Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Eslam El-Sawy and Version 1 by Mohamed Abdel-Aziz.
Asteltoxins belong to a group of polyene pyrone mycotoxins that are known to be potent inhibitors of mitochondrial ATP synthesis and ATP hydrolysis. Asteltoxin A was first isolated from the toxic maize cultures of Aspergillus stellatus. Several attempts have been made to synthesize asteltoxin A, starting with the synthesis of a bis(tetrahydrofuran) moiety that has been demonstrated previously in biosynthetic studies.
- asteltoxins
- sources
- biosynthesis
- synthesis
1. Introduction
Mycotoxins are secondary metabolites produced by fungi capable of causing disease and death in humans and animals [1]. There have been many different mycotoxins discovered, but the most prevalent mycotoxins that are harmful to both humans and animals are aflatoxins, ochratoxin A, patulin, fumonisins, zearalenone, and nivalenol/deoxynivalenol. When crops are infected with mold before and after harvest, mycotoxins enter the food chain. Mycotoxins can be ingested directly through contaminated food or indirectly through consuming animals fed contaminated feed [1]. Some mycotoxin derivatives have exceptional biological activity and are used as antibiotics (penicillin and citrinin) [2], to relieve migraine attacks (ergotamine) [2] and as plant growth stimulants (Fusarium metabolites) [3].
Asteltoxins are mycotoxins belonging to a structural group featuring an α-pyrone attached to a 2, 8-dioxabicyclo [3.3.0] octane ring via a triene linker [4] and that are structurally related to citreoviridin and aurovertin [5], except for asteltoxin G which contains a tetrahydrofuran ring instead of a 2, 8-dioxabicyclo [3.3.0] octane ring [6] (Figure 1).
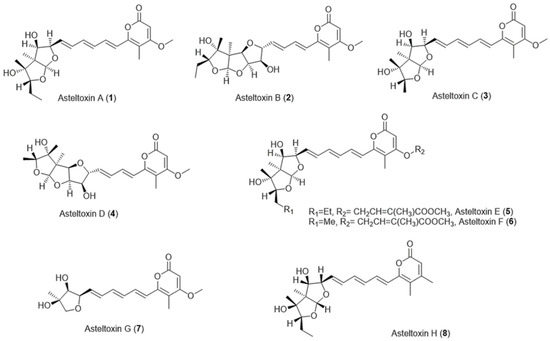
Figure 1. Chemical structures of asteltoxins A–H.
Asteltoxin A (1) has been isolated from the toxic maize meal cultures of Aspergillus stellatus Curzi (MRC 277) [7,8]. Asteltoxin A (1) was also isolated from Emericella variecolor [9]. Asteltoxin A (1) was the first identified member of the asteltoxins, which was isolated and chemically elucidated via spectroscopic methods and single-crystal X-ray analysis as mentioned by Rabie and his coworkers [7].
Other asteltoxin families such as asteltoxins B (2), C (3), and D (4) were produced by Pochonia bulbillosa 8-H-28 [10], whereas asteltoxins E (5) and F (6) were purified from Aspergillus sp. SCSIO XWS02F40 [11], and asteltoxin G (7) was obtained from Aspergillus ochraceopetaliformis [12]. Additionally, asteltoxin H (8) was isolated from the fungus Pochonia suchlasporia var. suchlasporia TAMA 87 [6] (Figure 1).
On the other hand, asteltoxins were reported to be isolated as secondary metabolites of Aspergillus karnatakaensis [13], Aspergillus aeneus [13], Aspergillus terreus [14], and Aspergillus alabamensis [14].
The chemical backbones of the natural asteltoxins A-G share the same α-pyrone moiety (4-methoxy-5-methyl-2-pyrone-based structure), but asteltoxin H has another type of α-pyrone moiety (3, 5-dimethyl-2-pyrone-based structure) (Figure 1).
Bao et al. (2013) [15] and Wu et al. (2015) [16] isolated an analog of asteltoxin A (1) from the gorgonian-derived fungus Aspergillus sp. SCSGAF 0076 and a sponge-associated fungus E. variecolor, respectively. They identified this analog as asteltoxin B with an epoxy group at C-7/C-8, which contradicts the known chemical form of the known asteltoxin B. They chemically elucidated its structure by spectroscopy, including affinities for 1H-1H COZY, HMBC, and NOESY.
Interestingly, similar asteltoxins have been identified that include asteltoxin-bearing dimers and prenylated asteltoxins (Figure 2).
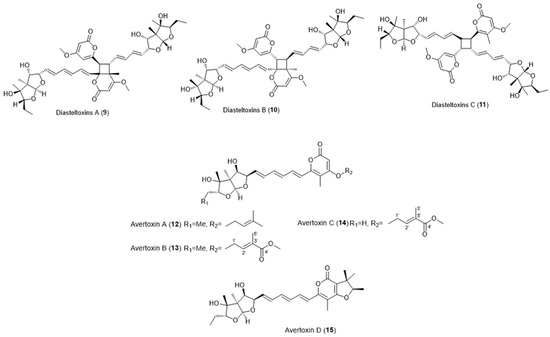
Figure 2. Chemical structures of similar asteltoxins. Diasteltoxin A (9), Diasteltoxin B (10), Disasteltoxin C (11), Avertoxin A (12), Avertoxin B (13), Avertoxin C (14), and Avertoxin D (15).
Three novel asteltoxin-bearing dimers, named diasteltoxins A−C (9–11) (Figure 2), were isolated from a mutated strain of a sponge-derived fungus Emericella variecolor XSA-07-2 [17]. Four new prenylated asteltoxin derivatives, named avertoxins A (12), B (13), C (14), and D (15) (O-prenyl and C-prenyl asteltoxins) (Figure 2), were isolated from Aspergillus versicolor Y10, an endophytic fungus isolated from Huperzia serrata [18].
Asteltoxin A (1) possesses a range of biological activities, including potent inhibition against bacterial ATP synthesis and ATP hydrolysis catalyzed by mitochondrial enzyme systems [19]. On the other hand, asteltoxin and its similar compounds showed various biological activities, viz., antiviral [11], antiproliferative [10], and insecticidal [6] activities, as well as human acetylcholinesterase inhibition [18].
Evidence suggests that the bis(tetrahydrofuran) moiety is responsible for the inhibition and binding properties of asteltoxins [8].
2. Biological Activities
Asteltoxin A (1) was the most important of all known asteltoxins, as it showed various biological activities, which included inhibition of Escherichia coli BFI-ATPase activity, quorum sensing (QS) inhibitor, extracellular vesicle (EV) inhibitor, and antiviral activity. Asteltoxin A (1) was found to inhibit Escherichia coli BFI-ATPase activity and can serve as a valuable fluorescent probe for mitochondrial F, bacterial BF, and ATPases [6]. Evidence suggests that the bis(tetrahydrofuran) moiety is responsible for the inhibition and binding properties of asteltoxin A [6]. Asteltoxin A (1), a respiratory toxin from Emericella variecolor, was examined to determine its inhibitory effect on mitochondrial function. Mitochondrial respiration is divided into different states, where each represents a different stage of oxygen consumption. State 3 of respiration represents oxygen consumption after ADP addition in the presence of a certain substrate, while state 4 represents oxygen consumption after ADP is completely transformed into ATP (i.e., in the absence of ADP). It was reported that the addition of asteltoxin (15 nmol) to mitochondria isolated from rat liver, before adding ADP and succinate, prevented state 3 and state 4 respiration processes. Hence, asteltoxin was shown to be able to inhibit the ATP synthesis system in mitochondria, leading to state 3 suppression; it was also found that asteltoxin A strongly decreases Mg2+-ATPase activity in mitochondria and slightly affects Na+- and K+-activated ATPases in microsomes at the concentration range for the inhibition of mitochondrial respiration [13]. This confirms that asteltoxin A (1) inhibits the energy transfer system in mitochondria, specifically inhibiting Mg2+-ATPase activity [13]. Asteltoxin A (1) is considered as a known quorum sensing (QS) inhibitor from the marine fungus Penicillium sp. QF046. It exhibits potent inhibition of violacein compared to the positive control, (Z-)-4-bromo-5-(bromomethylene)-2(5H)-furanone, meaning that it decreases the expression of multiple QS-related genes (lasA, lasB, vioB, vioI, cynS, and hcnB) [31]. Asteltoxin A (1) was represented as a new type of extracellular vesicle (EV) inhibitor that controls the fate of multi-vesicular bodies (MVBs). In detail, after treatment with asteltoxin at low concentrations (0.1, 1.0, and 10 µg/mL), the adenosine monophosphate/adenosine triphosphate (AMP/ATP) ratio increases due to the inhibition of ATP synthase, and this increase induces AMPK-mediated suppression of mTORC1. Inactivation of the mammalian target of rapamycin complex 1 (mTORC1) promotes nuclear translocation of the microphthalmia/transcription factor E (MiT/TFE) family members, thereby inducing transcription of lysosome-related genes and activation of the lysosomal function. MVBs are then degraded, and the number of MVBs fused with the plasma membrane is low, resulting in the suppression of EV secretion (Figure 3) [34].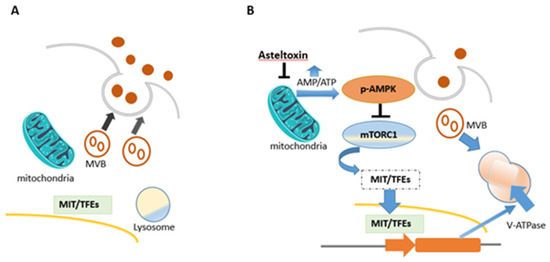
Figure 3. Schematic flowsheet considering the role of asteltoxin in regulating the fate of multi-vesicular bodies (MVBs) and regulation of EV secretion. (A) MVBs fuse with the plasma membrane and endorse EV secretion in cancer cells. (B) After asteltoxin A (1) treatment.
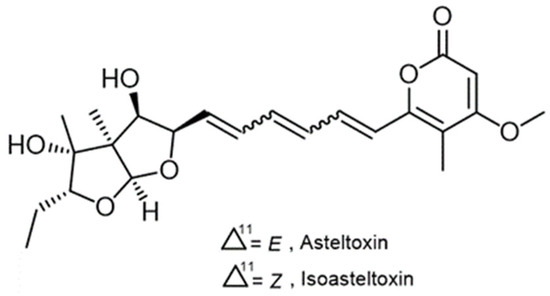
Figure 4. Chemical structures of asteltoxin A (1) and isoasteltoxin.
Table 1. Various biological activity of asteltoxins and their similar compounds.
| Asteltoxin Compounds | Activity | Ref |
|---|---|---|
| Asteltoxin C (3) | Antiproliferative activity against NIAS-SL64 cells derived from the body fat of Spodoptera litura larvae. | [10] |
| Asteltoxin H (8) | Insecticidal activity against prepupae of the blowfly, Lucilia sericata, with an LD50 value of 0.94 μg/mg of prepupal body weight. | [6] |
| Asteltoxins E (5) and F (6) | Antiviral activity against H3N2 with the prominent IC50 values of 6.2 ± 0.08 and 8.9 ± 0.3 µM, respectively. | [11] |
| asteltoxin F (6) | Antiviral activity with inhibitory activity against H1N1 with an IC50 value of 3.5 ± 1.3 µM. | [11] |
| Diasteltoxins A-C (66–68) | Antiproliferative effects against H1299 (human lung cancer) and MCF7 (human breast cancer) cells. Applicable inhibition against thioredoxin reductase (TrxR) with IC50 12.8 ± 0.8, 11.1 ± 0.2, and 7.2 ± 0.2 µM, respectively. | [17] |
| Avertoxin B (70) | Human acetylcholinesterase inhibition with the IC50 value of 14.9 μM. | [18] |
