You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Andrei Belousov.
Carbohydrate-based biomaterials are a unique platform for active molecular transport and targeted drug delivery, providing biocompatibility, biodegradability, and a reduction in toxic side effects.
- nanoparticles
- natural polymers
- polysaccharides
- glioblastoma
- drug delivery
1. Introduction
Glioma is a type of brain cancer that is nearly incurable due to the ineffectiveness of chemotherapy drugs, invasive growth, and the blood–brain barrier (BBB) that limits drug delivery [1]. The standard treatment for glioblastoma is surgical resection, followed by simultaneous radiotherapy and chemotherapy with temozolomide (TMZ) for 6 weeks, followed by adjuvant therapy with TMZ for 6 months. In addition to TMZ, there are four FDA-approved chemotherapy drugs for glioblastoma and high-grade gliomas treatment (Figure 1): lomustine, carmustine, taxol (paclitaxel, PTX), and bevacizumab [2,3][2][3]. All these drugs are administered intravenously. In addition, carmustine is used in the form of Carmustine wafer implants. The wafer is based on polifeprosan 20 (a copolymer of 1,3-bis-(p-carboxyphenoxy) propane and sebacic acid), which is biodegradable and can be loaded with chemotherapy agents such as carmustine or TMZ for a limited topical application directly into the area where the tumor has been removed. This approach has shown a significantly higher therapeutic impact and a reduced number of side effects [4,5][4][5].
Drug delivery to brain tumors is complicated by multiple factors, including poor drug solubility, low drug concentration at the tumor site, irregular tumor vasculature, intratumoral hypoxia and selective permeability of BBB, difficulty of surgical access to the tumor focus, infiltrative tumor growth, and metastasis [6].
Recent advancements in nanotechnology have led to the development of nanoparticles (NPs) that can bypass the BBB, efficiently deliver drugs to the tumor site, and penetrate glioma cell membranes [7,8][7][8]. They should be less than 200 nanometers, or even less depending on the surface charge, chemical properties, structure [9], and nanomechanical properties [10].
Important advantages are NPs’ safety and biodegradability. Non-organic and metal NPs, such as gold ones, showed a significant toxic effect on cancer and healthy cells [11]. A critical property of drug delivery systems is their ability to instigate the reversible immobilization of active molecules and their controlled release, to provide a constant therapeutic concentration, to maximize efficiency and minimize negative side effects. Additionally, a good option to facilitate the non-invasive monitoring of glioma progression and treatment response is the presence of non-invasive imaging capabilities [12].
To transfer through the BBB, NPs need to stimulate receptor-mediated transcytosis, which can be activated by some glycoproteins and antibodies. There is evidence from experiments with liposomal or peptide carriers [13], and carbohydrate transporters [14] showing their efficacy in vitro and in vivo on xenograft models. Carbohydrate NPs have emerged as a promising tool for anticancer therapy due to their excellent biocompatibility, biodegradability, and ability to target specific cancer cells. NPs can be produced from various natural and synthetic polysaccharides such as cellulose and its derivatives [15], chitosan [16], pectin [17], hyaluronic acid [18], and alginate [19]. Carbohydrate NPs can be loaded with chemotherapeutic agents, peptides, and vectors to enable the target cytotoxic effect [20,21][20][21].
2. Glucose-Based
Cellulose is the most abundant polysaccharide in nature. It is water-insoluble and chemically stable, so one of the approaches for functional material design is a high-intensity mechanic or ultrasonic treatment and the production of cellulose nanocrystals (CNCs) characterized by various shapes and sizes [24][22]. Chemical stability supplemented with morphological durability and persistence leads to various applications of surface functionalization, changing the hydrophilicity and charge, and providing new interaction sites for bio-affinity and biodegradability [25][23]. CNCs are usually isolated from lignocellulose biomass by inorganic acid hydrolysis (Figure 1). Some papers describe the process with sulfur acid [26][24], hydrochloric acid [27][25], nitric acid in pure form, and combinations [28][26]. The shape and size of the CNC and a wide range of chemical modifications suggest many ways to use them for the treatment of cancer [29][27].
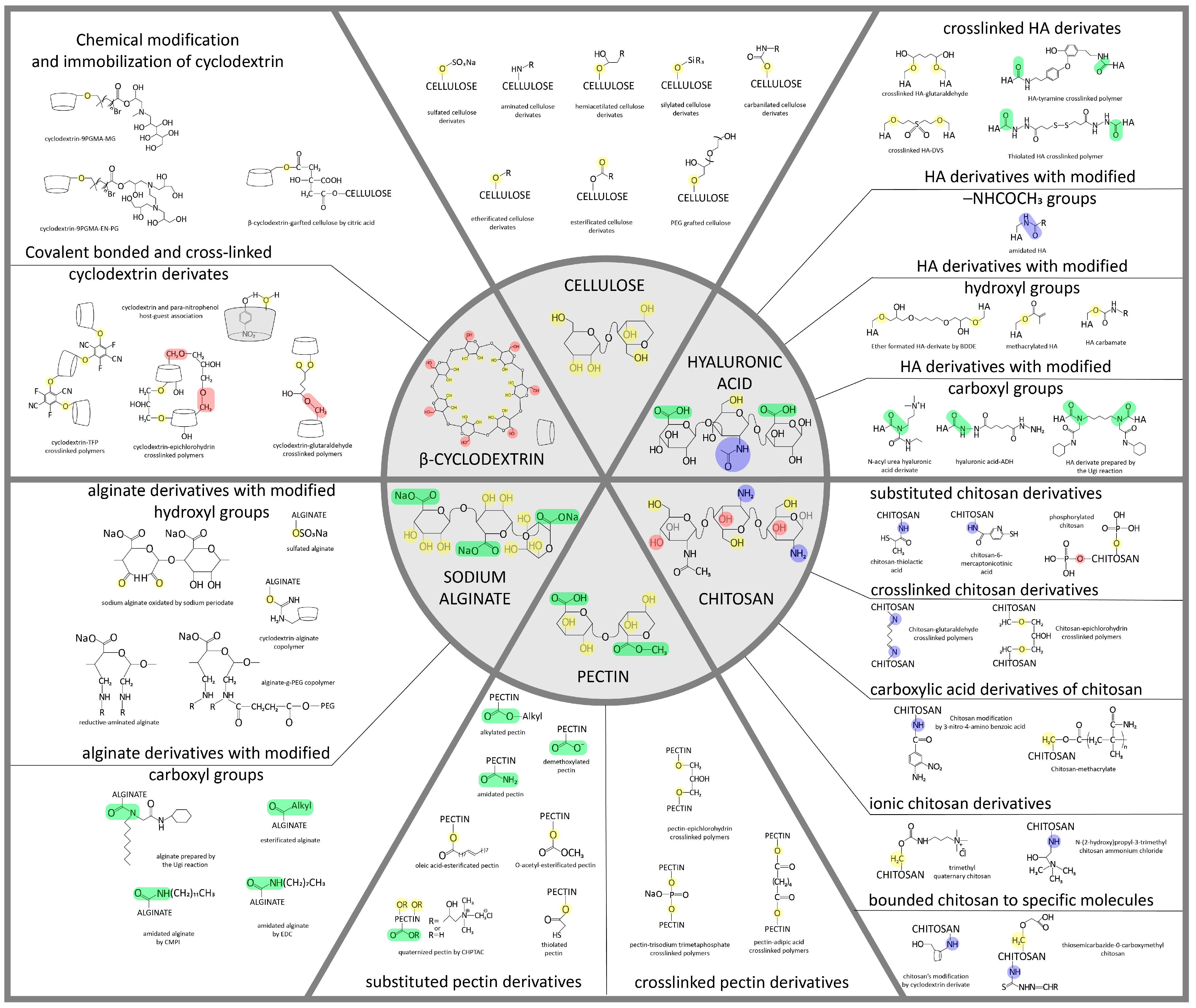
Figure 1. Structural formulas of some carbohydrates (cellulose and its derivatives, chitosan, pectin, hyaluronic acid, and alginate) and their possible modifications, on the basis of which it is possible to obtain various materials, including NPs [30,31,32,33,34,35,36,37,38][28][29][30][31][32][33][34][35][36]. The chemical modifications of polysaccharide nanoparticles are necessary to enhance their ability to cross the BBB and reach the target brain cells and for the attachment of targeting moieties and drugs. These modifications promote charge and active site setting, which allows nanoparticles with controlled drug release and biodegradation ability to be designed.
Cyclodextrins (CDs) are α-1,4-linked macrocyclic oligosaccharides obtained from cellulose by enzymatic hydrolysis. CDs are biocompatible, biodegradable, and non-toxic materials. The chemical structure of CDs is a truncated cone containing glucopyranose units with an outer hydrophilic exterior and inner hydrophobic pocket. It has been an attractive material for pharmaceutical applications due to this hydrophobic core to load various hydrophobic drug molecules by forming complexes through van der Waals force and hydrogen bonds. In that way, it is possible to overcome the undesirable physico-chemical properties of drugs: poor solubility and instability. Additionally, hydroxyl groups of the CDs cone’s exterior provide its solubility and the ability to form chemical modifications by functional molecules [39][37].
There are three most widely used CD native forms: α-CD, β-CD, and γ-CD, containing six, seven, and eight glucopyranose units, respectively. α-CD has a relatively small cavity that can only entrap small molecules. The drug capture of β-CD and γ-CD is better. Since γ-CD has a high production cost, β-CD with a moderate cavity and low production cost is the most widely applied CD in pharmaceutical research. The aqueous solubility of native α-CD, β-CD, and γ-CD is 13%, 2%, and 26% w/w, respectively. The low solubility of β-CD is due to the strong interaction of the inner hydrogen bonding formation among the secondary hydroxyl groups. This limit can be overcome by the disorganization of this strong hydrogen bonding [40,41][38][39].
3. Chitin and Chitosan
Chitin is the most abundant polysaccharide in nature after cellulose. It occurs in a number of eukaryotic species, such as crustaceans, mollusks, insects, and fungi. Chitin consists of 2-acetamido- (acetylated glucosamine, Figure 1 and Figure 2) and 2-amino-2-deoxy-β-D-glucoside (glucosamine, Figure 1 and Figure 2) residues connected by β-1,4-glycoside bonds. It is a homopolysaccharide, but usually, macromolecules include both types of residues [42][40].
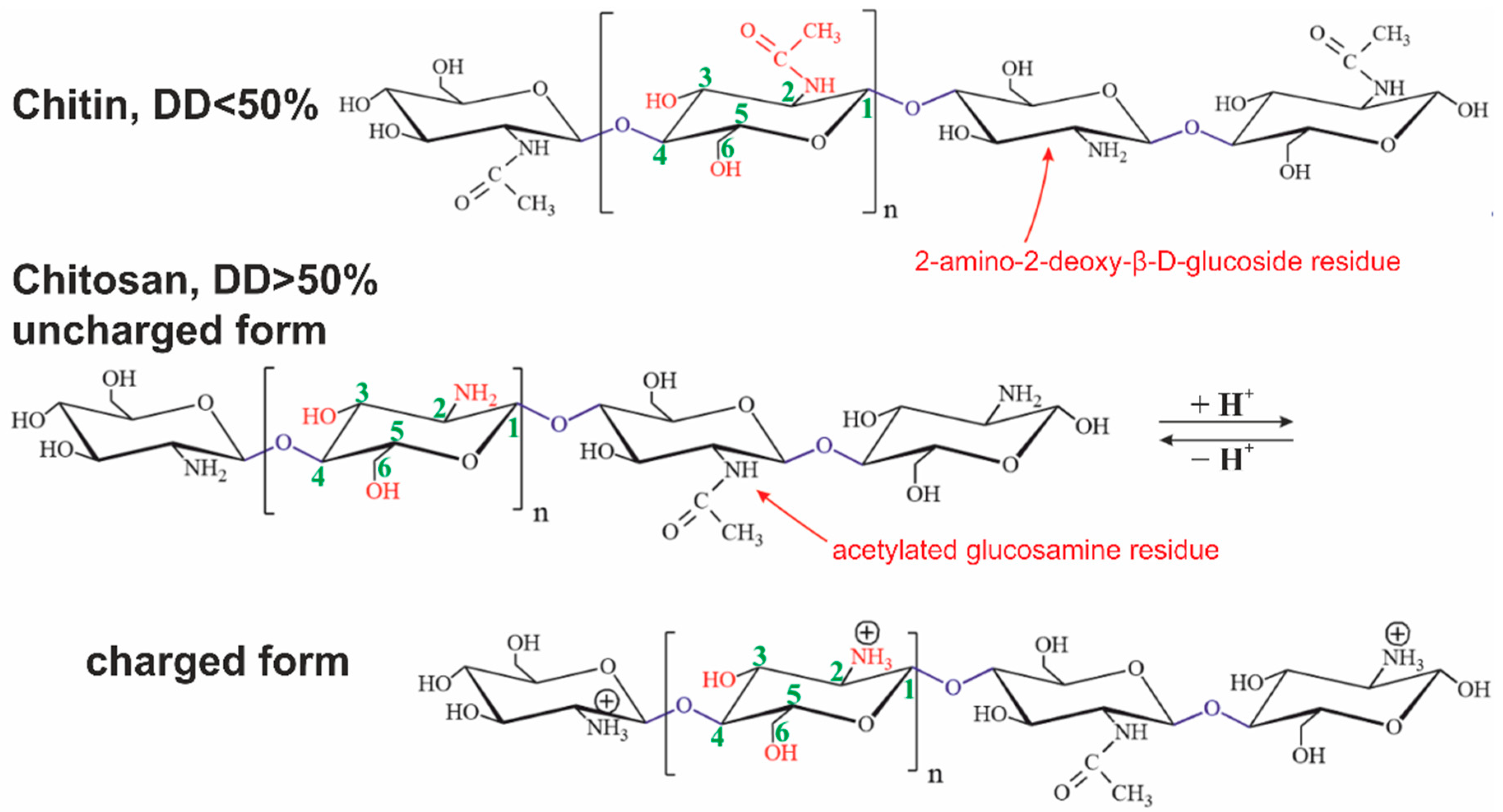
Figure 2. Structural formulas of chitin and chitosan in uncharged and charged forms. The presented polysaccharides can contain both types of glucosamine residues connected by O-glycoside bonds simultaneously.
Chitosan is derived from chitin by an N-deacetylation reaction. The reaction never goes through completely. The degree of deacetylation (DD) is always less than 95%. A polysaccharide with less than 50% deacetylated glucosamine residues is conventionally considered to be chitin, while a larger one is chitosan [43][41].
Chitin is soluble in a very limited number of organic solvents or under harsh conditions. The European Chitin Society suggests that a polysaccharide that is insoluble in 0.1 M acetic acid solution be considered chitin. Chitosan is soluble in aqueous solutions of most acids [44][42]. Its solubility is due to the polyelectrolyte effect because of charged amino groups in the macromolecule (Figure 1 and Figure 2) [45][43]. Polyelectrolytes are polymers containing functional groups capable of dissociation in aqueous solution. In this case, not one but many charges appear on their macromolecules.
Chitosan is known as a biologically active compound that exhibits numerous biological properties, such as antitumor, immune-strengthening, antifungal, antimicrobial, antioxidant, and wound-healing activities. The dependence of the effectiveness of the above properties on the DD was experimentally confirmed [46][44].
The above features as well as some exceptional properties such as non-toxicity, biodegradability, biocompatibility, and non-antigenicity have led to a wide application of this biopolymer in pharmaceutics, including biomedicine with the possibility of clinical use in drug delivery systems, tissue engineering [47][45], and food technology [48][46]. Moreover, interest in chitosan due to its biomedical applications in the central nervous system (CNS) has increased because of its potential ability to cross the BBB [49][47].
4. Pectin
Pectin primarily serves as a structural component of plant tissue’s cell wall. Citrus, apple, pear, and other fruits are used to extract pectin [50][48]. The chemical structure of this biopolymer has not yet been fully determined as it is a complicated natural molecule. Pectin is derived from linear polysaccharides, such as chains containing hundreds to thousands of saccharide units with average molecular weights ranging from 50 to 150 kDa. It is a heteropolysaccharide that consists of (1,4)-α-D-galacturonic acid residues branched with different neutral sugars (Figure 1 and Figure 3). There are four major building subunits, namely, homogalacturonan (~65% of pectin in plants), which consists of linear chains of α-1,4-linked methylated or acetylated galacturonic acid residues, rhamnogalacturonan-I (~20 to 35% of pectin in plants), xylogalacturonan (~10% of pectin in plants), and rhamnogalacturonan-II [51][49].
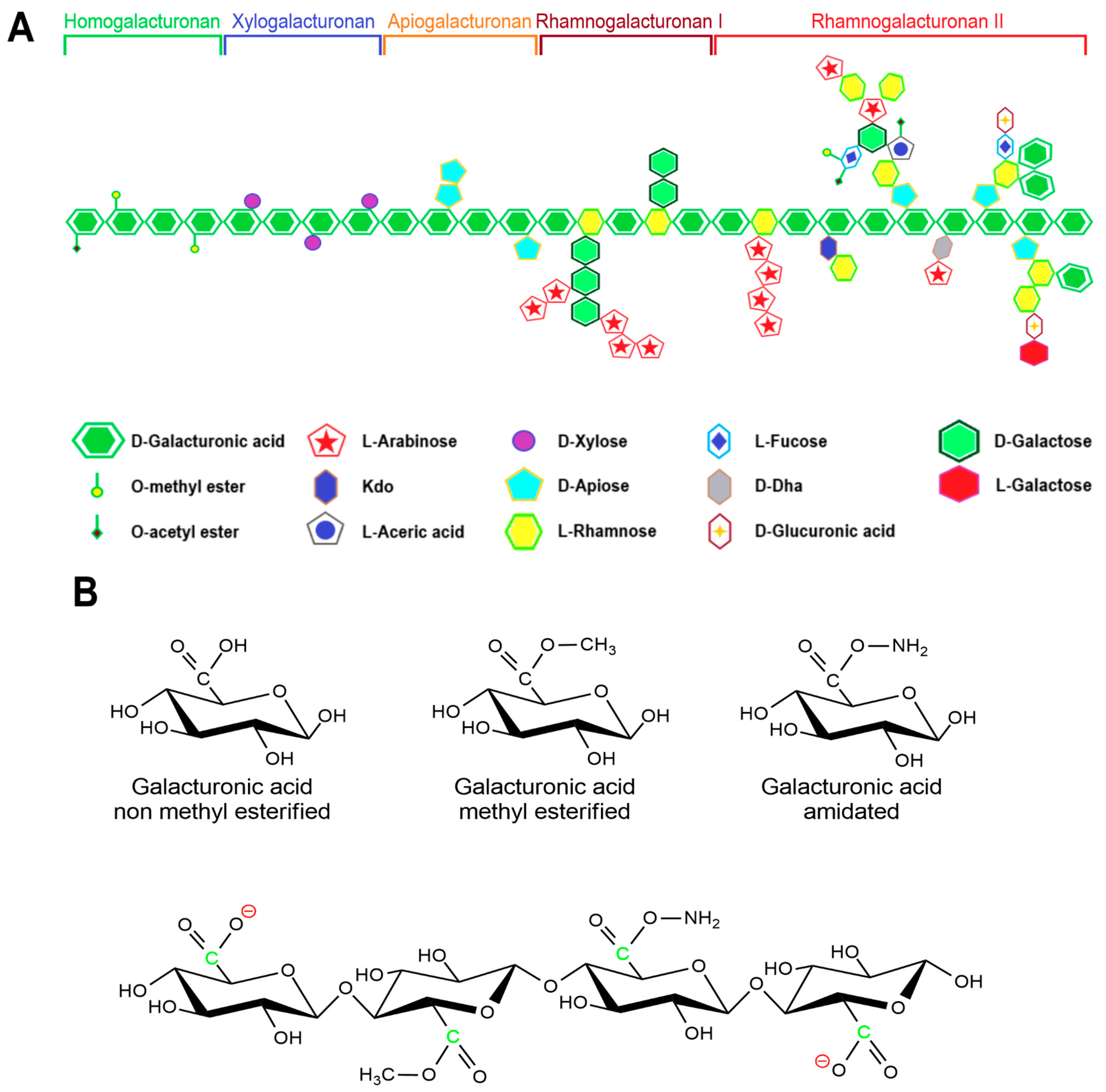
Pectin can be further classified by the ratio of esterified galacturonic acid groups to the total galacturonic acid group called degree of esterification (DE), which has a significant effect on the properties of pectin, particularly its solubility and gel formation, and biomedical and drug delivery applications [53][51].
Hydroxyl, amide, carboxyl, and methyl functional groups are the major functional groups in the pectin chain. Under different pH conditions, the -COOH of the pectin molecule can exist in different forms of -COO−, -COOH, and -COOH2+ [54][52]. Thus, pectin can shape into a composite with different feedstocks with opposite charges by adjusting the pH value of the medium.
Pectins that contain more than half of their carboxylate units as methyl esters have a relatively high carboxyl group ratio and are referred to as having a high methyl esterification rate. They are primarily used in gelation, require large amounts of sugar, and are very sensitive to acidity. Pectins that have less than 50% of the carboxylate units as methyl esters are usually referred to as having a low methyl esterification rate. This is obtained by slightly alkalizing a high ester pectin. It shows less sensitivity toward acidity and requires divalent ions to form gel [52][50]. Moreover, amidated pectin is produced through an alkaline process using ammonia to form a high ester polysaccharide. In this process, some of the remaining carboxylic acid groups are converted to acid amides. The properties of the amidated pectins can vary depending on the ratio of ester to amide unit ratio and the degree of polymerization. Polygalacturonic acid is partially esterified with methyl groups, and the free acid group is neutralized partially or completely with sodium, potassium, or ammonia ions [55][53].
Pectins have been reported to have a variety of bioactive properties including anticancer, anti-inflammatory, antioxidant, anti-diabetic, anti-cholesterol, anti-tumoral, and chemopreventive activities [54][52].
5. Hyaluronic Acid
Hyaluronic acid (HA) is a non-sulfated glycosaminoglycan consisting of disaccharides of D-glucuronic acid and N-acetyl-D-glucosamine, which are linked by β(1,4) and β(1,3) glycosidic bonds (Figure 1). HA is an anionic polymer (pKa = 3–4) [56][54], which allows it to interact with cationic polymers, surfactants, and lipids to form various nanostructures. HA has carboxyl and hydroxyl groups as well as an N-acetyl group, which gives a wide potential for further modification [57][55].
The key advantages of hyaluronic acid are its biocompatibility, complete biodegradability, and non-immunogenicity, which characterize hyaluronate as a biocompatible material for various biomedical applications. Interpenetrating networks of HAs facilitate the production of self-assembly aggregates, NPs, and hydrogels [58][56]. However, its short biological half-life and poor stability limit areas of its application [59][57]. In addition, HA fragments stimulate inflammation, tumor invasion, and P-glycoprotein-mediated multidrug resistance [60,61][58][59]. Fragments (25–50 disaccharide units) accumulate at the focus of inflammation and act as endogenous danger signals, reflecting oxidative stress in tissues [62][60]. HA oligosaccharides alter the expression of macrophages, chemokines, cytokines, and growth factors and can reduce the proliferation of endothelial fibroblasts and smooth muscle cells [63][61].
HA chemical modifications are mainly performed in aqueous solution involving two sites of the biopolymer: the hydroxyl and the carboxyl groups. Each disaccharide unit contains four hydroxyls, one amide, and one carboxyl group, and the resulting hydrogen bond associations prevent its hydrophobic modification in polar organic solvents. It limits the use of HA in the creation of permanent implants and other durable hydrophobic materials for biomedicine [64][62]. Alternative strategies are based on the partial protection of functional groups with hydrophobic blocking agents and the protection of anionic groups through complexation with cationic residues. Carboxyl groups are used for amidation and esterification, while hydroxyl groups form ester and ester bonds [65][63]. Dialdehyde groups formed after HA treatment with periodate can be used for chemical modification by reductive amination [66][64].
Hydrophobic modification allows for changing the rate of its degradation and hydrophilic properties depending on the degree of substitution. The attachment of amino acids with carboxyl or hydroxyl groups on HA provides strong resistance to its enzymatic cleavage. The biomedical application of HA is associated with its high hydrophilicity, unique rheological behavior, and its inherent pharmacological properties [67][65].
6. Alginate
Alginate is an anionic polysaccharide produced by brown algae and bacteria and consists of residues of α-L-guluronic acid (G-Block) and β-D-mannuronic acid (M-Block), linearly linked by 1,4-glycoside bonds. Alginate is non-toxic, biodegradable, low cost, and readily available, and has been found to be a mucoadhesive, biocompatible, and non-immunogenic substance. The composition and sequence of G and M residues depend on the source of the algae used and how this affects the properties of the alginate [68][66]. Alginate, a water-soluble unbranched polysaccharide, can also be chemically modified to change its properties (amidation, esterification, etc.) [32][30]. Alginate’s structure is shown in Figure 1 and Figure 4.
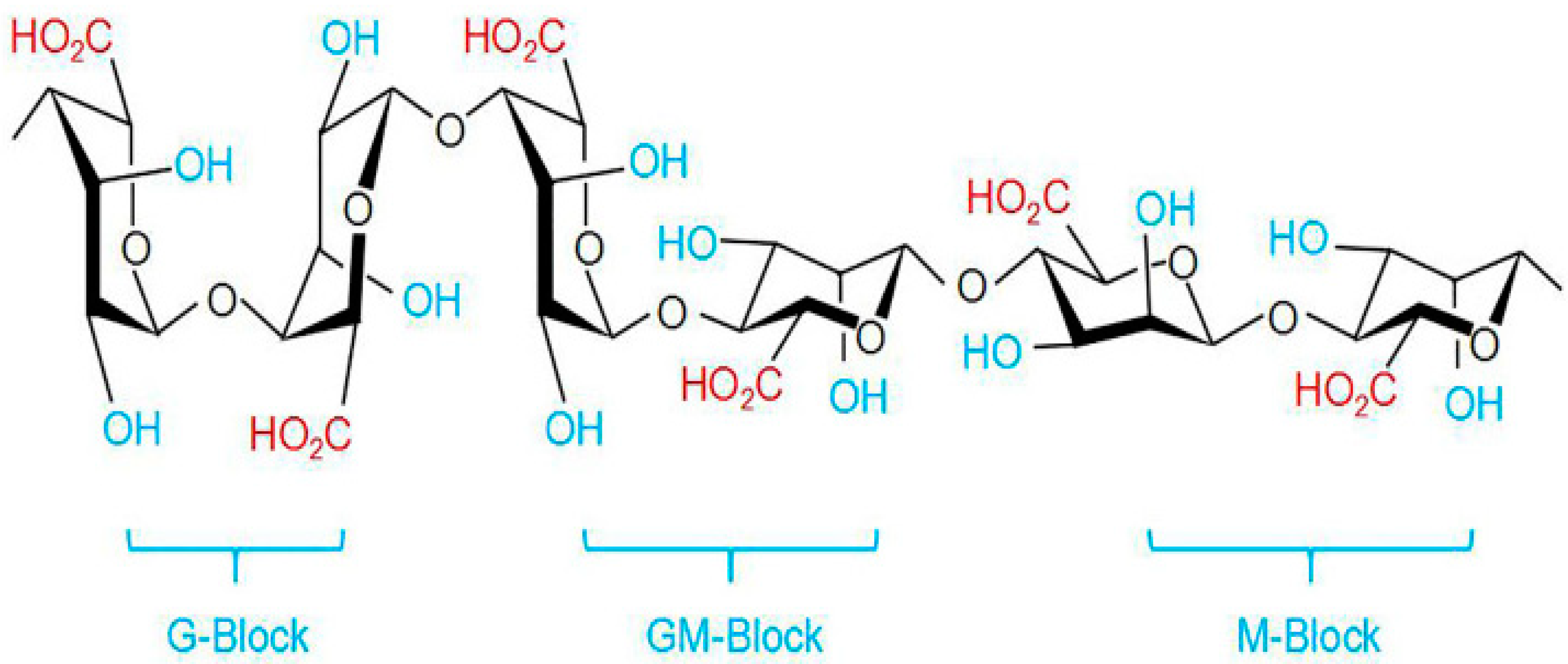
Alginates, which are anionic polymers with carboxyl groups, easily form gels in the presence of calcium ions and other divalent cations. Ionic and coordination crosslinking between alginate and divalent cations promotes interactions between alginate G-blocks and cations. Thus, alginates with a high content of guluronate can give more durable gels [70][68]. The addition of polycations such as chitosan or polylysine leads to the formation of a polyelectrolyte complex. At the same time, chitosan is considered preferable, since it causes a smaller immune response and is less toxic [71][69]. Alginates have an anti-anaphylaxis effect, immunomodulatory activity, antioxidant activity, and an anti-inflammatory effect [69][67]. The fields of application of alginate functional materials are regenerative engineering [72][70], wound dressing [73][71], and drug delivery [70,74][68][72].
References
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538.
- Fisher, J.P.; Adamson, D.C. Current FDA-approved therapies for high-grade malignant gliomas. Biomedicines 2021, 9, 324.
- Drugs Approved for Brain Tumors. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/brain (accessed on 5 May 2023).
- Nishikawa, R.; Iwata, H.; Sakata, Y.; Muramoto, K.; Matsuoka, T. Safety of gliadel implant for malignant glioma: Report of postmarketing surveillance in Japan. Neurol. Med. Chir. 2021, 61, 536–548.
- Kleinberg, L. Polifeprosan 20, 3.85% carmustine slow release wafer in malignant glioma: Patient selection and perspectives on a low-burden therapy. Patient Prefer. Adherence 2016, 10, 2397–2406.
- Deepti, R.D.; Archana, D.K.; Madhuri, A.C.; Shilpa, R.G. A review: Brain specific delivery. GSC Biol. Pharm. Sci. 2020, 13, 68–79.
- Raposo, C.D.; Conceição, C.A.; Barros, M.T. Nanoparticles based on novel carbohydrate-functionalized polymers. Molecules 2020, 25, 1744.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Ribovski, L.; Hamelmann, N.M.; Paulusse, J.M.J. Polymeric nanoparticles properties and brain delivery. Pharmaceutics 2021, 13, 2045.
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of nanoparticle mechanical properties in cancer drug delivery. ACS Nano 2019, 13, 7410–7424.
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759.
- Silva, A.C.; Oliveira, T.R.; Mamani, J.B.; Malheiros, S.M.F.; Malavolta, L.; Pavon, L.F.; Sibov, T.T.; Amaro, E., Jr.; Tannús, A.; Vidoto, E.L.G.; et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. IJN 2011, 6, 591–603.
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B.; et al. Phototheranostics: Active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano 2019, 13, 386–398.
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021, 21, 1484–1492.
- Ünal, S.; Aktaş, Y.; Benito, J.M.; Bilensoy, E. Cyclodextrin nanoparticle bound oral camptothecin for colorectal cancer: Formulation development and optimization. Int. J. Pharm. 2020, 584, 119468.
- Gao, X.; Liu, N.; Wang, Z.; Gao, J.; Zhang, H.; Li, M.; Du, Y.; Gao, X.; Zheng, A. Development and optimization of chitosan nanoparticle-based intranasal vaccine carrier. Molecules 2021, 27, 204.
- Jonassen, H.; Treves, A.; Kjøniksen, A.-L.; Smistad, G.; Hiorth, M. Preparation of ionically cross-linked pectin nanoparticles in the presence of chlorides of divalent and monovalent cations. Biomacromolecules 2013, 14, 3523–3531.
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic acid in ocular drug delivery. Carbohydr. Polym. 2021, 264, 118006.
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.-S. Application of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149.
- Corrêa, L.B.; Pinto, S.R.; Alencar, L.M.R.; Missailidis, S.; Rosas, E.C.; Henriques, M.D.G.M.D.O.; Santos-Oliveira, R. Nanoparticle conjugated with aptamer anti-MUC1/Y for inflammatory arthritis. Colloids Surf. B Biointerfaces 2022, 211, 112280.
- Wang, B.; Lv, L.; Wang, Z.; Zhao, Y.; Wu, L.; Fang, X.; Xu, Q.; Xin, H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor A2-mediated endocytosis. Biomaterials 2014, 35, 5897–5907.
- Xu, Y.; Gao, M.; Zhang, Y.; Ning, L.; Zhao, D.; Ni, Y. Cellulose hollow annular nanoparticles prepared from high-intensity ultrasonic treatment. ACS Nano 2022, 16, 8928–8938.
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581.
- Lu, S.; Ma, T.; Hu, X.; Zhao, J.; Liao, X.; Song, Y.; Hu, X. Facile extraction and characterization of cellulose nanocrystals from agricultural waste sugarcane straw. J. Sci. Food Agric. 2022, 102, 312–321.
- Shang, Z.; An, X.; Seta, F.T.; Ma, M.; Shen, M.; Dai, L.; Liu, H.; Ni, Y. Improving dispersion stability of hydrochloric acid hydrolyzed cellulose nano-crystals. Carbohydr. Polym. 2019, 222, 115037.
- Cheng, M.; Qin, Z.; Hu, J.; Liu, Q.; Wei, T.; Li, W.; Ling, Y.; Liu, B. Facile and rapid one–step extraction of carboxylated cellulose nanocrystals by H2SO4/HNO3 mixed acid hydrolysis. Carbohydr. Polym. 2020, 231, 115701.
- Lugoloobi, I.; Maniriho, H.; Jia, L.; Namulinda, T.; Shi, X.; Zhao, Y. Cellulose nanocrystals in cancer diagnostics and treatment. J. Control. Release 2021, 336, 207–232.
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702.
- Chen, J.; Liu, W.; Liu, C.-M.; Li, T.; Liang, R.-H.; Luo, S.-J. Pectin modifications: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698.
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39.
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23.
- Köse, K.; Tüysüz, M.; Aksüt, D.; Uzun, L. Modification of cyclodextrin and use in environmental applications. Environ. Sci. Pollut. Res. 2022, 29, 182–209.
- Borah, H.J.; Gogoi, M.; Das, D.B.; Hazarika, S. Cyclodextrine-glutaraldehyde cross-linked nanofiltration membrane for recovery of resveratrol from plant extract. J. Environ. Chem. Eng. 2020, 8, 103620.
- Luo, Q.; He, L.; Wang, X.; Huang, H.; Wang, X.; Sang, S.; Huang, X. Cyclodextrin derivatives used for the separation of boron and the removal of organic pollutants. Sci. Total Environ. 2020, 749, 141487.
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489.
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. IJMS 2014, 15, 11922–11940.
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754.
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50.
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in host–guest self-assembled cyclodextrin carriers: Implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 2020, 30, 1909049.
- Ley, C.; Elvers, B. Ullmann’s Encyclopedia of Industrial Chemistry, 1st ed.; Wiley: Weinheim, Germany, 2003; pp. 471–481. ISBN 978-3-527-30385-4.
- Kumirska, J.; Weinhold, M.X.; Czerwicka, M.; Kaczyski, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Influence of the chemical structure and physicochemical properties of chitin- and chitosan-based materials on their biomedical activity. In Biomedical Engineering, Trends in Materials Science; Laskovski, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 25–65. ISBN 978-953-307-513-6.
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin, 2. Effect of deacetylation on solubility. Makromol. Chem. 1976, 177, 3589–3600.
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678.
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174.
- Zhao, Y.; Tian, C.; Liu, Y.; Liu, Z.; Li, J.; Wang, Z.; Han, X. All-in-one bioactive properties of photothermal nanofibers for accelerating diabetic wound healing. Biomaterials 2023, 295, 122029.
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194.
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front. Bioeng. Biotechnol. 2020, 8, 389.
- Einhorn-Stoll, U.; Archut, A.; Eichhorn, M.; Kastner, H. Pectin—Plant protein systems and their application. Food Hydrocoll. 2021, 118, 106783.
- Kim, M.-S.; Chandika, P.; Jung, W.-K. Recent advances of pectin-based biomedical application: Potential of marine pectin. J. Mar. Biosci. Biotechnol. 2021, 13, 28–47.
- Martínez-Sabando, J.; Coin, F.; Melillo, J.H.; Goyanes, S.; Cerveny, S. A review of pectin-based material for applications in water treatment. Materials 2023, 16, 2207.
- Kedir, W.M.; Deresa, E.M.; Diriba, T.F. Pharmaceutical and drug delivery applications of pectin and its modified nanocomposites. Heliyon 2022, 8, e10654.
- Li, D.; Li, J.; Dong, H.; Li, X.; Zhang, J.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65.
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389.
- Cadete, A.; Alonso, M.J. Targeting cancer with hyaluronic acid-based nanocarriers: Recent advances and translational perspectives. Nanomedicine 2016, 11, 2341–2357.
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56.
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224.
- Buckley, C.; Murphy, E.J.; Montgomery, T.R.; Major, I. Hyaluronic acid: A review of the drug delivery capabilities of this naturally occurring polysaccharide. Polymers 2022, 14, 3442.
- Donelan, W.; Dominguez-Gutierrez, P.R.; Kusmartsev, S. Deregulated hyaluronan metabolism in the tumor microenvironment drives cancer inflammation and tumor-associated immune suppression. Front. Immunol. 2022, 13, 971278.
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908.
- Hu, L.; Nomura, S.; Sato, Y.; Takagi, K.; Ishii, T.; Honma, Y.; Watanabe, K.; Mizukami, Y.; Muto, J. Anti-inflammatory effects of differential molecular weight hyaluronic acids on UVB-induced calprotectin-mediated keratinocyte inflammation. J. Dermatol. Sci. 2022, 107, 24–31.
- Petrey, A.C.; De La Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101.
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571.
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical modification of hyaluronan and their biomedical applications. Front. Chem. 2022, 10, 830671.
- Pandit, A.H.; Mazumdar, N.; Ahmad, S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019, 137, 853–869.
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Improvement of hyaluronic acid enzymatic stability by the grafting of amino-acids. Carbohydr. Polym. 2012, 87, 2211–2216.
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171.
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628.
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823.
- Verma, M.L.; Dhanya, B.S.; Sukriti; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412.
- Chae, T.; Yang, H.; Leung, V.; Ko, F.; Troczynski, T. Novel biomimetic hydroxyapatite/alginate nanocomposite fibrous scaffolds for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 1885–1894.
- Stojkovska, J.; Djurdjevic, Z.; Jancic, I.; Bufan, B.; Milenkovic, M.; Jankovic, R.; Miskovic-Stankovic, V.; Obradovic, B. Comparative in vivo evaluation of novel formulations based on alginate and silver nanoparticles for wound treatments. J. Biomater. Appl. 2018, 32, 1197–1211.
- Ahmad, Z.; Khuller, G. Alginate-based sustained release drug delivery systems for tuberculosis. Expert Opin. Drug Deliv. 2008, 5, 1323–1334.
More
