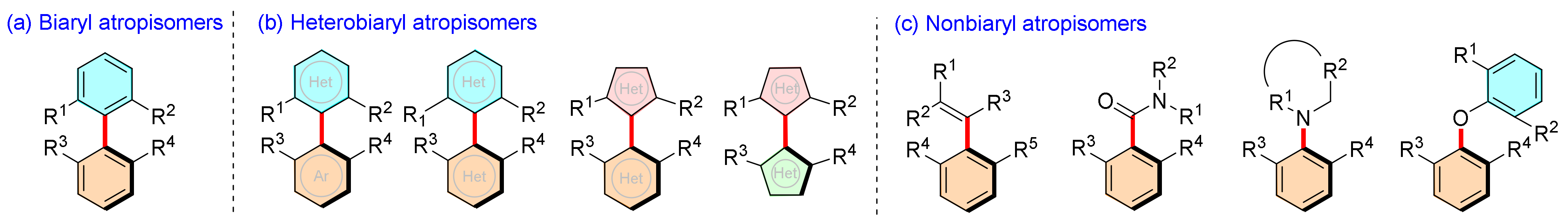
Atropisomeric molecules are present in many natural products, biologically active compounds, chiral ligands and catalysts. Many elegant methodologies have been developed to access axially chiral molecules. Among them, organocatalytic cycloaddition and cyclization have attracted much attention because they have been widely used in the asymmetric synthesis of biaryl/heterobiaryls atropisomers via construction of carbo- and hetero-cycles. This strategy has undoubtedly become and will continue to be a hot topic in the field of asymmetric synthesis and catalysis. This review aims to highlight the recent advancements in this field of atropisomer synthesis by using different organocatalysts in cycloaddition and cyclization strategies. The construction of each atropisomer, its possible mechanism, the role of catalysts, and its potential applications are illustrated.
- organocatalytic
- cycloaddition and cyclization
- atropisomer construction
1. Introduction
2. Chiral Phosphoric Acids (CPAs)
The chiral part of chiral phosphoric acid mainly consists of BINOL, H8-BINOL, SPINOL and TADDOL (Scheme 3) [36,37][36][37]. Different catalysts can be derived from different substituents on aromatic rings. Each CPA catalyst has a slight difference in steric hindrance and inductive effect [38]. The functional part of the catalyst contains both hydroxyl sites as proton donors and oxygen sites as proton acceptors [39]. These enable CPA catalyst to adapt to a variety of reactions, and to change its skeleton and substituents according to the needs of different reactions, so that it can be better combined with the substrates and catalyze the reaction.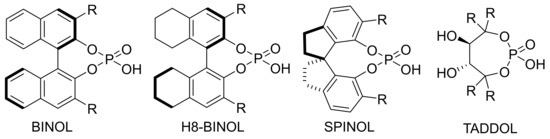
References
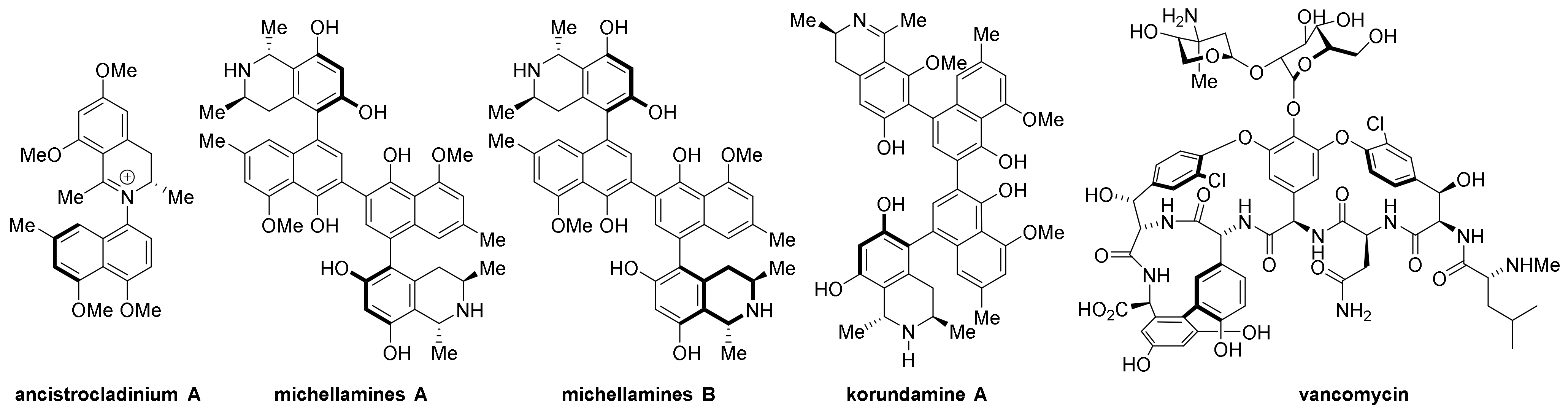
- Lombe, B.K.; Feineisa, D.; Bringmann, G. Dimeric naphthylisoquinoline alkaloids: Polyketide derived axially chiral bioactive quateraryls. Nat. Prod. Rep. 2019, 36, 1513–1545.
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639.
- Kozlowski, M.C.; Miller, S.J.; Perreault, S. Atropisomers: Synthesis, Analysis, and Applications. Acc. Chem. Res. 2023, 56, 187–188.
- Bringmann, G.; Kajahn, I.; Reichert, M.; Pedersen, S.E.H.; Faber, J.H.; Gulder, T.; Brun, R.; Christensen, S.B.; Ponte-Sucre, A.; Moll, H.; et al. Ancistrocladinium A and B, the First N,C-Coupled Naphthyldihydroisoquinoline Alkaloids, from a Congolese Ancistrocladus Species. J. Org. Chem. 2006, 71, 9348–9356.
- Boyd, M.R.; Hallock, Y.F.; Cardellina II, J.H.; Manfredi, K.P.; Blunt, J.W.; McMahon, J.B.; Buckheit, R.W., Jr.; Bringmann, G.; Schäffer, M.; Cragg, G.M.; et al. Anti-HIV Michellamines from Ancistrocladus korupensis. J. Med. Chem. 1994, 37, 1740–1745.
- Hallock, Y.F.; Cardellina, J.H., II; Schäffer, M.; Bringmann, G.; François, G.; Boyd, M.R. Korundamine A, a novel HIV-inhibitory and antimalarial “hybrid” naphthylisoquinoline alkaloid heterodimer from Ancistrocladus korupensis. Bioorganic Med. Chem. Lett. 1998, 8, 1729–1734.
- Hubbard, B.K.; Walsh, C.T. Vancomycin Assembly: Nature’s Way. Angew. Chem. Int. Ed. 2003, 42, 730–765.
- Christie, G.H.; Kenne, J. LXXI.—The molecular configurations of polynuclear aromatic compounds. Part I. The resolution of γ-6:6′-dinitro- and 4:6:4′:6′-tetranitro-diphenic acids into optically active components. J. Chem. Soc. Trans. 1922, 121, 614–620.
- Oki, M. Recent Advances in Atropisomerism. Top. Stereochem. 1983, 14, 1–81.
- Cheng, J.K.; Xiang, S.H.; Li, S.; Ye, L.; Tan, B. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 2021, 121, 4805–4902.
- Mei, G.; Koay, W.; Guan, C.; Lu, Y. Atropisomers beyond the C–C axial chirality: Advances in catalytic asymmetric synthesis. Chem 2022, 8, 1855–1893.
- Noyori, R.; Ohkuma, T.; Kitamura, M. Asymmetric Hydrogenation of β-Keto Carboxylic Esters. A Practical, Purely Chemical Access to β-Hydroxy Esters in High Enantiomeric Purity. J. Am. Chem. Soc. 1987, 109, 5856–5858.
- Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid. Angew. Chem. Int. Ed. 2004, 43, 1566–1568.
- Uraguchi, D.; Terada, M. Chiral Brønsted Acid-Catalyzed Direct Mannich Reactions via Electrophilic Activation. J. Am. Chem. Soc. 2004, 126, 5356–5357.
- Brown, J.M.; Woodward, S. Selective ortho lithiation of (2,5-dimethoxyphenyl)diphenylphosphine oxide and trapping of the resulting aryllithium with electrophiles. J. Org. Chem. 1991, 56, 6803–6809.
- Cheng, D.; Shao, Y. Advances in the Catalytic Asymmetric Synthesis of Atropisomeric Hexatomic N-Heterobiaryls. Adv. Synth. Catal. 2020, 362, 3081–3099.
- He, X.; Wang, C.; Wen, Y.; Wang, Z.; Qian, S. Recent Advances in Catalytic Atroposelective Construction of Pentatomic Heterobiaryl Scaffolds. ChemCatChem 2021, 13, 3547–3564.
- Cortright, S.B.; Huffman, J.C.; Yoder, R.A.; Coalter, J.N.; Johnston, J.N. IAN Amines: Chiral C2-Symmetric Zirconium(IV) Complexes from Readily Modified Axially Chiral C1-Symmetric β-Diketimines. Organometallics 2004, 23, 2238–2250.
- Terauchi, J.; Curran, D.P. N-Allylation of Anilides with Chiral Palladium Catalysts: The First Catalytic Asymmetric Synthesis of Axially Chiral Anilides. Tetrahedron Asymmetry 2003, 14, 587–592.
- Ponte-Sucre, A.; Gulder, T.; Wegehaupt, A.; Albert, C.; Rikanovic, C.; Schaeflein, L.; Frank, A.; Schultheis, M.; Unger, M.; Holzgrabe, U.; et al. Structure-Activity Relationship and Studies on the Molecular Mechanism of Leishmanicidal N,C-Coupled Arylisoquinolinium Salts. J. Med. Chem. 2009, 52, 626–636.
- Guenzi, A.; Johnson, C.A.; Cozzi, F.; Mislow, K. Dynamic Gearing and Residual Stereoisomerism in Labeled Bis(9-triptycyl)methane and Related Molecules. Synthesis and Stereochemistry of Bis(2,3-dimethyl-9-triptycyl)methane, Bis(2,3-dimethyl-9-triptycyl)carbinol, and Bis(1,4-dimethyl-9-triptycyl)methane1. J. Am. Chem. Soc. 1983, 105, 1438–1448.
- Fuji, K.; Oka, T.; Kawabata, T.; Kinoshita, T. The first synthesis of an optically active molecular bevel gear with only two cogs on each wheel. Tetrahedron Lett. 1998, 39, 1373–1376.
- Bao, H.; Cheng, Y.; Yang, X. Catalytic Asymmetric Synthesis of Axially Chiral Diaryl Ethers through Enantioselective Desymmetrization. Angew. Chem. Int. Ed. 2023, 135, e202300481.
- Wang, Y.; Tan, B. Construction of Axially Chiral Compounds via Asymmetric Organocatalysis. Acc. Chem. Res. 2018, 51, 534–547.
- Zilate, B.; Castrogiovanni, A.; Sparr, C. Catalyst-Controlled Stereoselective Synthesis of Atropisomers. ACS Catal. 2018, 8, 2981–2988.
- Wencel-Delord, J.; Panossian, A.; Lerouxb, F.R.; Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430.
- Yang, H.; Chen, J.; Zhou, L. Construction of Axially Chiral Compounds via Central-to-Axial Chirality Conversion. Chem Asian J. 2020, 15, 2939–2951.
- Lemaitre, C.; Perulli, S.; Quinonero, O.; Bressy, C.; Rodriguez, J.; Constantieux, T.; Mancheño, O.G.; Bugaut, X. Enantioselective synthesis of atropisomers by oxidative aromatization with central-to-axial conversion of chirality. Molecules 2023, 28, 3142.
- Link, A.; Sparr, C. Stereoselective arene formation. Chem. Soc. Rev. 2018, 47, 3804–3815.
- Metrano, A.J.; Miller, S.J. Peptide-Based Catalysts Reach the Outer Sphere through Remote Desymmetrization and Atroposelectivity. Acc. Chem. Res. 2019, 52, 199–215.
- Moyano, A.; Rios, R. Asymmetric Organocatalytic Cyclization and Cycloaddition Reactions. Chem. Rev. 2011, 111, 4703–4832.
- Sun, H.; Sharif, A.; Chen, J.; Zhou, L. Atroposelective Synthesis of Heterobiaryls through Ring Formation. Chem. Eur. J. 2023, 29, e202300183.
- Akiyama, T. Stronger Brønsted Acids. Chem. Rev. 2007, 107, 5744–5758.
- Volla, C.M.R.; Atodiresei, I.; Rueping, M. Catalytic C–C Bond-Forming Multi-Component Cascade or Domino Reactions: Pushing the Boundaries of Complexity in Asymmetric Organocatalysis. Chem. Rev. 2014, 114, 2390–2431.
- Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014, 114, 9047–9153.
- Gashaw, A.; Debeli, D.K. Recent progress on asymmetric multicomponent reactions via chiral phosphoric acid catalysis. J. Iran. Chem. Soc. 2022, 19, 1593–1611.
- Wang, L.; Yang, L.; Chen, J.; Zhou, L. Chiral Phosphoric Acid Catalyzed Asymmetric Cycloadditions: From Alkenes to Alkynes. Synlett 2023.
- Rahman, A.; Lin, X. Development and application of chiral spirocyclic phosphoric acids in asymmetric catalysis. Org. Biomol. Chem. 2018, 16, 4753–4777.
- Phipps, R.J.; Hamilton, G.L.; Toste, F.D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 2012, 4, 603–614.
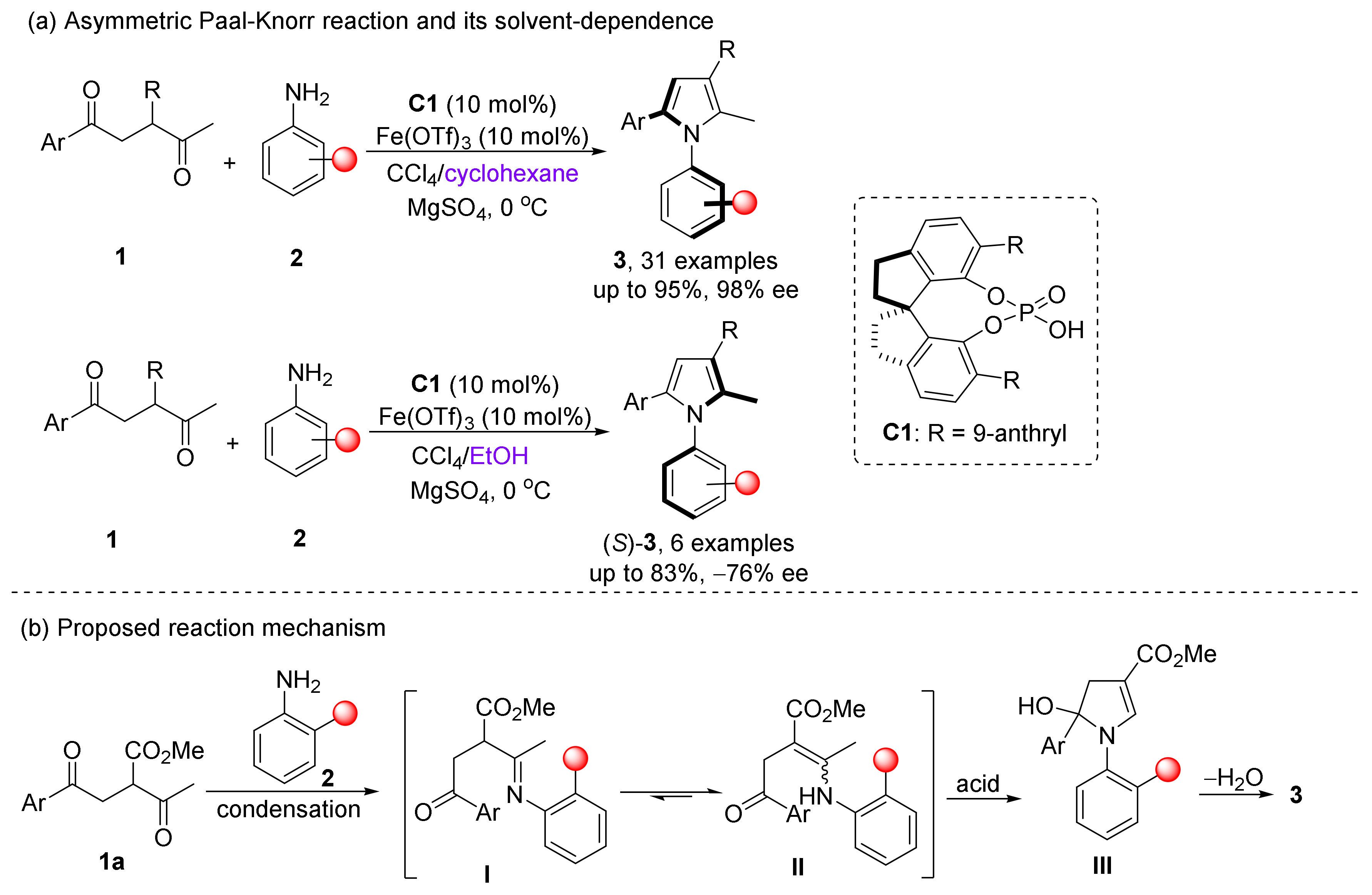
- Zhang, L.; Zhang, J.; Ma, J.; Cheng, D.; Tan, B. Highly Atroposelective Synthesis of Arylpyrroles by Catalytic Asymmetric Paal-Knorr Reaction. J. Am. Chem. Soc. 2017, 139, 1714–1717.
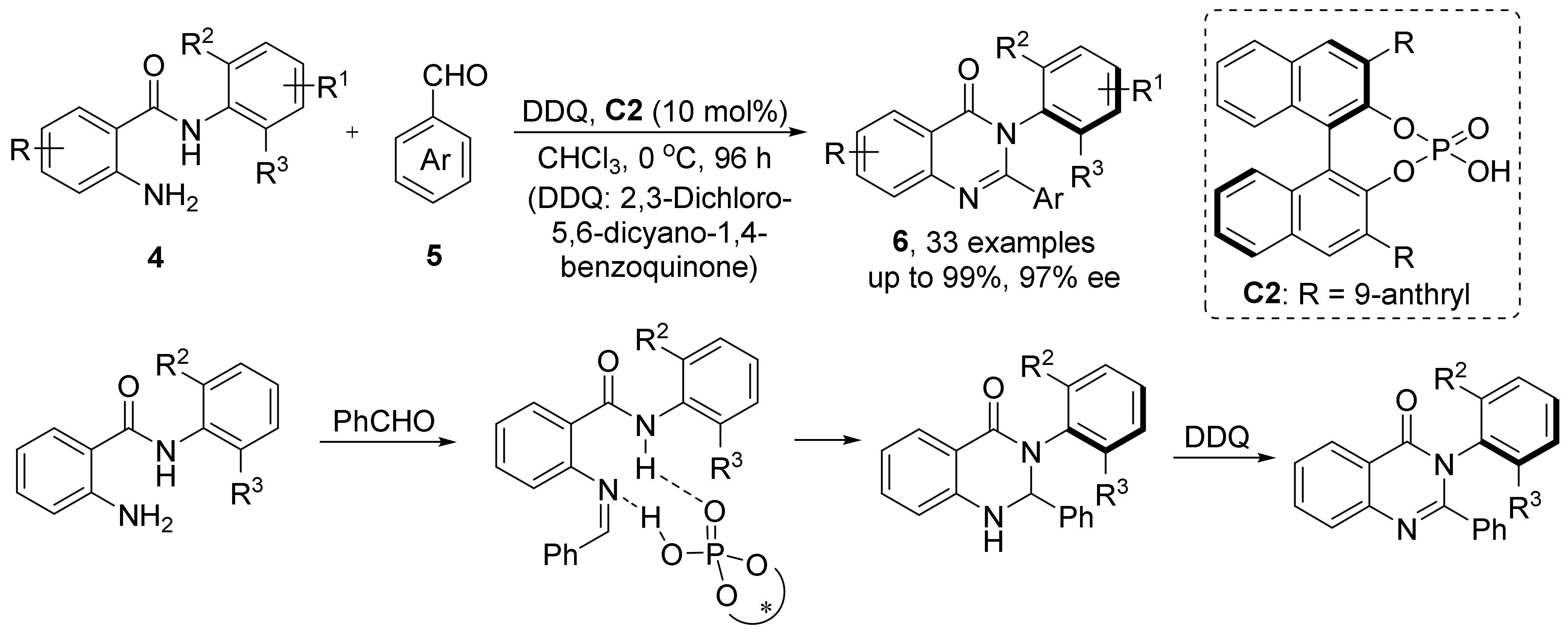
- Wang, Y.; Zheng, S.; Hu, Y.; Tan, B. Brønsted acid-catalysed enantioselective construction of axially chiral arylquinazolinones. Nat. Commun. 2017, 8, 15489.
