Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Pinelopi Samara.
Within the intricate realm of the mucosal immune system resides a captivating duo: the adenoids (or pharyngeal tonsils) and the tonsils (including palatine, tubal, and lingual variations), which harmoniously form the Waldeyer’s ring. As they are strategically positioned at the crossroads of the respiratory and gastrointestinal systems, these exceptional structures fulfill a vital purpose. They function as formidable “gatekeepers” by screening microorganisms—both bacteria and viruses—with the mission to vanquish local pathogens via antibody production. However, under specific circumstances, their function can take an unsettling turn, inadvertently transforming them into reservoirs for pathogen incubation.
- adenoids
- tonsils
- Waldeyer’s ring
- microbiome
- antibiotics
1. Introduction
Waldeyer’s ring, named after the distinguished German anatomist, Heinrich Wilhelm Gottfried von Waldeyer-Hartz (6 October 1836–23 January 1921), is a remarkable collection of lymphoid tissues that spans the naso- and oropharynx [1]. It appears during the fifth month of gestation and consists of four distinct structures: (1) pharyngeal tonsils or adenoids, located on the roof of the nasopharynx under the sphenoid bone; (2) tubal tonsils, found bilaterally surrounding the opening of the Eustachian tube in the lateral wall of the nasopharynx; (3) palatine tonsils, which are traditionally referred to as “the tonsils”, situated within the tonsillar clefts of the oropharynx; and (4) lingual tonsils, a group of lymphatic tissue characterized by numerous protrusions at the posterior third of the tongue [2][3] (Figure 1). Moreover, mucosa-associated lymphoid tissue (MALT) can be found within these tonsils and throughout the naso- and oropharynx [4][5]. All these entities form the mucosal immune system, which is strategically placed at the intersection between the respiratory and digestive systems [6]. They play a crucial role in defending against pathogens introduced through inhalation and digestion, serving as primary sites for antigen sampling and triggering essential immune responses [7][8].
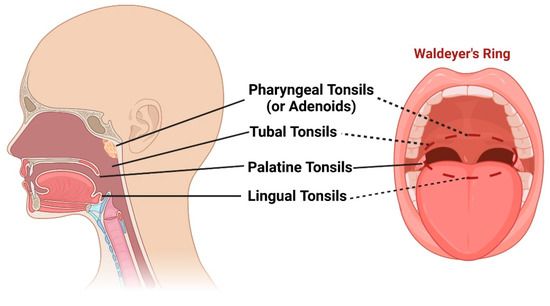
Figure 1. Schematic representation of pharyngeal tonsils (or adenoids), tubal tonsils, palatine tonsils, and lingual tonsils, as well as the “intelligible ring” that they form (Waldeyer’s ring). Created with BioRender.com (accessed on 29 May 2023).
However, sometimes, chronic inflammation caused by microbes, mainly bacteria, can lead to adenotonsillar disease [9]. Ιn these cases, an appropriate treatment must be administered, including proper antibiotics or, if indicated, the surgical removal of implicated structures that create “more harm than good” [10][11]. The accurate diagnosis and effective management of adenotonsillar infections highly depend on in-depth knowledge of the anatomy, physiology, immunology, microbiology, and pathophysiology of the area and its underlying tissues. In the past, there were two schools of thought concerning the necessity of these surgical interventions (adenoidectomy, tonsillectomy, or adenotonsillectomy) [12]. Nowadays, it is widely accepted that there are specific indications for their removal, with the primary objective of improving the patients’ quality of life. In fact, adenoidectomy and tonsillectomy are among the most commonly performed surgical procedures for the treatment of children worldwide [13].
2. Anatomy and Physiology of Adenoids and Tonsils
For pediatric otolaryngologists, the pharyngeal and palatine tonsils are of utmost importance in their daily clinical practice [14].
2.1. Anatomy of Pharyngeal Tonsils (Adenoids)
The pharyngeal tonsils, which are also called adenoids, are lymphoid tissue located in the central area where the sphenoid and occipital bones meet along the roof and posterior wall of the nasopharynx. Below the lower border of the pharyngeal tonsil, the pharyngobasilar fascia is present, which extends downward and is associated with the pharyngeal constrictor muscles [15]. Grisel’s syndrome, a rare complication that typically occurs after an adenoidectomy, can result from inflammation caused by aggressive adenoid removal and/or excessive backward bending in patients with certain anatomical predispositions [16]. The blood supply to the pharyngeal tonsils comes from various sources, including the ascending pharyngeal artery, ascending palatine artery, tonsillar branch of the facial artery, pharyngeal branch of the internal maxillary artery, and artery of the pterygoid canal [17].
2.2. Anatomy of Palatine Tonsils
The palatine tonsils are lymphoid structures located within the tonsillar fossa. This region is bordered by the anterior and posterior tonsil pillars, which consist of the palatoglossus and palatopharyngeus muscles [18]. The lateral side of the tonsillar fossa is formed of the superior constrictor muscle. The buccopharyngeal fascia and a layer of loose connective tissue separate the tonsil from the parapharyngeal space [3]. Blood supply to the tonsil is provided by branches of the external carotid artery system, including the tonsillar artery and the ascending palatine artery. The superior pole of the palatine tonsil receives blood from the tonsillar branches of the ascending pharyngeal artery and the descending palatine artery. During procedures involving the palatine tonsils, healthcare providers must be cautious of nearby neurovascular structures, such as the internal carotid artery and the glossopharyngeal nerve [19][20]. In adults, the internal carotid artery is typically located 2.5 cm posterolateral to the tonsillar fossa. However, in children weighing under 11 kg, it can be as close as 1.5 cm to the fossa [21].
2.3. Physiology of Adenoids and Tonsils
It is important to briefly discuss the embryological development of the palatine and pharyngeal tonsils in order to gain a better understanding of their physiology. The medial epithelial surface of the tonsil originates from the second branchial pouch, where solid epithelial cores penetrate into the surrounding mesenchyme. Over time, these cores undergo canalization and form small invaginations called crypts. At around the 16th–17th week of embryological development, lymphocytes and lymphoid stem cells invade the deepest layers of the connective tissue, forming follicles and germinal centers [22]. The growing lymphoid components merge the deepest layers of the connective tissue to create a thin tonsillar capsule. After birth, the tonsils develop multiple branching crypts, with approximately 10–30 per tonsil. These crypts have a fibrovascular core surrounded by lymphoid tissue. The tonsillar epithelial surface is composed of non-keratinized stratified squamous epithelium, while the lining of the crypts consists of stratified squamous epithelium and lymphoepithelium [23]. The invaginated structure of the crypts increases the surface area for antigen sampling and the direct trapping of foreign materials. Unlike lymph nodes and the spleen, the palatine tonsils lack lymphatic vessels for fluid drainage [24].
As for the pharyngeal tonsils, they exhibit mucosal folds, but have fewer crypts compared to those of palatine tonsils. Histologically, the pharyngeal tonsils are primarily composed of pseudostratified ciliated columnar epithelium with a smaller number of lymphoid follicles. A capsule separates the pharyngeal tonsil from the surrounding bones, and connective tissue septa divide the tissue into 4–6 segments [24].
3. Immunology of Adenoids and Tonsils: A Regionalized Immune System
The unique anatomical arrangement of adenoids and tonsils, combined with specialized immune cell populations, creates a regionalized immune system that helps prevent the spread of pathogens and elicits immune responses [25]. This section will briefly present the structural organization of adenoids and tonsils, examine their immune cell populations, and delve into their functional significance in terms of mucosal immunity.
The epithelium covering these lymphoid structures contains specialized cells, such as M cells and intraepithelial lymphocytes, which contribute to the immune surveillance of luminal antigens. Below the epithelium, the lamina propria harbors various immune cell populations, including B cells, T cells, dendritic cells (DCs), and macrophages, forming an organized network of lymphoid follicles and interfollicular areas. B cells, which are abundant, undergo germinal center reactions within the adenoids and tonsils, leading to antibody production and affinity maturation. T cells, including CD4+ helper T cells and CD8+ cytotoxic T cells, play a critical role in coordinating immune responses and eliminating infected cells [26]. DCs that are strategically positioned at the interface between the lumen and lymphoid tissue capture and present antigens to T and B cells, initiating adaptive immune responses. Macrophages distributed throughout the lymphoid tissue phagocytose pathogens and provide important regulatory signals to other immune cells [7].
The regionalized immune system of the adenoids and tonsils serves several important functions. Firstly, it acts as a physical barrier, preventing pathogens from gaining access to the underlying tissues. The tonsillar crypts and M cells facilitate antigen uptake and transport, initiating immune responses. Secondly, adenoids and tonsils participate in the generation of adaptive immune responses. B cells within germinal centers undergo class switch recombination and somatic hypermutation, resulting in the production of high-affinity antibodies. T cells recognize and eliminate infected cells, thus limiting the dissemination of pathogens. Thirdly, adenoids and tonsils contribute to the development of immune memory, promoting faster and more efficient immune responses upon re-exposure to previously encountered pathogens [27][28]. Despite them being physically close and having similar tissue characteristics, a study conducted by Stanisce et al. [29] has revealed significant variations in the cellular composition of adenoids and tonsils, specifically in functionally important immune and stromal subsets. These differences hold significant implications in terms of immunology, pathology, and physiology.
The immune system of these organs undergoes alterations following inflammation, which could contribute to the development of “local issues” like peritonsillar abscess, which is discussed further below. Specifically, chronic or recurrent tonsillitis disrupts the tonsillar immune system, resulting in the shedding of M cells and weakening of the immunologic response to antigens [30]. However, the exact role of the abnormal local immune response or immunological impairments as risk factors for peritonsillar abscess development remains unclear. Peritonsillar abscesses are rarely observed in patients with general immunodeficiency, but they have been associated with infectious mononucleosis. Epstein–Barr virus (EBV) infection impairs humoral immunity, potentially affecting the coating of bacteria with immunoglobulins on tonsillar tissue. It may also lead to a temporary decrease in T-cell-mediated immunity, increasing bacterial attachment and colonization in the tonsils. Two studies have investigated the immunological changes or impairments in peritonsillar abscess patients without concurrent EBV infection. The first study discovered that only a small portion of bacteria in the pus showed opsonization via immunoglobulin or complement components. This could be due to the rapid phagocytosis or encapsulation of the infection, preventing immune cells from effectively attacking the bacteria within. The second study showed that human beta-defensins, which possess antimicrobial properties, were also detected in the tonsillar surface and abscess, but not in the lymphatic follicles. However, their precise function remains largely unexplored [31]. Further investigations into the immunological aspects of adenoids and tonsils will provide valuable insights into the mechanisms underlying mucosal immune responses and may lead to the development of novel strategies for immune-based interventions.
4. Microbiology of Adenoids and Tonsils
While the immunological role of adenoids and tonsils has been extensively studied, understanding their microbiology is equally crucial. These lymphoid tissues harbor a diverse microbial community, collectively known as the microbiota, which interacts with the local immune system and contributes to the maintenance of mucosal homeostasis [32]. Investigating the microbiology of adenoids and tonsils can provide valuable insights into the complex interplay between microbes and the host immune system, influencing health and disease outcomes (Figure 2).
Figure 2. Schematic representation of the numerous microbial communities inhabiting the oral and oropharyngeal cavity, highlighting the intricate “equilibrium” between healthy and diseased states. Created with BioRender.com (accessed on 29 May 2023).
4.1. The Normal Microbiome and the Microbiome in Adenotonsillar Disease
The colonization of the nasopharynx starts in infancy and develops over months [33]. The adenoids and tonsils host a diverse microbiome that contributes to achieving an immune balance and mucosal health. However, the composition of this microbiome can differ between individuals due to factors like age, environmental exposures, and overall health [34]. Commonly, potentially pathogenic bacteria can be found in the nasopharynx of healthy children, either as transient or regular components of the nasopharyngeal flora. These bacteria may include Neisseria, Streptococcus pyogenes, Haemophilus influenzae, Staphylococcus aureus, Actinomyces, Bacteroides, Prevotella, Porphyromonas, Peptostreptococci, and Fusobacterium species [35][36][37].
Recent studies have provided insights into the composition and function of the microbiome in adenotonsillar disease. These investigations employed various techniques, including in vitro cultures, 16S rRNA gene sequencing, and tissue sample analysis, to explore microbial communities [38][39]. Several studies utilizing cultivation and PCR techniques suggest that Helicobacter pylori exposure occurs during early childhood and may contribute to the development of chronic adenotonsillitis, particularly in regions with a high prevalence [40][41]. It is important to note that swab-based culture methods only identify bacteria present on the surface of the sampled tissue area, potentially missing those residing in deep crypts or intracellular regions. Consequently, culture swabs have limitations in clinical practice as they can only grow bacteria with specific metabolic requirements that align with the culture media and atmospheric conditions employed. By comparing the microbiomes of healthy individuals and those of patients with adenotonsillar disease, researchers aim to uncover crucial differences and understand the microbial factors involved in disease development. Importantly, the adenotonsillar microbiota’s composition may vary and have specific roles in both surface and core tissues [42].
In pediatric populations, the microbial composition has been observed to differ between patients with tonsillar hyperplasia and recurrent tonsillitis. Jensen et al. [43] reported variations in the tonsillar crypt microbiota based on age and health status. Haemophilus influenzae, Neisseria species, and Streptococcus pneumoniae are exclusively found in children, while Prevotella, Actinomyces, Parvimonas, Veillonella, and Treponema are more abundant in adults. Children with tonsillar hyperplasia exhibit a predominance of Streptococcus, Neisseria, Prevotella, Haemophilus, Porphyromonas, Gemella, and Fusobacterium species within the tonsillar crypts. On the other hand, Fusobacterium necrophorum, Streptococcus intermedius, and Prevotella melaninogenica/histicola are associated with recurrent tonsillitis in adults. Notably, significant differences in phylogenetic community structures were observed between healthy adults and those with recurrent tonsillitis, as well as between children with recurrent tonsillitis and those with tonsillar hyperplasia.
In a study by Kim and Min [44], the microbiomes of adenotonsillar tissues in pediatric patients who snore were analyzed. The tonsil tissue samples were primarily composed of Proteobacteria (mainly the Haemophilus genus), Firmicutes (predominantly the Streptococcus genus), and Bacteroidetes (dominated by the Prevotella genus). Adenoid tissue samples were dominated by Proteobacteria (mostly the Haemophilus genus), Firmicutes (mainly the Streptococcus genus), and Fusobacteria (predominantly the Fusobacterium genus). Furthermore, Swidsinski et al. [45] examined the tonsil and adenoid tissues and found that many patients had multiple areas of ongoing purulent infection within these tissues. The traditional notion that chronic adenotonsillitis is solely caused by a single bacterial species colonizing the tissue surface is being challenged [46]. Instead, tissue hyperplasia and enlargement may be influenced by a variety of opportunistic, commensal, and pathogenic microorganisms, as well as the immune system’s response to them.
It would be an oversight to disregard the wide range of viruses that colonize the tonsils and adenoids. The list of respiratory viruses associated with upper airway infections is extensive and includes well-known agents, such as adenoviruses, bocavirus, coronaviruses, enteroviruses, EBV, human metapneumovirus, influenza viruses, parainfluenza viruses, respiratory syncytial virus, and rhinoviruses. Additionally, cytomegalovirus, human herpes viruses 6–8, herpes simplex virus, human papillomaviruses, human parvovirus B19, and polyomaviruses have been detected in the tonsillar and adenoidal tissues of asymptomatic children [47]. Remarkably, it has been reported that up to 97% of tonsils and adenoids harbor detectable viruses, and co-infections with multiple viral types have also been observed. While approximately 80% of adenoidal tissues may contain multiple viruses, slightly lower rates ranging between 59% and 68% have been reported in tonsillar tissues. These findings indicate that the presence of viruses in the tonsils and adenoids can be considered as a “normal viral flora”, suggesting that certain respiratory viruses may exhibit more chronic behavior [48]. It is worth noting that data from the COVID-19 pandemic have shown that tonsils and adenoids could serve as significant sites of SARS-CoV-2 infection in asymptomatic children [49].
In HIV patients, specific alterations in their tonsils and adenoids have been documented. Bacteria and yeast isolates, including Staphylococcus aureus, Streptococcus pyogenes, Klebsiella pneumoniae, Escherichia coli, Proteus mirabilis, Candida albicans, and Candida tropicalis, have been found in the respiratory tracts of HIV-positive children in Cambodia and Kenya. Additionally, a significant percentage of these children exhibited HIV-like sequences in these bacteria and yeasts, suggesting the potential for horizontal gene transfer between eukaryotic and prokaryotic cells, which could impact the progression of HIV disease [50].
All the aforementioned findings propose a potential connection between dysregulated microbiomes and the development or progression of adenotonsillar disease. Further research utilizing advanced sequencing techniques and metagenomic analyses is necessary to gain a comprehensive understanding of the adenoids and tonsils’ microbiomes and their potential implications in immune regulation and disease susceptibility.
4.2. The “Pathogen Reservoir” Hypothesis Relates to Biofilm Formation
The “Pathogen Reservoir” Hypothesis suggests that tonsils and adenoids serve as reservoirs for bacteria, contributing to recurrent upper respiratory tract infections. These lymphatic tissues can harbor microorganisms, leading to the formation of protective biofilms. Biofilms develop when bacteria adhere to surfaces and create a matrix that defends against the immune response and antimicrobial treatments. Several studies have highlighted the role of biofilms in chronic [51] and recurrent [52] tonsillitis, demonstrating their persistence and involvement in infection recurrence. Furthermore, biofilms contribute to antibiotic resistance by reducing the susceptibility to antimicrobial agents.
Bacterial biofilms also play a role in chronic adenoiditis and its associated complications, such as middle ear diseases [53]. Biofilms are present throughout the nasopharyngeal mucosa, particularly in the lateral region of adenoidal pads near the Eustachian tube, suggesting their involvement in chronic inflammation and middle ear problems [54]. Antibiotic therapy often fails in chronic adenoiditis cases due to biofilm resistance, which is facilitated by the physical barrier of the extracellular matrix and unique biofilm characteristics. Nasopharyngeal biofilms are associated with persistent or recurrent middle ear diseases, including chronic otitis media [55]. These biofilms impede antibiotic diffusion, exhibit reduced bacterial replication, and acquire resistance mechanisms. Young children with recurrent acute otitis media have a higher prevalence of nasopharyngeal biofilm-producing bacteria. Some studies challenge the assumption of sterile conditions in otitis media, as bacterial DNA has been found in middle ear effusion. Scanning electron microscopy reveals extensive biofilm coverage on adenoids in children with recurrent acute otitis media [56]. The specific location of biofilms near the Eustachian tube or tonsils further underscores their significance in various upper respiratory tract conditions.
These findings greatly contribute to our understanding of the “Pathogen Reservoir” Hypothesis and emphasize the importance of exploring strategies that target biofilms for the effective management of recurrent infections in the tonsils, adenoids, and ears.
References
- Perry, M.; Whyte, A. Immunology of the tonsils. Immunol. Today 1998, 19, 414–421.
- Fossum, C.C.; Chintakuntlawar, A.V.; Price, D.L.; Garcia, J.J. Characterization of the oropharynx: Anatomy, histology, immunology, squamous cell carcinoma and surgical resection. Histopathology 2017, 70, 1021–1029.
- Kharbanda, O.P. Orthodontics: Diagnosis and Management of Malocclusion and Dentofacial Deformities, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2021; p. 10.
- Kuper, C.F.; Koornstra, P.J.; Hameleers, D.M.; Biewenga, J.; Spit, B.J.; Duijvestijn, A.M.; Vriesman, P.J.v.B.; Sminia, T. The role of nasopharyngeal lymphoid tissue. Immunol. Today 1992, 13, 219–224.
- Kracke, A.; Hiller, A.S.; Tschernig, T.; Kasper, M.; Kleemann, W.J.; Tröger, H.D.; Pabst, R. Larynx-associated lymphoid tissue (LALT) in young children. Anat. Rec. 1997, 248, 413–420.
- Brandtzaeg, P. Function of Mucosa-Associated Lymphoid Tissue in Antibody Formation. Immunol. Investig. 2010, 39, 303–355.
- van Kempen, M.; Rijkers, G.; van Cauwenberge, P. The Immune Response in Adenoids and Tonsils. Int. Arch. Allergy Immunol. 2000, 122, 8–19.
- Brandtzaeg, P. Immune Functions of Nasopharyngeal Lymphoid Tissue. Adv. Otorhinolaryngol. 2011, 72, 20–24.
- Zautner, A.E. Adenotonsillar disease. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 121–129.
- Zautner, A.E.; Krause, M.; Stropahl, G.; Holtfreter, S.; Frickmann, H.; Maletzki, C.; Kreikemeyer, B.; Pau, H.W.; Podbielski, A. Intracellular Persisting Staphylococcus aureus Is the Major Pathogen in Recurrent Tonsillitis. PLoS ONE 2010, 5, e9452.
- Mitchell, R.B.; Archer, S.M.; Ishman, S.L.; Rosenfeld, R.M.; Coles, S.; Finestone, S.A.; Friedman, N.R.; Giordano, T.; Hildrew, D.M.; Kim, T.W.; et al. Clinical Practice Guideline: Tonsillectomy in Children (Update)—Executive Summary. Otolaryngol. Neck Surg. 2019, 160, 187–205.
- Sprinkle, P.M.; Veltri, R.W. The tonsils and adenoids. Clin. Otolaryngol. Allied Sci. 1977, 2, 153–167.
- Randall, D.A. Current Indications for Tonsillectomy and Adenoidectomy. J. Am. Board Fam. Med. 2020, 33, 1025–1030.
- Arambula, A.; Brown, J.R.; Neff, L. Anatomy and physiology of the palatine tonsils, adenoids, and lingual tonsils. World J. Otorhinolaryngol.-Head Neck Surg. 2021, 7, 155–160.
- Mnatsakanian, A.; Heil, J.R.; Sharma, S. Anatomy, Head and Neck: Adenoids; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Driweesh, T.A.; Altheyab, F.; Alenezi, M.; Alanazy, S.; Aldrees, T. Grisel’s syndrome post otolaryngology procedures: A sys-tematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110225.
- Standring, S. Pharynx. In Gray’s Anatomy, 40th ed.; Elsevier Press: Amsterdam, The Netherlands, 2021; pp. 702–716.e2.
- Meegalla, N.; Downs, B.W. Anatomy, Head and Neck, Palatine Tonsil (Faucial Tonsils); StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Ford, L.C.; Cruz, R.M. Bilateral Glossopharyngeal Nerve Paralysis after Tonsillectomy: Case Report and Anatomic Study. Laryngoscope 2004, 114, 2196–2199.
- Uzun, C.; Adali, M.K.; Karasalihoglu, A.R. Unusual complication of tonsillectomy: Taste disturbance and the lingual branch of the glossopharyngeal nerve. J. Laryngol. Otol. 2003, 117, 314–317.
- Deutsch, M.D.; Kriss, V.M.; Willging, J.P. Distance Between the Tonsillar Fossa and Internal Carotid Artery in Children. Arch. Otolaryngol. Neck Surg. 1995, 121, 1410–1412.
- Standring, S. Gray’s Anatomy, 40th ed.; Elsevier Press: Amsterdam, The Netherlands, 2021; pp. 273–291.e4.
- Isaacson, G.; Parikh, T. Developmental anatomy of the tonsil and its implications for intracapsular tonsillectomy. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 89–96.
- Brandtzaeg, P. Immunology of tonsils and adenoids: Everything the ENT surgeon needs to know. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, S69–S76.
- Brandtzaeg, P. Regionalized immune function of tonsils and adenoids. Immunol. Today 1999, 20, 383–384.
- Wu, R.-Q.; Zhang, D.-F.; Tu, E.; Chen, Q.-M.; Chen, W. The mucosal immune system in the oral cavity—An orchestra of T cell diversity. Int. J. Oral Sci. 2014, 6, 125–132.
- Nave, H.; Gebert, A.; Pabst, R. Morphology and immunology of the human palatine tonsil. Anat. Embryol. 2001, 204, 367–373.
- Scadding, G.K. Immunology of the Tonsil: A Review. J. R. Soc. Med. 1990, 83, 104–107.
- Stanisce, L.; Sims, E.; Hou, C.; Koshkareva, Y.; Gaughan, J.P.; Kuzin, I.; Bottaro, A. Differential cellular composition of human palatine and pharyngeal tonsils. Arch. Oral Biol. 2018, 96, 80–86.
- Castagnini, L.A.; Goyal, M.; Ongkasuwan, J. Tonsillitis and Peritonsillar Abscess. Infect. Dis. Pediatr. Otolaryngol. 2015, 14, 137–150.
- Klug, T.E. Peritonsillar abscess: Clinical aspects of microbiology, risk factors, and the association with parapharyngeal abscess. Dan. Med. J. 2017, 64, 354–359.
- Johnston, J.J.; Douglas, R. Adenotonsillar microbiome: An update. Postgrad. Med. J. 2018, 94, 398–403.
- Winther, B.; Gross, B.C.; Hendley, J.O.; Early, S.V. Location of Bacterial Biofilm in the Mucus Overlying the Adenoid by Light Microscopy. Arch. Otolaryngol. Neck Surg. 2009, 135, 1239–1245.
- Esposito, S.; Principi, N. Impact of nasopharyngeal microbiota on the development of respiratory tract diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 37, 1–7.
- Brook, I. The role of anaerobic bacteria in tonsillitis. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 9–19.
- Subtil, J.; Rodrigues, J.C.; Reis, L.; Freitas, L.; Filipe, J.; Santos, A.; Macor, C.; Duarte, A.; Jordao, L. Adenoid bacterial coloni-zation in a paediatric population. Eur. Arch. Otorhinolaryngol. 2017, 274, 1933–1938.
- Brook, I.; Shah, K.; Jackson, W. Microbiology of Healthy and Diseased Adenoids. Laryngoscope 2000, 110, 994–999.
- Ren, T.; Glatt, D.U.; Nguyen, T.N.; Allen, E.K.; Early, S.V.; Sale, M.; Winther, B.; Wu, M. 16S rRNA survey revealed complex bacterial communities and evidence of bacterial interference on human adenoids. Environ. Microbiol. 2012, 15, 535–547.
- Stępińska, M.; Olszewska-Sosińska, O.; Lau-Dworak, M.; Zielnik-Jurkiewicz, B.; Trafny, E.A. Identification of Intracellular Bacteria in Adenoid and Tonsil Tissue Specimens: The Efficiency of Culture Versus Fluorescent In Situ Hybridization (FISH). Curr. Microbiol. 2013, 68, 21–29.
- Vilarinho, S.; Guimarães, N.M.; Ferreira, R.M.; Gomes, B.; Wen, X.; Vieira, M.J.; Carneiro, F.; Godinho, T.; Figueiredo, C. Helicobacter pylori colonization of the adenotonsillar tissue: Fact or fiction? Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 807–811.
- Wu, X.; Wang, W.; Fang, L.; Shi, L.; Rao, X. Is Helicobacter pylori colonization associated with chronic tonsillitis?—A meta-analysis and systematic review. Am. J. Otolaryngol. 2022, 43, 103515.
- Cho, S.W.; Yang, S.K. What Does the Microbiome in the Tonsil Tell Us? Clin. Exp. Otorhinolaryngol. 2021, 14, 247–248.
- Jensen, A.; Fagö-Olsen, H.; Sørensen, C.H.; Kilian, M. Molecular Mapping to Species Level of the Tonsillar Crypt Microbiota Associated with Health and Recurrent Tonsillitis. PLoS ONE 2013, 8, e56418.
- Kim, K.S.; Min, H.J. Correlations Between the Adenotonsillar Microbiome and Clinical Characteristics of Pediatric Patients with Snoring. Clin. Exp. Otorhinolaryngol. 2021, 14, 295–302.
- Swidsinski, A.; Goktas, O.; Bessler, C.; Loening-Baucke, V.; Hale, L.P.; Andree, H.; Weizenegger, M.; Holzl, M.; Scherer, H.; Lochs, H. Spatial organisation of microbiota in quiescent adenoiditis and tonsillitis. J. Clin. Pathol. 2006, 60, 253–260.
- Kostić, M.; Ivanov, M.; Babić, S.S.; Tepavčević, Z.; Radanović, O.; Soković, M.; Ćirić, A. Analysis of tonsil tissues from patients diagnosed with chronic tonsillitis-microbiological profile, biofilm-forming capacity and histology. Antibiotics 2022, 11, 1747.
- Faden, H.; Callanan, V.; Pizzuto, M.; Nagy, M.; Wilby, M.; Lamson, D.; Wrotniak, B.; Juretschko, S.; George, K.S. The ubiquity of asymptomatic respiratory viral infections in the tonsils and adenoids of children and their impact on airway obstruction. Int. J. Pediatr. Otorhinolaryngol. 2016, 90, 128–132.
- Sato, M.; Li, H.; Ikizler, M.R.; Werkhaven, J.A.; Williams, J.V.; Chappell, J.D.; Tang, Y.-W.; Wright, P.F. Detection of Viruses in Human Adenoid Tissues by Use of Multiplex PCR. J. Clin. Microbiol. 2009, 47, 771–773.
- Miura, C.S.; Lima, T.M.; Martins, R.B.; Jorge, D.M.M.; Tamashiro, E.; Anselmo-Lima, W.T.; Arruda, E.; Valerab, F.C.P. Asymptomatic SARS-COV-2 infection in children’s tonsils. Braz. J. Otorhinolaryngol. 2022, 88, 9.
- Zajac, V.; Matelova, L.; Liskova, A.; Mego, M.; Holec, V.; Adamcikova, Z.; Stevurkova, V.; Shahum, A.; Krcmery, V. Con-firmation of HIV-like sequences in respiratory tract bacteria of Cambodian and Kenyan HIV-positive pediatric patients. Med. Sci. Monit. 2011, 17, CR154–CR158.
- Bakar, M.A.; McKimm, J.; Haque, S.Z.; Majumder, M.A.A.; Haque, M. Chronic tonsillitis and biofilms: A brief overview of treatment modalities. J. Inflamm. Res. 2018, 11, 329–337.
- Nazzari, E.; Torretta, S.; Pignataro, L.; Marchisio, P.; Esposito, S. Role of biofilm in children with recurrent upper respiratory tract infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 34, 421–429.
- Torretta, S.; Drago, L.; Marchisio, P.; Ibba, T.; Pignataro, L. Role of Biofilms in Children with Chronic Adenoiditis and Middle Ear Disease. J. Clin. Med. 2019, 8, 671.
- Nistico, L.; Kreft, R.; Gieseke, A.; Coticchia, J.M.; Burrows, A.; Khampang, P.; Liu, Y.; Kerschner, J.E.; Post, J.C.; Lonergan, S.; et al. Adenoid Reservoir for Pathogenic Biofilm Bacteria. J. Clin. Microbiol. 2011, 49, 1411–1420.
- Bakaletz, L.O. Bacterial biofilms in the upper airway—Evidence for role in pathology and implications for treatment of otitis media. Paediatr. Respir. Rev. 2012, 13, 154–159.
- Torretta, S.; Marchisio, P.; Drago, L.; Baggi, E.; De Vecchi, E.; Garavello, W.; Nazzari, E.; Pignataro, L.; Esposito, S. Nasopha-ryngeal biofilm-producing otopathogens in children with nonsevere recurrent acute otitis media. Otolaryngol. Head Neck Surg. 2012, 146, 991–996.
More
