Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Milica Kalaba and Version 2 by Rita Xu.
The biological properties, mechanisms of action, and available data supporting the potential role of isoflavones in the prevention of breast cancer.
- isoflavones
- breast cancer
1. Introduction
At least three facts support the thesis that the Western dietary pattern featuring high consumption of processed red meat and fat and limited intake of plant-based foods may be associated with the increased risk of breast cancer and prostate cancer. First, these oncopathologies are most common in geographical areas with predominant Western-style nutrition and quite rare in parts of Asia where soy and unrefined plant foods are the main dietary components. Second, extensive literature suggests markedly low incidence rates of breast cancer and prostate cancer among great apes, who are herbivores and the closest evolutionary cousins of modern humans. Third, Homo sapiens was obligatory herbivore until it began to introduce meat into the diet. This significant change in evolutionary dietary trajectory is now hypothesized to be an important factor in the rising incidence of breast cancer and prostate cancer in humans [1][2][1,2]. Exploration of nutritional factors and diet-derived mediators is a rapidly growing area of anticancer research. Remarkable advancements in high-throughput genomic technologies, molecular epidemiology and biochemical techniques have paved the way for a more holistic and precise approach to deciphering the relationship between bioactive food compounds and cancer at the cellular, individual and population level [3].
The similarity between breast and prostate tissue is reflected in the presence of connective stroma and glandular secretory epithelium. Through constant interaction, the stroma and glandular epithelium control the secretory activity of these organs. Sex hormones, testosterone and estrogen, mostly activate stromal cells via the androgen receptor (AR) and two estrogen receptors (ER): ER-α and ER-β. The ER-α mediates stroma proliferation in both normal and malignant tissue, while the ER-β opposes the action of ER-α [4]. With functional features and structural elements correspondent to the superfamily of nuclear hormone receptors, ERs modulate the transcription of the specific target genes through complex genomic and non-genomic mechanisms and additional convergent cascades, thereby controlling a wide array of physiological functions in respective organ systems, including reproductive, cardiovascular, skeletal and central nervous systems [5]. Being encoded with distinct genes (ESR1 and ESR2), these receptor subtypes have diverse spatial and temporal tissue expression patterns and differential ligand specificities and regulate different physiological processes [6]. The variable absolute expression level and the context-specific variants-ratio are profoundly associated with their biological functions: the alpha subtype is reported to have a more significant role in the cell growth and differentiation in the uterus, prostate and mammary glands as well as the maintenance of metabolic and skeletal homeostasis, whereas the beta form dominantly affects the immune and central nervous systems and counteracts the ERα-induced cell proliferation in estrogen-sensitive tissues [7].
Isoflavones are an important subgroup of flavonoids, a large class of naturally occurring phenolic compounds with great structural and functional diversity. Aglycons genistein, daidzein and glycitein, and their glycosides genistin, daidzin and glycitin are considered to be the most important representatives of isoflavones. Major sources of isoflavones are soybeans and other legumes. In plants, isoflavones play a critical role in the formation of phytoalexins during defense against microbes, while in humans, due to their structural similarity to 17β-estradiol, isoflavones exert significant estrogenic activity [8][9][8,9]. In contrast with estradiol, which activates both ERs equally, isoflavones are more selective. For example, genistein, the most important isoflavone, has a substantially higher binding affinity for the ER-β isoform than for the ER-α isoform. Genistein competes with estradiol and acts as an ER-α antagonist as well as a potent ER-β agonist [10].
2. Breast Cancer and Isoflavones
2.1. Breast Cancer—Epidemiology and Risk Factors
With extraordinary heterogeneity in terms of occurrence rates, clinical presentation, available preventive and curative interventions, and therapeutic response, the overall magnitude of the public health burden related to cancer incidence and mortality is substantially increasing worldwide. The increasing cancer occurrence and mortality are the results of the cumulative effects of aging and the growth of the global population, as well as the transition of distribution and prevalence of major risk factors. Breast cancer is the most frequently occurring invasive cancer in the female population. With an annual estimation of 2.3 million new cases based on the Global Cancer Statistics—GLOBOCAN report for 2020, breast cancer surpassed lung cancer as the most commonly diagnosed malignancy accounting for 11.7% of total cases. Furthermore, breast cancer is considered to be the leading cause of cancer death among women and ranks fifth in combined-sex mortality estimates [11][46]. There is prominent geographic variability in breast cancer registry data. Although the age-standardized incidence rates are approximately 88% higher in transitioned countries (i.e., Australia, New Zealand, and other countries in North America, Western and Northern Europe) compared to transitioning countries (55.9 versus 29.7 per 100,000, respectively), mortality rates are 19% higher in regions with a lower level of socioeconomic development [11][46]. These patterns may be attributed to both variances in exposure to risk factors and to worldwide disparities across the continuum of healthcare [12][47]. Furthermore, global temporal-trend figures show that incidence rates have been increasing in virtually all regions over the last three decades. Although overall lethality indices continuously decline, case-fatality proportions remain markedly higher in lower-income countries, particularly in Africa and among small island nations in Melanesia, Micronesia, Polynesia and the Caribbean [13][14][48,49]. Current patterns in breast cancer incidence and survival trends are affected by: alterations in environmental and individual-level variables and risk factors, public health determinants (such as widespread mammography screening programs), and general advancements in diagnostic and treatment infrastructure [15][16][50,51]. Pronounced differences in mortality-to-incidence ratios warrant concern and an urgent need for methodical and harmonized dissemination and implementation of evidence-based, contextually suitable policies and interventions targeting awareness, education, risk identification, and early detection, as well as reinforcement of equitable resources and capacities for high-quality multimodal treatment and survivorship care [17][18][52,53]. Nevertheless, the rising incidence in developing countries in Africa, South America, and Asia, as well as in certain affluent Asian countries, where rates are traditionally low, is associated with the so-called “Westernization” phenomenon. This term refers to dramatic changes in sociocultural circumstances, economic development, urbanization, and demographic and workforce shifts that are accompanied by behavioral and lifestyle modifications. Similar incidence trend variations were observed in migration studies. Sedentary culture with less physical activity, increased prevalence of obesity, nutritional transition towards high-fat and (ultra) processed foods, postponement of childbirth and lower parity, and accessibility to hormonal birth control and replacement therapy are all contributing to the convergence towards the industrialized Western countries breast cancer risk-profile and the narrowing of the international gap between historically low and high morbidity areas [8][14][8,49]. Having a remarkably heterogeneous oncopathology, breast cancer comprises a wide spectrum of tumors with a high level of diversity in molecular alterations, cellular composition, histological features, clinical presentation, metastatic potential and dissemination patterns, treatment sensitivity and patient outcomes [19][54]. The classification of breast cancer is challenging due to the versatility of cancer cell phenotypes and their perplexing interaction with a plethora of molecular and cellular mediators of both tumoral and microenvironmental plasticity. The complexity of inter and intra-tumor variability is only partially epitomized by routinely applied principal clinical parameters (patient age, lymph node involvement, neoplasm size, tumor stage and grading) and pathobiological markers such as estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2/ERBB2) [20][55]. The traditional framework for clinical breast cancer classification encompasses three major types: (1) luminal ER-positive and PR-positive (additionally subdivided into luminal types A and B based on histochemical staining for the proliferation marker protein Ki-67 (MKI67) or genetic profiling); (2) HER2-positive; (3) triple-negative breast cancer (TNBC) [21][56]. Nevertheless, the appreciation of the tumor-immune dynamic relationship, breakthroughs in omics technologies and next-generation sequencing, and inclusion of predictive and prognostic genomic and immunomarkers have enabled better comprehension of the malignant ecosystem and progression pathways, refined diagnostic and patient-stratification algorithms, and most importantly, revolutionized the therapeutic landscape, thus providing novel opportunities for breast cancer management [22][57]. Etiopathology of breast cancer is rather complex. A variety of intricately interrelated modifiable and non-modifiable factors, including genetics, environmental exposures, and physiological, sociobiological and lifestyle determinants, may increase susceptibility or encourage disease development [23][58]. Female sex is one of the major risk factors, predominantly due to elevated hormonal stimulation and increased vulnerability of breast tissue to estrogen-progesterone balance disruption. Male breast cancer accounts for approximately 1% of all cases, and the most common being the ER-positive type [24][59]. Currently, nearly 80% of breast cancer patients are older than 50, while more than 40% of affected individuals are older than 65 years. Epidemiological data reveal associations between age at diagnosis and certain cancer molecular subtypes. Aggressive, recurrence-prone, resistant TNBC is most frequently detected in populations less than 40 years of age, whereas among patients above 70 years the luminal A type is most frequently detected [25][60]. A significantly higher incidence rate of breast cancer is observed among individuals with a positive family history, with 13-19% of patients having a first-degree relative affected with the same or related malignancy [26][61]. Familial clustering of breast cancer was estimated to be approximately 73% attributed to heritable components and 27% to environmental triggers. Most inherited cases due to predisposing genetic mutations are associated with two autosomal dominant high-risk genes: BRCA1 (BReast CAncer gene 1) and BRCA2 (BReast CAncer gene 2), located on chromosomes 17(17q21.31) and 13(13q13.1), respectively. Several pathogenic variants in highly penetrant (e.g., TP53, CDH1, PTEN and STK11) and moderately penetrant susceptibility genes (e.g., CHECK2, PALB2, ATM and BRIP1) were also reported to be involved in breast carcinogenesis [27][62]. Although large-scale genotyping analysis within genome-wide association studies (GWAS) provided significant advancement in this area, a considerable proportion remains unclear. Further research is needed to elucidate the intricate breast cancer genetic background. Extensive scientific evidence corroborates the relationship between an individual’s reproductive history and the risk of breast cancer. Timing, duration and accompanying hormonal fluctuations of particular entities, such as menstruation, pregnancy and breastfeeding may have a considerable impact on the potential induction of oncogenesis in the breast microenvironment. Early menarches, advanced age of full-term pregnancy, absence or short-period breastfeeding, nulliparas or low parity, and late menopause (with a high total lifetime count of menstrual cycles) elevate the breast cancer risk. Additionally, certain breast properties such as high tissue density, personal history of cancerous and non-cancerous lesions, as well as previous chest exposure to radiation were correlated with increased risk of breast cancer occurrence. Other environmental and lifestyle risk factors include smoking, a high-fat diet, excessive alcohol consumption, hormonal replacement therapy, oral contraceptives, obesity and low physical activity [28][29][63,64].2.2. Breast Cancer Chemoprevention and Dietary Isoflavone Intake
Accumulating evidence and enhanced comprehension of intricate mechanisms involved in cancerogenesis have directed scientific attention toward the immense potential of chemopreventive strategies and, in particular, compounds that may suppress, arrest or reverse various molecular pathways associated with cancer development and progression. Chemoprevention encompasses a myriad of molecular targets related to distinct stages of the carcinogenic process (i.e., initiation, promotion, and progression) and may be broadly categorized into three major types: primary, secondary and tertiary [30][31][65,66]. Primary prevention refers to the exposure of healthy individuals or populations exerting particular risk features but without the overt disease to certain natural or synthetic agents in order to prevent cancer occurrence. Secondary prevention pertains to premalignant lesions and the administration of selected agents to perturb the transition to invasive cancer. Tertiary prevention applies to patients who successfully underwent cancer treatment in order to prevent recurrence or the development of a new primary tumor [32][67]. Compounds that impede the initial neoplastic transformation are termed as “blocking agents”, whereas those that counteract the progression of benign lesions to advanced-stage cancers are denominated as the “suppressing agents”. Mechanistically, these agents effectuate their chemopreventive properties by a wide spectrum of functions, including the inhibition of carcinogen uptake, induction of cellular detoxifying enzymes and genomic repair pathways, antioxidant activity and free radical scavenging, modulation of epigenetic alterations, downregulation of chronic inflammation, as well as interaction with cellular signaling networks responsible for maintaining the delicate balance between cell survival, differentiation, proliferation, senescence, and death [33][68]. A multitude of epidemiological studies has associated isoflavones and their dietary sources with various health benefits, including protection against hormone-dependent oncopathologies (such as breast and prostate cancer), cardiovascular disease, disturbances in glycoregulation, osteoporosis, support to weight management/obesity reduction and attenuation of physical and emotional menopausal symptoms [8][34][8,69]. Available dietary surveys indicate a great level of variability regarding the intake of total phytoestrogens, particular subclasses of these plant-derived secondary metabolites, and their food sources with regard to geographical and sociocultural determinants. Consumption of foods containing high levels of isoflavones, such as soybeans and soy-based products and other legume seeds (beans, peas, lentils, clover), is commonly associated with traditional Asian dietary patterns rather than with nutritional preferences of the Western world. Despite the considerable inter-study variations, a substantially higher average daily intake of isoflavones per capita is reported for South and East Asian countries (i.e., 20–50 mg/day) than for European countries (0.37–4.5 mg/day) and the USA (0.73–3.3 mg/day) [35][36][37][38][70,71,72,73]. Interestingly, however, in the last couple of decades, noteworthy changes in isoflavone-related dietary habits were observed: simultaneously with the decreased consumption of soy in favor of animal-sourced protein in Asia due to the adoption of Westernized nutrition, the intake of soy is growing among health-conscious individuals in the USA and Europe. Based on dietary analyses, vegans and vegetarians are a Western-world subgroup with the highest isoflavone intake of approximately 7–12 mg/day [39][74]. Dominant isoflavone sources in traditional Asian diet are fermented and non-fermented soybeans and first-generation soy products, including tofu, tempeh, miso, soy milk, natto and cheonggukjang. In contrast, soy-based meat and dairy substitutes, as well as various food derivatives with soy flour or soy proteins added as fillers or extenders, prevail in modern Western habitual nutrition [8][40][8,75]. Given the biological properties of isoflavones and a striking concomitant geographical discrepancy in isoflavone intake and breast cancer prevalence, researchers have hypothesized the potential role of these compounds in the prevention of mammary tumorigenesis among susceptible populations and among women with or without previous history. In addition to epidemiological-level research, numerous studies explored the benefits of isoflavones in cell cultures, animal and human breast cancer scientific models trying to: (1) elucidate mechanisms of action and tissue-specific dose-dependent response; (2) validate relevant biomarkers; (3) determine efficacy, safety, opportunities, and challenges of controlled preventive and curative interventions. Meta-analyses indicated a positive association between the consumption of high levels of isoflavones and a reduced breast cancer risk in Asian populations, but failed to detect a significant correlation in Western populations, presumably due to low isoflavone intake levels [41][42][43][44][76,77,78,79]. Nevertheless, in these meta-analyses, there was considerable between-study heterogeneity that may have compromised the reported observations. Key limitations comprise the case-control design in the majority of included studies, inherent drawbacks of the employed exposure evaluation methods, varied range definitions and intake cut-off values, as well as residual confounding related to unreported yet relevant factors. Contrary to other reports, the China Kadoorie Biobank (CKB) study, which enrolled more than 300,000 women residing in 10 diverse regions in China, found no association between soy intake and breast cancer incidence overall. However, based on the further dose–response meta-analysis integrating the CKB study with other prospective studies, each 10 mg/day of soy isoflavone led to a 3% risk reduction [45][80]. Literature reports accounting for menopausal status and hence endogenous estrogen environment are also conflicting. Although a significant inverse association between the isoflavone intake and breast cancer risk in both pre- and post-menopausal Asian women was reported [43][44][78,79], the menopausal status may be an important effect modifier with the protective effect of soy isoflavone intake existing among postmenopausal subjects only [41][46][76,81]. Recent meta-analyses summarizing findings from eight prospective studies from 2003 to 2021 concluded that elevated soy consumption and, consequently, isoflavone intake are associated with better breast cancer prognosis and lower occurrence risk regardless of menopause status [47][82]. According to the systematic review and meta-analysis by combining data derived from 12 scientific articles and 37,275 cases of women with breast cancer, pre-diagnosis soy and isoflavone intake were favorably correlated with the overall survival and reduced recurrence risk among post-menopausal patients exclusively [48][83]. A number of epidemiological studies evaluated the potential association between isoflavone intake and breast cancer risk stratified by hormone receptor (ER and PR) and HER2 status in the general population. The Shanghai Breast Cancer Study reported a superior risk reduction for ER+/PR+ tumors than for other subtypes [49][84]. However, in several case-control and cohort studies the protective effect of soy products against breast cancer remained comparable across all ER/PR status subtypes. Concerning the HER2 status, a Japanese case–control study [50][85] found that elevated intake of soy-based products significantly reduced the risk of HER2-negative breast cancer by 21%, whereas no significant differences with respect to HER2 status were found in another study [51][86]. A recent study examined the association between the isoflavone intake and breast cancer risk by molecular subtype in 1,709 Korean females featuring a high risk of hereditary breast cancer (i.e., BRCA1/2 mutation carriers and non-carriers with family history and early-onset breast cancer).2.3. Isoflavone Mechanisms of Action and Impact on Breast Cancer
Isoflavones can mediate the majority of their biological effects via estrogen receptor (ER) signaling pathways due to a significant structural resemblance with the mammalian 17-β-estradiol (E2). Although the importance of estrogens and their receptors in breast cancerogenesis and subsequent tumor development is well-established, the implication of various environmental and synthetic receptor modulators (i.e., phytoestrogens and xenoestrogens) is still a controversial subject and a matter of debate. Isoflavones may interact with both alpha (ERα) and beta receptor (ERβ) isoforms but exert markedly stronger affinity towards the ERβ. Even though the ligand binding cavities of ERα and ERβ differ by only two amino acid positions (replacement of Leu-384 and Met-421 in ERα with Met-336 and Ile-373, respectively, in ERβ), the sequence diversity beyond the pocket residues confers the ERβ to the topology more suitable for the isoflavones [10]. In general, the ER binding potency of isoflavones ranges from 10−4 to 10−3 compared to the primary natural ligand, i.e., estradiol (E2). Appropriate orientation, hydrophobic core and planar chemical structure featuring p-hydroxy-substituted aromatic ring approximately 12 Å distant from the 2nd planar hydroxyl group enable isoflavones to mimic endogenous estrogen interaction with the hormone binding pockets of ERs. Specific structural elements shaping spatial, electronic, and topological characteristics of particular isoflavone compounds determine their potency as phytoestrogenic ligands and convey their qualitative and functional properties [52][88]. Quantitative structure-activity relationship (QSAR) and comparative molecular field research highlighted pharmacophore features significant for receptor binding and activation, including partial charges on atoms C2′, C4′ (ring B) and C7 (ring A), conformational rigidity determinants and molecular orientation [53][89]. Given that isoflavones may display both estrogenic and anti-estrogenic effects, they are sometimes regarded as natural selective ER modulators (SERMs). The ultimate actions are predisposed by a multitude of factors, including ambient estrogen concentrations and environmental hormonal milieu, relative levels of ERα and ERβ, the local concentration of the studied isoflavone compound(s), the presence of other phyto/xenoestrogens as well as the interference of present co-activators and co-repressors [54][90]. Nevertheless, the findings of various studies are rather inconsistent, and comprehensive characterization of the intricate bioactivity of isoflavones warrants further research. These incoherencies may be at least partly attributed to substantial qualitative and quantitative variability of isoflavones content and composition in food sources, the complexity of their metabolic transformations as well as inter and intra-individual genetic and physiological differences. Several preclinical rodent tumor models were applied in animal studies exploring the isoflavone influence on breast cancer. Experimental induction of breast cancer may be obtained through genetic engineering leading to spontaneous cancer development by administration of a chemical carcinogen (most commonly 1-methyl-1-nitrosourea (MNU) for protocols aiming to investigate more aggressive mammary oncopathologies or 7,12-dimethylbenz[a]anthracene (DMBA) or by exposure to excess estrogen levels [6]. The inoculation model refers to the transplantation of tumor cells or explants into susceptible murine hosts. The xenograft model is generated in immunodeficient hosts with immortalized human cancer cell lines (commonly MCF-7 or MDA-MB-231) or tumor explants of patient origin, whereas allograft models are created when allogenic cells/tissues are transplanted into immunocompetent syngeneic mice [55][91]. Each of these models is of great scientific significance, albeit their inherent imperfections and species-specific peculiarities limit the translational relevance of findings to humans. The deficiency of target-site exposure data undoubtedly remains the critical knowledge gap to be addressed in future studies. These data would contribute to better interpretation of in vitro results, translation of such findings into in vivo relevant context, and eventually their integration with human observational and interventional research in comprehensive risk-assessment analyses. Although the interaction with ER is the most commonly studied isoflavone mechanism of action, there is a growing body of evidence indicating that additional pathways and alternative cascades may also be affected. In addition to ER-mediated signaling, isoflavones may modulate the local hormone levels in breasts and ovaries in a tissue-specific and concentration-dependent manner by affecting the activity or expression of steroidogenic enzymes implicated in estrogen synthesis and metabolism (e.g., cytochrome P450—aromatase (CYP19A1), 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase, cytochrome P450 1A1 and 1B1 and sulfotransferase, thereby altering the conversion of androgenic precursors to estrogens and the dehydrogenation of estrone to estradiol [56][92]. Furthermore, isoflavones deliver antitumorigenic effects through several ER-independent activities, including apoptosis induction, inhibition of cell proliferation and angiogenesis, support for antioxidant defense and DNA repair mechanisms, inflammation downregulation, and interference in other signal-transduction systems (Figure 1) [40][75].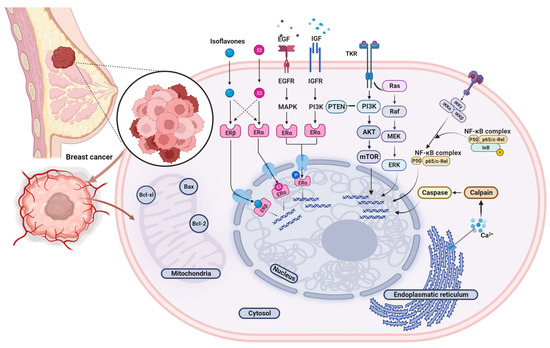
Figure 1. Graphical overview of the isoflavones’ possible molecular targets and cellular signaling pathways in the breast cancer model. Abbreviations: AKT, Protein kinase-B; Bcl-2, B-cell lymphoma 2; Bcl-xL, B-cell lymphoma-extra large; Bax, Bcl-2-associated X-protein; E2, Estradiol; EGF, epidermal growth factor; EGFR, Epidermal growth factor receptor; ERα, Estrogen receptor alpha; ERβ, Estrogen receptor beta; ERK, Extracellular-signal-regulated kinase; IGF, insulin-like growth factor; IGFR, insulin-like growth factor receptor; IKKα, IKKβ and IKKγ, IκB ki-nases; MAPK, Mitogen-activated protein kinases; MEK, Mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; PI3K, Phosphoinositide 3-kinase; PTEN, Phosphatase and tensin homolog; Raf, Rapidly accelerated fibrosarcoma; Ras, Rat sarcoma, TKR; Tyrosine kinase receptor.
