO33− and F−. It was also reported that the eluate of S-PRG filler suppressed the adherence of S. mutans [22][6], and thus, S. mutans biofilm formation was inhibited on the surface of resin composites containing S-PRG filler. An in situ study assessing plaque accumulation on resin composites placed in human mouths for 24 h showed significant suppression of dental plaque maturation on the surface of resin composites containing S-PRG filler [29][7].
An investigation of demineralization in the tooth structure surrounding filling materials revealed that S-PRG filler-containing resin composites inhibited the demineralization of wall and outer lesions in enamel and dentin and increased the surrounding tooth hardness [30][8]. Lai et al. [31][9] investigated the anti-demineralization effects of restorations using S-PRG filler-containing resin composites (Beautifil II) and adhesives (FL-Bond II) on the root surfaces. Using an oral biofilm reactor and pH cycling artificial caries model, they demonstrated that the combination of Beautifil II and FL-Bond II exhibited a cumulative effect to inhibit demineralization of root dentin adjacent to restoration.
It has been reported that S. mutans contains esterases that can potentially degrade resin-based restorative materials [32][10]. Gautam et al. [33][11] revealed by in vitro test that degradation by S. mutans was reduced for resin composites containing S-PRG filler (Beautifil II) compared with other commercial resin composites. On the other hand, Yoshihara et al. [34][12] reported production of many holes on the surface of S-PRG filler-containing resin composites after immersion in lactic acid at pH 4.0 for 3 days, while no change in surface integrity was detected for other conventional resin composites. They described that such surface alteration was caused by dissolution of S-PRG filler. Although several clinical studies revealed their excellent performance [35[13][14],36], long-term surface durability of S-PRG filler-containing resin composites remains to be investigated in detail.
Recently, CAD/CAM resin composite blocks containing S-PRG filler have been developed for the restoration of primary molar teeth. Nakase et al. [37][15] reported that S-PRG filler-containing CAD/CAM resin composite blocks demonstrated acceptable physical properties and wear performance for clinical use. Moreover, an S-PRG filler-containing CAD/CAM resin composite crown is advantageous for primary tooth restoration because it can be cemented using a conventional glass-ionomer cement through the chelation reaction between Sr and polycarboxylic acid [38][16].
2.2. Caries Prevention/Management
The S-PRG filler modulates the pH of the surrounding medium and shifts it toward neutral and weak alkaline values [14][17]. Additionally, the release of F− and Sr2+ from S-PRG filler strengthens the tooth substrate, as described above. These effects are beneficial for the prevention and management of caries. Many studies have demonstrated that ions released from S-PRG filler in various materials are taken up by adjacent enamel and dentin to prevent demineralization, including adhesives [39][18], endodontic sealers [40][19], orthodontic adhesives [41][20], pit/fissure sealants [42][21], coating materials [43[22][23],44], resinous vanishes [45][24], and denture base resins [46][25].
A resinous coating material containing S-PRG filler (PRG Barrier Coat, Shofu Inc.) protects the enamel surface from demineralization caused by acidic attack [44,47][23][26]. Ions released from S-PRG filler in PRG Barrier Coat exert acid-neutralizing effects near the coated surface. Uptake of F and Sr released from PRG Barrier Coat by the tooth substrate can be effective to inhibit demineralization [48][27]. Ma at al. [43][22] demonstrated that PRG Barrier Coat containing S-PRG filler can protect root surfaces from demineralization by producing a coating layer with a thickness of approximately 200 µm. It was also shown that the pH drop in S. mutans clumps caused by glucose on the surface of the PRG Barrier Coat was reduced, possibly because the glucose metabolism was hindered by the released ions as well as acid buffering [43][22].
The incorporation of S-PRG filler into a resin-based pit and fissure sealant provides acid neutralization capacity without deteriorating the sealing effectiveness [49,50][28][29] or the ability to release and recharge fluoride ions [51][30]. Some clinical studies demonstrated caries preventive effects of commercially available S-PRG filler-containing pit/fissure sealant (BeautiSealant, Shofu Inc.) [52,53][31][32]. Penha et al. [53][32] conducted a randomized clinical trial to compare the evolution of caries in newly erupted permanent molars and determined that the S-PRG sealant group presented higher sound teeth predominance compared with another fluoride-releasing sealant after 12 months.
A polishing paste containing S-PRG filler, including a commercial one containing 5% S-PRG filler (PRG Pro-Care Gel, Shofu Inc.), is useful for controlling caries incidence and remineralization. Iijima et al. [54][33] reported that human enamel polished with S-PRG filler-containing paste, following immersion in the demineralizing solution at pH 4.5, exhibited greater surface hardness and smoothness than those polished with fluoridated paste or nano-hydroxyapatite-containing paste. Furthermore, the surface hardness of demineralized dentin polished with the S-PRG filler-containing paste recovered after immersion in mineralizing solution for 1 month, indicating that the S-PRG filler-containing paste can promote dentin remineralization [55][34]. A recent study revealed that PRG Pro-Care Gel has a similar potential to remineralize the demineralized root dentin as a 38% silver diamine fluoride solution [56][35].
Amaechi et al. [57][36] examined the effectiveness of experimental toothpastes containing S-PRG filler in preventing tooth surface demineralization using Featherstone’s pH cycling model to develop early caries. It was determined that S-PRG filler-containing dentifrice was more effective in preventing tooth demineralization than 1100 ppm F-containing toothpaste, highlighting the potential of S-PRG filler to assist individuals at high risk of developing caries [58][37]. The potential of S-PRG filler-containing toothpaste to remineralize the demineralized enamel surface has also been shown in situ [59][38]. Experimental toothpaste containing 5% S-PRG filler showed remineralizing effects similar to those of toothpaste containing 1500 ppm F, even showing greater activity to recover mineral loss in the subsurface regions.
Moecke et al. [60][39] investigated the remineralizing effects of experimental varnishes containing S-PRG filler. They revealed that the varnish containing 40% S-PRG filler more effectively promoted the remineralization of demineralized bovine enamel compared with the varnish containing 5% sodium fluoride.
2.3. Vital Pulp Therapy
S-PRG filler can promote tertiary dentinogenesis. As pulp capping materials, several attempts have been made to incorporate S-PRG filler into adhesives [61[40][41],62], self-adhesive resins [63][42], and inorganic cements [64,65][43][44].
Experimental inorganic cement, prepared by mixing S-PRG filler with copolymers of acrylic acid and tricarboxylic acid, induces complete tertiary dentin formation at 2 and 4 weeks after pulp exposure in rat molars [64][43]. Strontium released from S-PRG cement was determined to transfer into the pulpal tissue and contribute to the induction of mineralization and dentinogenesis [65][44]. These functions are based on biological action because the S-PRG cement enhanced the expression of genes related to osteo/dentinogenic differentiation, such as CXCL-12 and TGF-β1, which contribute to tertiary dentin formation during the healing process in pulpal tissue [65][44].
It has been reported that lithium ions can activate the Wnt/β-catenin signaling pathway and induce dentin formation in pulpotomized teeth [66][45]. Therefore, trials to add LiCl to S-PRG cement were conducted, revealing that reparative dentin formation in rat teeth was enhanced through activation of the Wnt/β-catenin canonical signaling pathway without adversely affecting the sealing property of the cement [67,68][46][47].
2.4. Endodontic Treatment
Apical periodontitis is an infectious disease, and the treatment requires careful bacterial eradication inside filled root canals. However, the complete elimination of bacterial infection in root canals is difficult to achieve using only mechanical instrumentation, irrigation, and medication. Therefore, endodontic filling materials capable of eliminating residual bacteria inside root canals have been proposed as a possible solution to this problem. An experimental zinc oxide-based endodontic sealer containing S-PRG filler demonstrated the sustained release of multiple ions (i.e., Sr2+, BO33−, F−, Na+, SiO32−, and Al3+) [40][19] and antibacterial effects against E. faecalis and P. gingivalis [69,70][48][49].
The eluate obtained from experimental root canal sealer containing S-PRG filler was reported to downregulate mRNA expression levels of proinflammatory cytokines, such as interleukin (IL)-1α, IL-6, and TNF-α, in LPS-stimulated RAW264.7 cells, suggesting its anti-inflammatory effects [71][50]. Moreover, an experimental sealer containing S-PRG filler can promote the healing of periapical lesions in vivo [70,72,73][49][51][52].
2.5. Prevention/Treatment of Periodontal Disease
The S-PRG filler eluate suppresses the activity of periodontitis-related bacteria [21,22][6][53] and inhibits penetration of P. gingivalis virulence factors into gingival epithelial cells [27][54]. It also promotes recovery of migration activity in epithelial cells infected with P. gingivalis. In recent years, several animal studies have verified the ability of S-PRG filler to help prevent the progression of periodontal disease. Iwamatsu-Kobayashi et al. [74][55] investigated the effects against tissue destruction induced in a mouse periodontal disease model and revealed that periodical dripping of the S-PRG filler eluate suppressed the bone loss and resulted in greater volume and density of alveolar bone. It has also been reported that ultrasonic irrigation with S-PRG filler dispersion can improve periodontal parameters, such as gingival index, bleeding on probing, probing pocket depth, and clinical attachment level when applied to a periodontal defect in a dog [75,76][56][57]. Irrigation with S-PRG filler dispersion reduced the ratio of red complex (i.e., Tannerella forsythia, P. gingivalis, and Treponema denticola) among the bacterial flora in the periodontal pocket [76][57].
2.6. Prevention of Denture Stomatitis
A denture base with antifungal effects may be useful for the prevention of denture stomatitis. The morphology of C. albicans cells remains in the yeast form in a normal resident flora, but it grows from the yeast form to the hyphal form by creating a longer germ tube when grown in an inflammatory environment in a human host [77][58]. The eluate of S-PRG filler prevents adherence of C. albicans to denture base resin and inhibits the dimorphism conversion of C. albicans [23][59]. The incorporation of S-PRG filler into polymethyl methacrylate-based resin effectively inhibited biofilm formation by C. albicans, with reduced length of the hyphae [78][60]. Similar results have been reported for the addition of S-PRG filler to other materials in the field of removable prosthodontics, such as tissue conditioners [79,80][61][62] and poly(methyl vinyl ether-alt-maleic anhydride)-based denture adhesives [81][63]. The multiple-ion release characteristics of S-PRG filler may contribute to the oral health of the elderly population by preventing the onset of oral candidiasis.
2.7. Perforation Repair/Root End Filling
Hirata-Tsuchiya et al. [72][51] reported that the application of commercially available resin composites containing S-PRG filler for sealing root perforation can facilitate periodontal tissue healing and support good clinical outcomes. Inorganic cement containing S-PRG filler exhibits excellent cytocompatibility for osteoblastic cells, similar to a calcium silicate-based cement. Considering the enhancement of cell activity by released ions, S-PRG filler-containing materials may be useful for perforation repair and root end filling, for which calcium silicate-based cements have been widely used.
References
- Ito, S.; Iijima, M.; Hashimoto, M.; Tsukamoto, N.; Mizoguchi, I.; Saito, T. Effects of surface pre-reacted glass-ionomer fillers on mineral induction by phosphoprotein. J. Dent. 2011, 39, 72–79.
- Naoum, S.; Ellakwa, A.; Martin, F.; Swain, M. Fluoride release, recharge and mechanical property stability of various fluoride-containing resin composites. Oper. Dent. 2011, 36, 422–432.
- Imazato, S.; Kohno, T.; Tsuboi, R.; Thongthai, P.; Xu, H.H.; Kitagawa, H. Cutting-edge filler technologies to release bio-active components for restorative and preventive dentistry. Dent. Mater. J. 2020, 39, 69–79.
- Imazato, S.; Kitagawa, H. Dental resin-based materials with antibacterial properties: Contact inhibition and controlled release. In Oral Biofilms and Modern Dental Materials: Advances toward Bioactivity; Ionescu, A.C., Hahnel, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 127–140.
- Miki, S.; Kitagawa, H.; Kitagawa, R.; Kiba, W.; Hayashi, M.; Imazato, S. Antibacterial activity of resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent. Mater. 2016, 32, 1095–1102.
- Yoneda, M.; Suzuki, N.; Masuo, Y.; Fujimoto, A.; Iha, K.; Yamada, K.; Iwamoto, T.; Hirofuji, T. Effect of S-PRG eluate on biofilm formation and enzyme activity of oral bacteria. Int. J. Dent. 2012, 2012, 814913.
- Saku, S.; Kotake, H.; Scougall-vilchis, R.J.; Ohashi, S.; Hotta, M.; Yamamoto, K. Antibacterial activity of composite resin with glass-ionomer filler particles. Dent. Mater. J. 2010, 29, 193–198.
- Zhou, Y.; Hiraishi, N.; Shimada, Y.; Wang, G.; Tagami, J.; Feng, X. Evaluation of tooth demineralization and interfacial bacterial penetration around resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent. Mater. 2021, 37, 849–862.
- Lai, Y.J.; Takahashi, R.; Lin, P.Y.; Kuo, L.; Zhou, Y.; Matin, K.; Chiang, Y.C.; Shimada, Y.; Tagami, J. Anti-demineralization effects of dental adhesive-composites on enamel-root dentin junction. Polymers 2021, 13, 3327.
- Bourbia, M.; Ma, D.; Cvitkovitch, D.G.; Santerre, J.P.; Finer, Y. Cariogenic bacteria degrade dental resin composites and adhesives. J. Dent. Res. 2013, 92, 989–994.
- Gautam, A.K.; Thakur, R.; Shashikiran, N.D.; Shilpy, S.; Agarwal, N.; Tiwari, S. Degradation of resin restorative materials by Streptococcus mutans: A pilot study. J. Clin. Pediatr. Dent. 2017, 41, 225–227.
- Yoshihara, K.; Nagaoka, N.; Maruo, Y.; Sano, H.; Yoshida, Y.; Van Meerbeek, B. Bacterial adhesion not inhibited by ion-releasing bioactive glass filler. Dent. Mater. 2017, 33, 723–734.
- Ozer, F.; Patel, R.; Yip, J.; Yakymiv, O.; Saleh, N.; Blatz, M.B. Five-year clinical performance of two fluoride-releasing giomer resin materials in occlusal restorations. J. Esthet. Restor. Dent. 2022, 34, 1213–1220.
- Toz-Akalin, T.; Öztürk-Bozkurt, F.; Kusdemir, M.; Özsoy, A.; Yüzbaşıoğlu, E.; Özcan, M. Clinical evaluation of low-shrinkage bioactive material giomer versus nanohybrid resin composite restorations: A two-year prospective controlled clinical trial. Oper. Dent. 2023, 48, 10–20.
- Nakase, Y.; Yamaguchi, S.; Okawa, R.; Nakano, K.; Kitagawa, H.; Imazato, S. Physical properties and wear behavior of CAD/CAM resin composite blocks containing S-PRG filler for restoring primary molar teeth. Dent. Mater. 2022, 38, 158–168.
- Akimoto, N.; Sakamoto, T.; Kubota, Y.; Kondo, Y.; Momoi, Y. A novel composite-to-composite adhesive bond mechanism. Dent. Mater. J. 2011, 30, 523–527.
- Fujimoto, Y.; Iwasa, M.; Murayama, R.; Miyazaki, M.; Nagafuji, A.; Nakatsuka, T. Detection of ions released from S-PRG fillers and their modulation effect. Dent. Mater. J. 2010, 29, 392–397.
- Han, L.; Okamoto, A.; Fukushima, M.; Okiji, T. Evaluation of a new fluoride-releasing one-step adhesive. Dent. Mater. J. 2006, 25, 509–515.
- Han, L.; Okiji, T. Evaluation of the ions release / incorporation of the prototype S-PRG filler-containing endodontic sealer. Dent. Mater. J. 2011, 30, 898–903.
- Horiuchi, S.; Kaneko, K.; Mori, H.; Kawakami, E.; Tsukahara, T.; Yamamoto, K.; Hamada, K.; Asaoka, K.; Tanaka, E. Enamel bonding of self-etching and phosphoric acid-etching orthodontic adhesives in simulated clinical conditions: Debonding force and enamel surface. Dent. Mater. J. 2009, 28, 419–425.
- Kaga, M.; Kakuda, S.; Ida, Y.; Toshima, H.; Hashimoto, M.; Endo, K.; Sano, H. Inhibition of enamel demineralization by buffering effect of S-PRG filler-containing dental sealant. Eur. J. Oral Sci. 2014, 122, 78–83.
- Ma, S.; Imazato, S.; Chen, J.-H.; Mayanagi, G.; Takahashi, N.; Ishimoto, T.; Nakano, T. Effects of a coating resin containing S-PRG filler to prevent demineralization of root surfaces. Dent. Mater. J. 2012, 31, 909–915.
- Kawasaki, K.; Kambara, M. Effects of ion-releasing tooth-coating material on demineralization of bovine tooth enamel. Int. J. Dent. 2014, 2014, 463149.
- Shiiya, T.; Mukai, Y.; Tomiyama, K.; Teranaka, T. Anti-demineralization effect of a novel fluoride-releasing varnish on dentin. Am. J. Dent. 2012, 25, 347–350.
- Mukai, Y.; Kamijo, K.; Fujino, F.; Hirata, Y.; Teranaka, T.; Ten Cate, J.M. Effect of denture base-resin with prereacted glass-ionomer filler on dentin demineralization. Eur. J. Oral Sci. 2009, 117, 750–754.
- Murayama, R.; Nagura, Y.; Yamauchi, K.; Moritake, N.; Iino, M.; Ishii, R.; Kurokawa, H.; Miyazaki, M.; Hosoya, Y. Effect of a coating material containing surface reaction-type pre-reacted glass-ionomer filler on prevention of primary enamel demineralization detected by optical coherence tomography. J. Oral Sci. 2018, 60, 367–373.
- Funato, Y.; Matsuda, Y.; Okuyama, K.; Yamamoto, H.; Komatsu, H.; Sano, H. A new technique for analyzing trace element uptake by human enamel. Dent. Mater. J. 2015, 34, 240–245.
- Hirayama, K.; Hanada, T.; Hino, R.; Saito, K.; Kobayashi, M.; Arakaki, M. Material properties on enamel and fissure of surface pre-reacted glass-ionomer filler-containing dental sealant. Pediatr. Dent. J. 2018, 28, 87–95.
- Wang, Y.; Kaga, M.; Kajiwara, D.; Minamikawa, H.; Kakuda, S.; Hashimoto, M.; Yawaka, Y. Ion release and buffering capacity of S-PRG filler-containing pit and fissure sealant in lactic acid. Nano Biomed. 2011, 3, 275–281.
- Patil, S.S.; Kontham, U.R.; Kontham, R.K.; Patil, S.S.; Kamble, S.P. Fluoride release and fluoride-recharging ability of three different sealants. J. Indian Soc. Pedod. Prev. Dent. 2020, 38, 247–252.
- Hirayama, N.; Karaki, T.; Onaga, M.; Usuba, R.; Idaira, Y.; Asada, Y. Comparisons of retention rate and caries preventive effect between sealant containing S-PRG filler and resin-based sealant in children. Jpn. J. Pediatr. Dent. 2019, 57, 54–65. (In Japanese)
- Penha, K.J.S.; Roma, F.R.V.O.; Filho, E.M.M.; Ribeiro, C.C.C.; Firoozmand, L.M. Bioactive self-etching sealant on newly erupted molars: A split-mouth clinical trial. J. Dent. 2021, 115, 103857.
- Iijima, M.; Kawaguchi, K.; Kawamura, N.; Ito, S.; Saito, T.; Mizoguchi, I. The effects of single application of pastes containing ion-releasing particles on enamel demineralization. Dent. Mater. J. 2017, 36, 461–468.
- Iijima, M.; Ishikawa, R.; Kawaguchi, K.; Ito, S.; Saito, T.; Mizoguchi, I. Effects of pastes containing ion-releasing particles on dentin remineralization. Dent. Mater. J. 2019, 38, 271–277.
- Okuwaki, T.; Sugimura, R.; Kurokawa, H.; Tsujimoto, A.; Takamizawa, T.; Miyazaki, M.; Garcia-Godoy, F. Effect of ion-releasing filler-containing gel application on dentin remineralization using optical coherent tomography. Am. J. Dent. 2021, 34, 286–292.
- Amaechi, B.T.; Kasundra, H.; Joshi, D.; Abdollahi, A.; Azees, P.A.A.; Okoye, L.O. Effectiveness of S-PRG filler-containing toothpaste in inhibiting demineralization of human tooth surface. Open Dent. J. 2018, 12, 811–819.
- Amaechi, B.T.; Key, M.C.; Balu, S.; Okoye, L.O.; Gakunga, P.T. Evaluation of the caries-preventive effect of toothpaste containing surface prereacted glass-ionomer filler. J. Investig. Clin. Dent. 2017, 8, e12249.
- Vertuan, M.; França da Silva, J.; Ferreira, A.M.; Braga, A.S.; Magalhães, A.C. Effect of a toothpaste containing surface pre-reacted glass-ionomer filler on the remineralization of artificial carious enamel lesions in situ. Caries Res. 2022, 56, 447–454.
- Moecke, S.E.; Silva, A.G.C.S.; Andrade, A.C.M.; Borges, A.B.; Torres, C.R.G. Efficacy of S-PRG filler varnishes on enamel caries remineralization. J. Dent. 2022, 119, 104074.
- Kawashima, S.; Shinkai, K.; Suzuki, M. The effect of multi-ion releasing filler contents on the dentin bond strength of an adhesive resin developed for direct pulp-capping. Dent. Mater. J. 2015, 34, 841–846.
- Kawashima, S.; Shinkai, K.; Suzuki, M. Effect of an experimental adhesive resin containing multi-ion releasing fillers on direct pulp-capping. Dent. Mater. J. 2016, 35, 479–489.
- Sato, F.; Suzuki, M.; Shinkai, K. Pulp tissue reaction to a self-adhesive, resin-based direct pulp capping material containing surface pre-reacted glass-ionomer filler. Dent. Mater. 2021, 37, 972–982.
- Takahashi, Y.; Okamoto, M.; Komichi, S.; Imazato, S.; Nakatsuka, T.; Sakamoto, S.; Kimoto, K.; Hayashi, M. Application of a direct pulp capping cement containing S-PRG filler. Clin. Oral Investig. 2019, 23, 1723–1731.
- Okamoto, M.; Ali, M.; Komichi, S.; Watanabe, M.; Huang, H.; Ito, Y.; Miura, J.; Hirose, Y.; Mizuhira, M.; Takahashi, Y.; et al. Surface pre-reacted glass filler contributes to tertiary dentin formation through a mechanism different than that of hydraulic calcium-silicate cement. J. Clin. Med. 2019, 8, 1440.
- Li, L.; Peng, X.; Qin, Y.; Wang, R.; Tang, J.; Cui, X.; Wang, T.; Liu, W.; Pan, H.; Li, B. Acceleration of bone regeneration by activating Wnt/β-catenin signalling pathway via lithium released from lithium chloride/calcium phosphate cement in osteoporosis. Sci. Rep. 2017, 24, 45204.
- Ali, M.; Okamoto, M.; Komichi, S.; Watanabe, M.; Huang, H.; Takahashi, Y.; Hayashi, M. Lithium-containing surface pre-reacted glass fillers enhance hDPSC functions and induce reparative dentin formation in a rat pulp capping model through activation of Wnt/β-catenin signaling. Acta Biomater. 2019, 96, 594–604.
- Ali, M.; Okamoto, M.; Watanabe, M.; Huang, H.; Matsumoto, S.; Komichi, S.; Takahashi, Y.; Hayashi, M. Biological properties of lithium-containing surface pre-reacted glass fillers as direct pulp-capping cements. Dent. Mater. 2022, 38, 294–308.
- Yassen, G.H.; Huang, R.; Al-Zain, A.; Yoshida, T.; Gregory, R.L.; Platt, J.A. Evaluation of selected properties of a new root repair cement containing surface pre-reacted glass ionomer fillers. Clin. Oral Investig. 2016, 20, 2139–2148.
- Miyaji, H.; Mayumi, K.; Miyata, S.; Nishida, E.; Shitomi, K.; Hamamoto, A.; Tanaka, S.; Akasaka, T. Comparative biological assessments of endodontic root canal sealer containing surface pre-reacted glass-ionomer (S-PRG) filler or silica filler. Dent. Mater. J. 2020, 39, 287–294.
- Thein, H.S.S.; Hashimoto, K.; Kawashima, N.; Noda, S.; Okiji, T. Evaluation of the anti-inflammatory effects of surface-reaction-type pre-reacted glass-ionomer filler containing root canal sealer in lipopolysaccharide-stimulated RAW264.7 macrophages. Dent. Mater. J. 2022, 41, 150–158.
- Hirata-Tsuchiya, S.; Suzuki, S.; Nakamoto, T.; Kakimoto, N.; Yamada, S.; Shiba, H. Surgical sealing of laterally localized accessory root canal with resin containing S-PRG filler in combination with non-surgical endodontic treatment: A case report. Dent. J. 2020, 8, 131.
- Xiong, B.; Shirai, K.; Matsumoto, K.; Abiko, Y.; Furuichi, Y. The potential of a surface pre-reacted glass root canal dressing for treating apical periodontitis in rats. Int. Endod. J. 2021, 54, 255–267.
- Kono, Y.; Tamura, M.; Cueno, M.E.; Tonogi, M.; Imai, K. S-PRG filler eluate induces oxidative stress in oral microorganism: Suppression of growth and pathogenicity, and possible clinical application. Antibiotics 2021, 10, 816.
- Takeuchi, H.; Kato, Y.; Sasaki, N.; Tanigaki, K.; Yamaga, S.; Mita, E.; Kuboniwa, M.; Matsusaki, M.; Amano, A. Surface pre-reacted glass-ionomer eluate protects gingival epithelium from penetration by lipopolysaccharides and peptidoglycans via transcription factor EB pathway. PLoS ONE 2022, 17, e0271192.
- Iwamatsu-Kobayashi, Y.; Abe, S.; Fujieda, Y.; Orimoto, A.; Kanehira, M.; Handa, K.; Venkataiah, V.S.; Zou, W.; Ishikawa, M.; Saito, M. Metal ions from S-PRG filler have the potential to prevent periodontal disease. Clin. Exp. Dent. Res. 2017, 3, 126–133.
- Mayumi, K.; Miyaji, H.; Miyata, S.; Nishida, E.; Furihata, T.; Kanemoto, Y.; Sugaya, T.; Shitomi, K.; Akasaka, T. Antibacterial coating of tooth surface with ion-releasing pre-reacted glass-ionomer (S-PRG) nanofillers. Heliyon 2021, 7, e06147.
- Miyaji, H.; Mayumi, K.; Kanemoto, Y.; Okamoto, I.; Hamamoto, A.; Kato, A.; Sugaya, T.; Akasaka, T.; Tanaka, S. Ultrasonic irrigation of periodontal pocket with surface pre-reacted glass-ionomer (S-PRG) nanofiller dispersion improves periodontal parameters in beagle dogs. J. Oral Biosci. 2022, 64, 222–228.
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324.
- Tamura, M.; Cueno, M.E.; Abe, K.; Kamio, N.; Ochiai, K.; Imai, K. Ions released from a S-PRG filler induces oxidative stress in Candida albicans inhibiting its growth and pathogenicity. Cell Stress Chaperones 2018, 23, 1337–1343.
- Tsutsumi, C.; Takakuda, K.; Wakabayashi, N. Reduction of Candida biofilm adhesion by incorporation of prereacted glass ionomer filler in denture base resin. J. Dent. 2016, 44, 37–43.
- Takakusaki, K.; Fueki, K.; Tsutsumi, C.; Tsutsumi, Y.; Iwasaki, N.; Hanawa, T.; Takahashi, H.; Takakuda, K.; Wakabayashi, N. Effect of incorporation of surface pre-reacted glass ionomer filler in tissue conditioner on the inhibition of Candida albicans adhesion. Dent. Mater. J. 2018, 37, 453–459.
- Tonprasong, W.; Inokoshi, M.; Tamura, M.; Uo, M.; Wada, T.; Takahashi, R.; Hatano, K.; Shimizubata, M.; Minakuchi, S. Tissue conditioner incorporating a nano-sized surface pre-reacted glass-ionomer (S-PRG) filler. Materials 2021, 14, 6648.
- Hatano, K.; Inokoshi, M.; Tamura, M.; Uo, M.; Shimizubata, M.; Tonprasong, W.; Wada, T.; Takahashi, R.; Imai, K.; Minakuchi, S. Novel antimicrobial denture adhesive containing S-PRG filler. Dent. Mater. J. 2021, 40, 1365–1372.
More
