Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Luca Bergamaschi.
Non-invasive imaging methods can assess coronary anatomy through coronary computed tomography angiography (CCTA) and/or inducible myocardial ischemia through functional stress testing (stress echocardiography, cardiac magnetic resonance imaging, single photon emission computed tomography—SPECT, or positron emission tomography—PET).
- chronic coronary syndrome
- echocardiography
- cardiac magnetic resonance
- coronary computed tomography angiography
- nuclear medicine
- ischemia
- CAD
1. A Multimodal Imaging Approach in Chronic Coronary Syndrome
Coronary artery disease (CAD) is a major cause of morbidity and mortality worldwide, and an accurate diagnostic assessment is pivotal for identifying patients that could potentially benefit from revascularization [1].
A multimodal non-invasive diagnostic approach for CAD detection includes anatomical (Coronary Computed Tomography Angiography, CCTA) and non-invasive functional imaging (stress echocardiography—SE, Cardiac Magnetic Resonance—CMR, nuclear imaging, stress CCTA, or CCTA derived Fractional Flow Reserve-FFR) [2,3][2][3].
While CCTA accurately depicts coronary anatomy, detects potential stenosis, and evaluates plaque features, other non-invasive functional imaging can demonstrate myocardial ischemia and the corresponding coronary territory. Integrating this complementary information is essential for the global risk assessment and the subsequential management of patients with suspected CAD [2].
The current European Society of Cardiology (ESC) guidelines recommend the use of either anatomical or non-invasive functional imaging as the initial test for diagnosing CAD after a global clinical risk assessment [4].
Which test to prescribe can be sometimes difficult to decide; typically CCTA is the preferred test in patients with a lower range of clinical likelihood of CAD, whereas the non-invasive functional tests for ischemia have better rule-in power and should be therefore preferred in those with higher clinical risk of coronary atherosclerosis [4].
2. The Role of Echocardiography
Functional tests designed to identify suspected CAD, with or without cardiac imaging, traditionally serve three important clinical purposes in the field of cardiology:-
Diagnosing CAD
-
Guiding appropriate therapy (revascularization and/or medical intervention) in cases where CAD is confirmed
-
Assessing the long-term outcomes and stratifying the risk for patients with CAD.
-
Cost-effectiveness and wide accessibility
-
Absence of ionizing radiation (environmentally friendly)
-
Ease of performance, potentially at the bedside
3. The Role of Cardiac Magnetic Resonance
Among noninvasive imaging modalities, CMR has been increasingly used in recent years due to its unique qualities in providing a complete assessment in patients with known or suspected CAD [30][5]. Indeed, CMR is able not only to evaluate the morphology, volume, and wall motion of the left ventricle (LV) but also allows precise tissue characterization. Furthermore, after gadolinium-based contrast medium (GBCM) injection, it enables the evaluation of stress-perfusion defects and the acquisition of late gadolinium enhancement (LGE) sequences for the detection of the extent of infarct scar [31,32,33][6][7][8]. In some cases, nuclear imaging may show false negative (balanced ischemia) or false positive results; in these circumstances, stress-CMR offers better sensibility and specificity in detecting functionally significant CAD [2]. In a few large randomized clinical trials, stress-CMR has been shown to be even superior in detecting ischemia compared to single photon emission computed tomography (SPECT) [34,35[9][10][11],36], and it resulted in a lower probability of unnecessary subsequent angiography [37,38][12][13]. The importance of stress-CMR is not only related to its high diagnostic accuracy but also to its ability to predict the patient’s prognosis. In fact, numerous studies have demonstrated that pathological stress-CMR is associated with a higher risk of cardiac death and adverse events during long-term follow-up [39,40,41,42][14][15][16][17]. In patients with prior revascularization, a stress-CMR reduces the need for further diagnostic imaging techniques, subsequent coronary angiography, and revascularizations without impairing patients’ prognosis [30][5].4. The Role of Nuclear Medicine (SPECT/PET)
The use of single photon emission computed tomography (SPECT) is currently an established approach in the initial evaluation of patients with suspected CAD thanks to wide availability, standardized protocols, and the extensive data established for diagnostic accuracy. Ischemia can be provoked by exercise or pharmacological stressors (dobutamine) that increase myocardial work and oxygen demand, as in other stress imaging protocols [2]. In case of left bundle branch block or ventricular paced rhythms, vasodilators (i.e., adenosine, regadenoson, or dipyridamole) should be preferred to identify heterogeneity in myocardial perfusion as well as in patients who are not able to achieve ≥85% of maximal age-predicted heart rate during exercise [59][18]. The mechanisms of action of the vasodilator substances and dobutamine are the same as already described in the CMR section. Single-photon emission computed tomography implicates the intravenous administration of gamma-emitting radiotracers, which are accumulated by cardiomyocytes in proportion to myocardial blood flow. This uptake occurs during periods of rest as well as during physical or pharmacological stress. The radionuclide agent is typically injected at the peak of exercise or during maximum vasodilation. During stress, a decrease in regional tracer uptake indicates relative myocardial hypoperfusion, whereas reduced uptake both during stress and at rest suggests the presence of a myocardial scar [60][19]. Two radiopharmaceuticals labeled with technetium-99m (99mTc) (sestamibi and tetrofosmin) and thallium-201 (201Tl) chloride are currently commercially available [59,61][18][20]. The particular pharmacokinetic characteristic of 201Tl is the prolonged retention within the cardiomyocytes allowing the evaluation of the coronary reserve in a single administration immediately after the provocation test. On the contrary, the perfusion study should be performed in two sessions (stress and rest test, usually 24 h apart) using the technetium-based tracers, with more favorable dosimetry and improved quality of gated images thanks to their shorter half-life [59][18].5. The Role of Coronary Computed Tomography Angiography
Coronary CT Angiography (CCTA) is the preferred imaging technique in symptomatic patients with a low-intermediate pre-test probability of CAD [4,69][4][21]. The current new-generation CT scanners enable high image quality with reduced contrast volume and radiation dosage, providing high diagnostic accuracy in the detection of CAD, even in the case of patients with high and/or irregular heart rates [20,70][22][23]. Integrating CCTA in the diagnostic algorithm of patients with stable chest pain was shown to be associated with a significant reduction in cardiovascular death and non-fatal myocardial infarction, due to proper diagnosis and tailoring of the treatment strategy [71,72][24][25]. Coronary CT Angiography allows risk stratification of patients with CAD based on the severity of coronary stenoses but also evaluation of the plaque characteristics (Figure 1). Importantly, baseline plaque burden is associated with the risk of major cardiovascular events independently of the presence of obstructive lesions [73,74,75][26][27][28] and predicts the progression to obstructive CAD [76][29]. High-risk plaque features (i.e., positive remodeling, low attenuated plaques, napkin-ring sign, spotty calcification) are strong predictors of future MI and focused the physician on secondary medical treatments [77,78,79][30][31][32].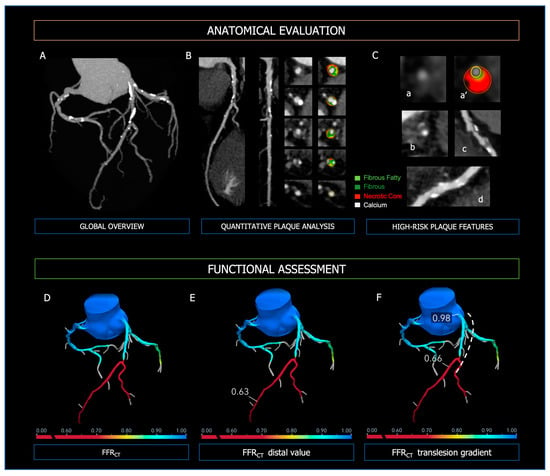
Figure 1. Anatomical evaluation and functional assessment of coronary plaques derived from CCTA. Panel (A)—3-vessel overview of the coronary tree for evaluation of calcium extent and distribution; Panel (B)—curved MPR, straight MPR, and cross-sections for quantitative plaque analysis; Panel (C)—high-risk plaque features; (a,a’): low attenuated plaque; (b): napkin-ring sign; (c): positive remodeling; (d): spotty calcifications. In the upper Panels, the figure legend refers to the colors used for quantitative plaque analysis; light green: fibrous fatty volume; dark green: fibrous volume; red: necrotic core volume; white: calcific volume. Panel (D): FFR-CT 3D model of the coronary tree, with distal FFR-CT (Panel (E)) and trans-lesion gradient (Panel (F), dashed line). At the bottom of Panels (D–F), the colorimetric scale of FFR-CT used in the 3D models is shown.
References
- Patel, M.R.; Calhoon, J.H.; Dehmer, G.J.; Grantham, J.A.; Maddox, T.M.; Maron, D.J.; Smith, P.K. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients with Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J. Nucl. Cardiol. 2017, 24, 1759–1792.
- Morrone, D.; Gentile, F.; Aimo, A.; Cameli, M.; Barison, A.; Picoi, M.E.; Guglielmo, M.; Villano, A.; DeVita, A.; Mandoli, G.E.; et al. Perspectives in noninvasive imaging for chronic coronary syndromes. Int. J. Cardiol. 2022, 365, 19–29.
- Muscogiuri, G.; Guglielmo, M.; Serra, A.; Gatti, M.; Volpato, V.; Schoepf, U.J.; Saba, L.; Cau, R.; Faletti, R.; McGill, L.J.; et al. Multimodality Imaging in Ischemic Chronic Cardiomyopathy. J. Imaging 2022, 8, 35.
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477.
- Baessato, F.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; Fusini, L.; Scafuri, S.; Babbaro, M.; Mollace, R.; Collevecchio, A.; Guaricci, A.I.; et al. Stress CMR in Known or Suspected CAD: Diagnostic and Prognostic Role. BioMed Res. Int. 2021, 2021, 1–12.
- Dweck, M.R.; Williams, M.C.; Moss, A.J.; Newby, D.E.; Fayad, Z.A. Computed Tomography and Cardiac Magnetic Resonance in Ischemic Heart Disease. J. Am. Coll. Cardiol. 2016, 68, 2201–2216.
- Kelle, S.; Roes, S.D.; Klein, C.; Kokocinski, T.; de Roos, A.; Fleck, E.; Bax, J.J.; Nagel, E. Prognostic Value of Myocardial Infarct Size and Contractile Reserve Using Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2009, 54, 1770–1777.
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and Prognostic Role of Cardiac Magnetic Resonance in MINOCA. JACC Cardiovasc. Imaging 2023, 16, 376–389.
- Schwitter, J.; Wacker, C.M.; van Rossum, A.C.; Lombardi, M.; Al-Saadi, N.; Ahlstrom, H.; Dill, T.; Larsson, H.B.W.; Flamm, S.D.; Marquardt, M.; et al. MR-IMPACT: Comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur. Heart J. 2008, 29, 480–489.
- Schwitter, J.; Wacker, C.M.; Wilke, N.; Al-Saadi, N.; Sauer, E.; Huettle, K.; Schönberg, S.O.; Luchner, A.; Strohm, O.; Ahlstrom, H.; et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: Perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: A comparative multicentre, multivendor trial. Eur. Heart J. 2013, 34, 775–781.
- Greenwood, J.P.; Maredia, N.; Younger, J.F.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Ridgway, J.P.; Radjenovic, A.; Dickinson, C.J.; et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet 2012, 379, 453–460.
- Greenwood, J.P.; Ripley, D.P.; Berry, C.; McCann, G.P.; Plein, S.; Bucciarelli-Ducci, C.; Dall’Armellina, E.; Prasad, A.; Bijsterveld, P.; Foley, J.R.; et al. Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scintigraphy, or NICE Guidelines on Subsequent Unnecessary Angiography Rates: The CE-MARC 2 Randomized Clinical Trial. JAMA 2016, 316, 1051.
- Nagel, E.; Greenwood, J.P.; McCann, G.P.; Bettencourt, N.; Shah, A.M.; Hussain, S.T.; Perera, D.; Plein, S.; Bucciarelli-Ducci, C.; Paul, M.; et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N. Engl. J. Med. 2019, 380, 2418–2428.
- Jahnke, C.; Nagel, E.; Gebker, R.; Kokocinski, T.; Kelle, S.; Manka, R.; Fleck, E.; Paetsch, I. Prognostic Value of Cardiac Magnetic Resonance Stress Tests: Adenosine Stress Perfusion and Dobutamine Stress Wall Motion Imaging. Circulation 2007, 115, 1769–1776.
- Lipinski, M.J.; McVey, C.M.; Berger, J.S.; Kramer, C.M.; Salerno, M. Prognostic Value of Stress Cardiac Magnetic Resonance Imaging in Patients with Known or Suspected Coronary Artery Disease. J. Am. Coll. Cardiol. 2013, 62, 826–838.
- Vincenti, G.; Masci, P.G.; Monney, P.; Rutz, T.; Hugelshofer, S.; Gaxherri, M.; Muller, O.; Iglesias, J.F.; Eeckhout, E.; Lorenzoni, V.; et al. Stress Perfusion CMR in Patients with Known and Suspected CAD. JACC Cardiovasc. Imaging 2017, 10, 526–537.
- Pontone, G.; Andreini, D.; Bertella, E.; Loguercio, M.; Guglielmo, M.; Baggiano, A.; Aquaro, G.D.; Mushtaq, S.; Salerni, S.; Gripari, P.; et al. Prognostic value of dipyridamole stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: A mid-term follow-up study. Eur. Radiol. 2016, 26, 2155–2165.
- Verberne, H.J.; Acampa, W.; Anagnostopoulos, C.; Ballinger, J.; Bengel, F.; De Bondt, P.; Buechel, R.R.; Cuocolo, A.; van Eck-Smit, B.L.F.; Flotats, A.; et al. EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT: 2015 revision. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1929–1940.
- Agostini, D.; Marie, P.-Y.; Ben-Haim, S.; Rouzet, F.; Songy, B.; Giordano, A.; Gimelli, A.; Hyafil, F.; Sciagrà, R.; Bucerius, J.; et al. Performance of cardiac cadmium-zinc-telluride gamma camera imaging in coronary artery disease: A review from the cardiovascular committee of the European Association of Nuclear Medicine (EANM). Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2423–2432.
- Bedetti, G.; Pizzi, C.; Gavaruzzi, G.; Lugaresi, F.; Cicognani, A.; Picano, E. Suboptimal Awareness of Radiologic Dose among Patients Undergoing Cardiac Stress Scintigraphy. J. Am. Coll. Radiol. 2008, 5, 126–131.
- Carrabba, N.; Migliorini, A.; Pradella, S.; Acquafresca, M.; Guglielmo, M.; Baggiano, A.; Moscogiuri, G.; Valenti, R. Old and New NICE Guidelines for the Evaluation of New Onset Stable Chest Pain: A Real World Perspective. BioMed Res. Int. 2018, 2018, 1–7.
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330.
- Andreini, D.; Mushtaq, S.; Pontone, G.; Conte, E.; Guglielmo, M.; Annoni, A.; Baggiano, A.; Formenti, A.; Ditali, V.; Mancini, M.E.; et al. Diagnostic performance of coronary CT angiography carried out with a novel whole-heart coverage high-definition CT scanner in patients with high heart rate. Int. J. Cardiol. 2018, 257, 325–331.
- Min, J.K.; Dunning, A.; Lin, F.Y.; Achenbach, S.; Al-Mallah, M.; Budoff, M.J.; Cademartiri, F.; Callister, T.Q.; Chang, H.-J.; Cheng, V.; et al. Age- and Sex-Related Differences in All-Cause Mortality Risk Based on Coronary Computed Tomography Angiography Findings. J. Am. Coll. Cardiol. 2011, 58, 849–860.
- Chang, H.-J.; Lin, F.Y.; Gebow, D.; An, H.Y.; Andreini, D.; Bathina, R.; Baggiano, A.; Beltrama, V.; Cerci, R.; Choi, E.-Y.; et al. Selective Referral Using CCTA Versus Direct Referral for Individuals Referred to Invasive Coronary Angiography for Suspected CAD. JACC Cardiovasc. Imaging 2019, 12, 1303–1312.
- Mortensen, M.B.; Dzaye, O.; Steffensen, F.H.; Bøtker, H.E.; Jensen, J.M.; Rønnow Sand, N.P.; Kragholm, K.H.; Sørensen, H.T.; Leipsic, J.; Mæng, M.; et al. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients with Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2020, 76, 2803–2813.
- Andreini, D.; Conte, E.; Mushtaq, S.; Magatelli, M.; Traversari, F.; Gigante, C.; Belmonte, M.; Gaudenzi-Asinelli, M.; Annoni, A.; Formenti, A.; et al. Plaque assessment by coronary CT angiography may predict cardiac events in high risk and very high risk diabetic patients: A long-term follow-up study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 586–595.
- The SCOT-HEART Investigators Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933.
- Lee, S.-E.; Sung, J.M.; Andreini, D.; Al-Mallah, M.H.; Budoff, M.J.; Cademartiri, F.; Chinnaiyan, K.; Choi, J.H.; Chun, E.J.; Conte, E.; et al. Differences in Progression to Obstructive Lesions per High-Risk Plaque Features and Plaque Volumes with CCTA. JACC Cardiovasc. Imaging 2020, 13, 1409–1417.
- Chang, H.-J.; Lin, F.Y.; Lee, S.-E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.W.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522.
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results from the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462.
- Paolisso, P.; Bergamaschi, L.; Saturi, G.; D’Angelo, E.C.; Magnani, I.; Toniolo, S.; Stefanizzi, A.; Rinaldi, A.; Bartoli, L.; Angeli, F.; et al. Secondary Prevention Medical Therapy and Outcomes in Patients with Myocardial Infarction with Non-Obstructive Coronary Artery Disease. Front. Pharmacol. 2020, 10, 1606.
- Hecht, H.S.; Shaw, L.; Chandrashekhar, Y.S.; Bax, J.J.; Narula, J. Should NICE guidelines be universally accepted for the evaluation of stable coronary disease? A debate. Eur. Heart J. 2019, 40, 1440–1453.
- Driessen, R.S.; Danad, I.; Stuijfzand, W.J.; Raijmakers, P.G.; Schumacher, S.P.; van Diemen, P.A.; Leipsic, J.A.; Knuuti, J.; Underwood, S.R.; van de Ven, P.M.; et al. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. J. Am. Coll. Cardiol. 2019, 73, 161–173.
- Patel, M.R.; Nørgaard, B.L.; Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Berman, D.S.; Raff, G.L.; Hurwitz Koweek, L.M.; Pontone, G.; Kawasaki, T.; et al. 1-Year Impact on Medical Practice and Clinical Outcomes of FFRCT. JACC Cardiovasc. Imaging 2020, 13, 97–105.
- Douglas, P.S.; Hoffmann, U.; Patel, M.R.; Mark, D.B.; Al-Khalidi, H.R.; Cavanaugh, B.; Cole, J.; Dolor, R.J.; Fordyce, C.B.; Huang, M.; et al. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. N. Engl. J. Med. 2015, 372, 1291–1300.
- Pontone, G.; Baggiano, A.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Fazzari, F.; Mushtaq, S.; Conte, E.; et al. Stress Computed Tomography Perfusion Versus Fractional Flow Reserve CT Derived in Suspected Coronary Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 1487–1497.
- Celeng, C.; Leiner, T.; Maurovich-Horvat, P.; Merkely, B.; de Jong, P.; Dankbaar, J.W.; van Es, H.W.; Ghoshhajra, B.B.; Hoffmann, U.; Takx, R.A.P. Anatomical and Functional Computed Tomography for Diagnosing Hemodynamically Significant Coronary Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 1316–1325.
- Lubbers, M.; Coenen, A.; Kofflard, M.; Bruning, T.; Kietselaer, B.; Galema, T.; Kock, M.; Niezen, A.; Das, M.; van Gent, M.; et al. Comprehensive Cardiac CT with Myocardial Perfusion Imaging Versus Functional Testing in Suspected Coronary Artery Disease. JACC Cardiovasc. Imaging 2018, 11, 1625–1636.
- Chen, M.Y.; Rochitte, C.E.; Arbab-Zadeh, A.; Dewey, M.; George, R.T.; Miller, J.M.; Niinuma, H.; Yoshioka, K.; Kitagawa, K.; Sakuma, H.; et al. Prognostic Value of Combined CT Angiography and Myocardial Perfusion Imaging versus Invasive Coronary Angiography and Nuclear Stress Perfusion Imaging in the Prediction of Major Adverse Cardiovascular Events: The CORE320 Multicenter Study. Radiology 2017, 284, 55–65.
More
