Multiple sclerosis (MS) is a disabling immune-mediated demyelinating neurodegenerative disease with an estimated prevalence of 1 in 1000 in populations of European descent. It primarily affects females (F:M = 2–3:1) mainly between the ages of 15 and 55 years.
- multiple sclerosis
- pediatric-onset multiple sclerosis
- nutrition
- diet
- gut microbiota
- gut-brain axis
- blood-brain barrier
- vitamin D
1. The Gut-Brain Axis in Multiple Sclerosis
1.1. The Intestinal Barrier
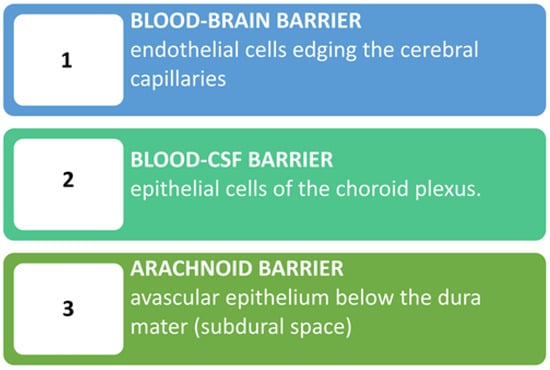
1.2. The Blood-Brain Barrier
2. Diets and Dietary Supplementations
2.1. Dietary Influence on Multiple Sclerosis
2.1. Dietary Influence on MS
| Diet Name | Main Characteristics |
|---|---|
| Allergen free/milk free | Hypoallergenic diet based on the unproven hypothesis of the association between MS and external allergens [65][76]. The milk protein butyrophilin has been implicated through antigenic mimicry with myelin oligodendrocyte glycoprotein in EAE [66][77] as well as in MS patients [67][78]. Some studies with questionnaires suggest an inverse relationship between total dairy intake and MS disability severity [68][69][79,80] with an inverse relationship between whole grain intake and MS-related disability [69][80]. |
| Gluten free | Among studies, only one clinical trial gave meaningful results, but there are methodological limitations [70][71][72][81,82,83]. All in all, the current level of evidence is inadequate to state whether gluten plays a role in MS [71][82]. |
| Mega Ascorbic | High in vitamin C diet. No well-defined link between MS and vitamin C [73][84]. |
| Multi Vitaminic | Multi vitaminic supplementation (e.g., A and D): quite convincing data show that higher vitamin intake/serum levels correlate with lower risk of MS development but not convincing on the contrary [74][85]. Possible detrimental effects of overdosing require vitamin-level monitoring [75][76][86,87]. |
| Hebener’s | Self-reported disease stability/amelioration in one study with fish oil and antioxidant drugs supplementation + Ω-6 restriction [77][88]. |
| Kousmine | High in polyunsaturated fats/low in animal fats diet to counteract a possibly increased membrane permeability [78][79][80][89,90,91]. |
| Swank (low saturated fats) | Low-saturated fats (<20 g fat/day or <20% total calories): reported lower death rates and better outcome in the more adherent patients and those with lower disability at entry [81][82][92,93]. |
| Mediterranean diets (MD) | Common features include emphasis on vegetables, fruits, beans, nuts, seeds, breads, unrefined grains, and olive oil; inclusion of fish and wine; minimal intake of full-fat dairy products and possibly lean meats [83][84][94,95]. Conflicting results on whether lean and unprocessed red meat is detrimental [85][86][87][96,97,98]. It is considered beneficial for its antioxidant properties. Negatively associated with neurological and fatigue symptoms. Adherence should be monitored through validated tests [e.g., Predimed for adults [83][94] and KidMed for children [88][99] |
| Mediterranean/DASH | It derives from the Mediterranean Dietary Approaches to Stop Hypertension (DASH) [89][90][100,101]. |
| MIND | The Mediterranean/Intervention for Neurodegenerative Delay (MIND) is a combination of MD and DASH [89][90][91][100,101,102]. |
| Paleolithic1 | Consists of high-quality foods full of nutrients and fiber and with less artificial sugar and salt compared to present-day diets [92][103]. Nutrients included in this diet are essential to myelin growth and repair. Typically, it does not permit consumption of dairy or grain products. |
| Modified Paleolithic (MD-PI intervention) |
This diet is rich in α-lipoic acid and polyphenols. It has commonalities with MD including avoidance of high-fat meats/ultra-processed foods with added sugar, sodium, and hydrogenated fats [93][94][95][104,105,106]. |
| Wahls™ Paleo diet | Differences from a traditional Paleo diet: exclusion of eggs; limited animal and fish protein. It allows legumes (e.g., soy milk), two servings of gluten-free grains (e.g., rice) per week; it specifies nine cups of fruits and vegetables (F/V)/day with 1/3 each from dark-green leafy vegetables, sulfur-rich vegetables, and deeply colored F/V; seaweed, algae and nutritional yeast are encouraged [96][97][107,108]. |
| Wahls/Elim Paleo | This is a paleo version modified by adding a restriction of lectins to reduce intestinal permeability and CNS inflammation [96][97][107,108]. |
| Overcoming MS (OMS) | Minimized saturated fats and plant-based, whole-food diet plus seafood [68][98][79,109]. |
| Ketogenic diet (KD) | Eliminating all/almost all carbohydrates and increasing the intake of proteins. KD combined with a modified MD have been suggested to improve neuroinflammation in MS [99][100][110,111]. |
| Energy restriction (ER) | Chronic ER/Intermittent energy restriction (IER) determines a switch from glucose to fatty acids and ketones as the major fuel source for cells [101][[104][112102][103],113,114,115]. Mice fed a “fasting mimicking” diet (very low-calorie diet lasting for 3 days every 7 days) exhibited delayed onset, reduced incidence, and decreased severity of EAE. Histological findings show reduced immune cell infiltration and demyelination in the spinal cord [105][116]. |
| McDougall Diet | A low-fat (10–15% of calories from fat), starch-based, vegan diet with no oils permitted. For 7 days, produced significant favorable changes in commonly tested biomarkers used to predict future risks for cardiovascular disease and metabolic diseases [64][106][75,117]. It appeared safe and effective in preventing clinical attacks/new MRI lesions. Drawback = long-term adherence [107][118]. |
2.2. Dietary Supplementations
2.3 Pediatric issues: Diet and Nutrition Related Issues in Pediatric-Onset Multiple Sclerosis
MS starting in childhood (Pediatric-Onset MS, POMS) is estimated to account for between 2–5% and 5–10% of the MS population worldwide. Although youth with POMS have a lower risk of disability within the first 10 years of diagnosis than those with adult-onset MS, the disease may negatively affect their school and emotional spheres. Moreover, they reach disability milestones earlier than adults, even though they tend to take a longer time to advance to the secondary progressive phase. Overall, quite common cognitive impairment requiring specific management, decrease in QOL, and an increase in economic burden in POMS have been shown to have profound impacts not only on patients but also on their families.
Childhood obesity has been identified as a potential risk factor for increased morbidity not only due to hepatic-cardiac-metabolic comorbidities but also from MS and clinically isolated syndrome (CIS) in adolescents, particularly in girls. The underlying mechanism may involve vitamin D deficiency, as obesity is associated with lower vitamin D levels. Of note, sedentary indoor lifestyles and reduced sunlight exposure, contribute to decreased vitamin D synthesis and increased hypovitaminosis D in children.
Unbalanced diets with increased fats, especially saturated fat content, are associated with a higher risk of unfavorable disease progression. A healthy diet characterized by the consumption of fruit, yogurt and legumes during childhood, appears associated with a lower probability of developing subsequent MS in adulthood. Specific dietary strategies may therefore aid children with POMS in slowing disease progression and improving their quality of life. Overall, these data are particularly worrying if one considers that a large proportion of adolescents with POMS have been found to have a non-self-perceived elevated BMI. To improve their disease progression, they should therefore receive more accurate counseling to improve their diet and physical activity as well.
The gut microbiota (GM) has been implicated also in POMS. Differences in microbial composition and metabolic pathways have been observed in children with POMS compared to healthy controls, and have been found to predict the likelihood of recurrence.
3. Perspectives
What is around the gastrointestinal/nutritional corner of MS ? A) Next generation (NG) engineered probiotics, obtained by modifying original probiotics through gene editing modalities, have hitherto been used in inflammatory bowel disease, and in a number of bacterial infections, tumors, and metabolic diseases, mainly in MS akin EAE murine models and/or in vitro. Promising preliminary results showing they are effective, with fewer side effects than traditional treatments or wild-type strains, suggest that they will probably be proposed soon for central nervous system (CNS) diseases as well, including much probably MS. Of note, the design of NG probiotics should specifically be directed towards the production of metabolites (e.g., SCFAs) and neurotransmitters (e.g., serotonin, GABA) which are known to affect the neurobiology of CNS inflammatory diseases. B) Fecal microbiota transplantation (FMT) represents a further interesting approach to modulate GM. FMT studies in animal models and in humans with MS are still scarce and preliminary. Some data available from a cohort of RRMS patients show that FMT is safe and was well-tolerated and may have also improved their gut dysbiosis and elevated small intestinal permeability. Moreover, single case reports and a case series in addition to confirming the safety of the treatment, also showed specific clinical improvements in MS-related neurological symptoms.
4. Conclusions
In conclusion, data show that (1) no universal best diet exists, (2) healthy/balanced diets are necessary to safeguard the adequate intake of all essential nutrients, (3) diets with high intakes of fruits, vegetables, whole grains, and lean proteins that limit processed foods, sugar, and saturated fat appear beneficial for their antioxidant and anti-inflammatory properties and their ability to shape a gut microbiota that respects the gut and brain barriers, (4) obesity may trigger MS onset and/or its less favorable course, especially in pediatric-onset MS. Vitamin D and polyunsaturated fatty acids are the most studied supplements for reducing MS-associated inflammation.
More in detail, several dietary/nutritional factors play an important role both in adult and pediatric MS development and progression. Several gut-oriented nutritional interventions aiming to improve the dysregulation of the so-called Gut Brain Axis through a proper diet appear to intervene beneficially mainly against the inflammatory pathomechanisms associated with MS. The efficacy of any dietary intervention in MS, however, remains difficult to prove due to spontaneous remissions (and relapses) with temporary clinical improvement occurring by chance alone. Pending more solid evidence on specific diets, experts suggest that individuals with MS should be taught to follow a “healthy” regime and possibly enter into nutrition education programs, which, however, are largely lacking as in most other neurological diseases at present.
Because of the high prevalence of overweight/obesity, and the evidence that obesity can worsen MS prognosis, education on weight management is still an unmet need. Pediatric interventions may be hampered by the lack of self-perceived BMI elevation at this age. Because there is, at present, no robust evidence, future research is also needed to identify appropriate study designs and intervention strategies targeting physical activity participation. New solid longitudinal and experimental designs are necessary not only to better elucidate the role of diet and other modifiable lifestyle factors in this population, but also to explore other modalities of support. These should include a closer monitoring of nutritional status of patients with moderate-advanced MS in order to prevent their tendency to be overweight secondary to the decrease in basal energy expenditure and loss of muscle mass.
Further detailed and up-to-date information is available inside the article by Mandato C, Colucci A, Lanzillo R, Staiano A, Scarpato E, Schiavo L, Operto FF, Serra MR, Di Monaco C, Napoli JS, Massa G, and Vajro P. Multiple Sclerosis—Related Dietary and Nutritional Issues: An Updated Scoping Review with a Focus on Pediatrics. Children. 2023; 10(6):1022. https://doi.org/10.3390/children10061022
