Among therapeutically valuable opioids, morphinans are of the utmost clinical importance as analgesic drugs because of their agonistic actions to the mu-opioid receptor. They include powerful pain relieving agents, such as naturally occurring alkaloids (e.g., morphine and codeine), semisynthetic analogues (e.g., hydrocodone, hydromorphone, oxycodone, oxymorphone and buprenorphine), and synthetic derivatives (e.g., levorphanol).
- pain
- analgesia
- opioid receptors
- peripheral analgesia
- peripherally restricted opioids
- morphinans
- morphine
1. Introduction

2. Peripheralization Strategies Applied to Morphinans
Different chemical strategies have been developed to limit the ability of opioids to cross the BBB, including (a) chemical modifications to the morphinan skeleton to increase hydrophilicity of known and new opioids, and (b) nanocarrier-based approaches to selectively deliver opioids, such as morphine to the peripheral tissue. The following sections discuss significant representatives, including design strategies, synthetical procedures, pharmacology and structure–activity relationships (SAR).2.1. Quaternization of the Morphinan Nitrogen
The first effort to minimize the CNS effects of opioids while retaining their actions in peripheral tissue was the quaternization of the nitrogen in the clinically used morphine, oxymorphone, nalorphine, naloxone and naltrexone (Figure 23) [27][28][27,28]. Peripheral selectivity of the quaternary derivative of morphine, N-methylmorphine, was described over 50 years ago [29]. The systemic intravenous (i.v.) administration of N-methylmorphine caused the inhibition of gastrointestinal transit because of its action on the opioid receptors in the gut. In the hot-plate test, centrally mediated antinociception was produced by 15 mg/kg morphine in mice after intraperitoneal (i.p.) administration but not by N-methylmorphine at the same dose [29]. Furthermore, N-methylmorphine proved to be ineffective in the hot-plate test even in a dose 100 mg/kg [30]. In an acetic-acid-induced writhing assay, N-methylmorphine produced antinociceptive effects in mice after i.p. administration in a dose of 45 mg/kg, being 30-fold less potent than morphine [30]. N-methylmorphine was also shown to selectively inhibited phase II in the formalin test following systemic i.p. administration [31]. The antinociceptive effect of N-methylmorphine after central intracerebroventricular (i.c.v.) administration was antagonized by systemically applied naloxone but not by peripheral antagonist N-methylnaloxone, showing the peripheral site of action of N-methylmorphine [31].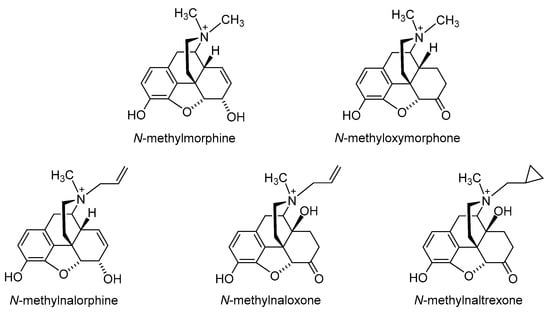
2.2. Introduction of Hydrophilic Substituents at Position 6
Polar or ionizable substitutions are able to increase polarity and inhibit the crossing of the BBB. Therefore, opioids with hydrophilic groups attached to the C-6 position of the morphinan skeleton were designed. The first examples of morphinans having ionizable residues at position 6 were reported more than 30 years ago. They were synthesized from β-oxymorphamine [33], β-naltrexamine [33] and β-funaltrexamine [34]. Such compounds with zwitterionic moieties showed significantly reduced access to the CNS without substantially decreased opioid receptor in vitro and in vivo activity [33][34][33,34]. Noteworthy are the 6-amide derivatives of β-oxymorphamine (a–e, Figure 34) reported as the first peripherally selective opioid agonists and effective antinociceptives [33]. All compounds have C-6 moieties that are ionized at the pH of the gut or at physiologic pH, accounting for a more restricted capability to enter the CNS than the unionized molecules. Compounds a, c and d were synthesized from β-oxymorphamine with the appropriate anhydride [33]. The fumaramic acid b was prepared by coupling the half-ester of fumaric acid with β-oxymorphamine and then subjecting the fumaramate esters to hydrolysis. The aspartyl derivative e was obtained through coupling BocAsp γ-benzyl ester with β-oxymorphamine, followed by deprotection with acid to remove the Boc group and hydrogenolysis of the benzyl function [33]. As regards biological activities, the ß-oxymorphamine derivatives a–e were all full agonists in the guinea pig ileum (GPI) bioassay with potencies that were 1.5- to 6-fold higher than the potency of morphine (Table 1) [33]. In a mouse model of acute thermal nociception, the tail-flick assay, all compounds possessed potent antinociceptive activity when administered by the i.c.v route (Table 1). They also were active in inducing antinociception when given systemically by i.v. administration to mice. When compared on a body weight basis, the i.v. ED50 doses were about 1000-fold higher than the i.c.v. ED50 values. Derivatives a and c were also active when given orally (p.o.) (Table 1) [33]. The attachment of polar groups, particularly zwitterionic moieties, at the C-6 position of the morphinan structure is effective in excluding such ligands from the CNS, thereby affording peripheral selectivity.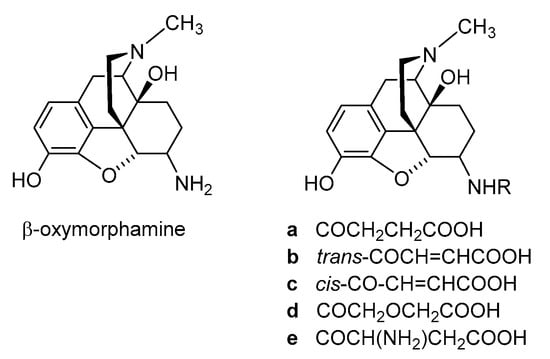
| Compound | Agonist Potencies IC50 × 108 (M) a | Antinociceptive Potencies ED50 b |
|---|---|---|
| a | 14.4 | 0.27 nmol/mouse, i.c.v. 9.8 µmol/kg, i.v. 30.3 µmol/kg, p.o. |
| b | 13.0 | - c |
| c | 3.9 | 0.17 nmol/mouse, i.c.v. 11 µmol/kg, i.v. ~20 µmol/kg, p.o. |
| d | 2.7 | 0.25 nmol/mouse, i.c.v. <20 µmol/kg, i.v. >25 µmol/kg, p.o. |
| e | 3.1 | 0.44 nmol/mouse, i.c.v. <0 µmol/kg, i.v. |
2.2.1. 6-Amino-acid-substituted 14-Alkoxymorphinans
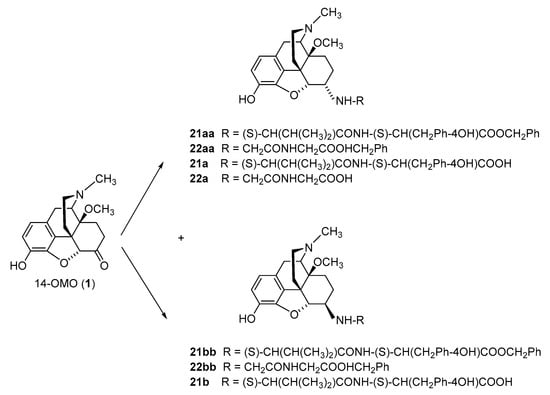
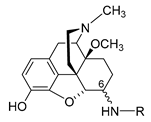
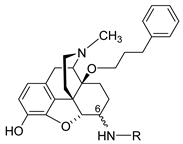
The first synthetic efforts directed towards the development of ionizable molecules in the class of 14-alkoxymorphinans as peripherally acting opioid analgesics started with the series of six 6-amino acids, i.e., Gly-, L-Ala- and L-Phe- substituted derivatives; 2a/b (HS-730/HS-731); 3a/b (HS-935/HS-936); and 4a/b (HS-937/HS-938), respectively, of the highly potent and centrally acting MOR agonist 14-O-methyloxymorphone (14-OMO, 1) (SchemeScheme 1) 1) [35][35]. A novel synthetic procedure for the synthesis of 6-amino-acid-substituted derivatives in the morphinan series was used. The tert-butyl ester derivatives 2aa/bb, 3aa/bb, and 4aa/bb were prepared from 14-OMO (1) by reductive amination with the respective tert-butyl ester hydrochlorides and sodium cyanoborohydride in ethanol. After separating the diastereoisomers by column chromatography, esters 2aa/bb, 3aa/bb, and 4aa/bb were treated with tetrafluoroboric acid in dichloromethane to afford 6-Gly (2a and 2b), 6-Ala (3a and 3b) and 6-Phe (4a and 4b) substituted derivatives, respectively (SchemeScheme 1) 1) [35][35].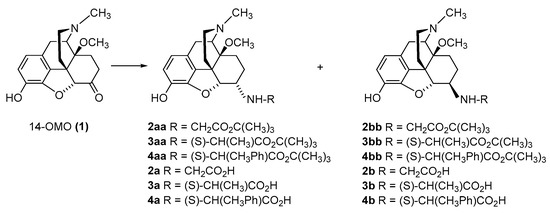
| Morphine | 6053 | 437 | 1613 | 1472 | |
| Fentanyl | 38.6 | ||||
| 14-OMO (1) | 14.9 | 3.26 | |||
| HS-730 (2a) | α-Gly | 58.5 | 35.7 | 72 | 110 |
| HS-731 (2b) | β-Gly | 29.0 | 27.5 | 125 | 204 |
| HS-935 (3a) | α-L-Ala | 68.9 | 16.0 |
| Pain Model | Route | ED50 | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Acute nociception Radiant heat tail-flick test (rat) |
i.c.v. s.c. |
0.030 nmol/rat 29.0 nmol/kg |
[40] [40] |
||||||
| Trigeminal nociception Eye wiping test (mouse) |
i.p. |
50 µg/kg a |
[43] |
||||||
| Visceral pain | |||||||||
| Acetic-acid-induced writhing assay (mouse) | i.c.v. s.c. |
0.49 pmol/mouse 51 nmol/kg |
[42] [42] |
||||||
| s.c. | 27.5 µg/kg | [36] | |||||||
| Inflammatory pain Formalin test (rat) Carrageenan-induced thermal and mechanical hyperalgesia (rat) |
i.pl. s.c. s.c. p.o. |
Phase I: 0.2 nmol; Phase II: 0.4 nmol Phase I: 125 nmol/kg; Phase II: 204 nmol/kg 20 µg/kg a 10 mg/kg a | |||||||
| HS-936 (3b) | β-L-Ala | 53.4 | 86.3 | ||||||
| HS-937 (4a) | α-L-Phe | 315 | 31.1 | 171 | 292 | ||||
| [ | 44 | HS-938 (4b) | β-L-Phe | >3600 | 579 | 79 | 107 | ||
| ] | [ | 40] | 5a | α-L-Ser | 32.1 | ||||
| 6b | β-L-Val | 117 | |||||||
| 7a | α-L-Lys | 20.6 | |||||||
| 8a | α-L-Tyr | 14.6 | |||||||
| 9b | β-L-Trp | 92.7 | |||||||
| [ | 41] [41] |
10a | α-L-Asn | 15.2 | |||||
| 12a | α-L-Asp | 38.2 | |||||||
| 13a | α-L-Glu | 36.1 | |||||||
| 15b | β-D-Val | 14.0 | |||||||
| 16a | α-D-Phe | 18.1 | |||||||
| Neuropathic pain, sciatic nerve ligation—Mechanical hyperalgesia (rat) | i.pl. | 441 nmol | [44] | 16b | β-D-Phe | 250 | |||
| 17a | α-L-Chg | 20.6 | |||||||
| 18a | α-L-Abu | 17.5 | |||||||
| 19a | α-β-Ala | 31.2 | |||||||
| 20a | α-GABA | 41.9 | |||||||
| 21a | β-L-Val-L-Tyr | 178 | |||||||
| 22a | β-Gly-Gly | 104 | |||||||
| 24a | α-Gly | 81.1 | |||||||
| 24b | β-Gly | 130 |
| Compound | R | Opioid Receptor Binding a | Agonist Activity b | clogD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | R | Opioid Receptor Binding, Ki (nM) a | clogD7.4 b | 7.4 c | ||||||||||
| MOR Ki (nM) |
DOR Ki (nM) |
KOR Ki (nM) |
Ki Ratio MOR/DOR/KOR |
MOR | ||||||||||
| MOR | EC | 50 | (nM) | MOR % stim. |
DOR EC50 (nM) |
DOR % stim. |
KOR EC50 (nM) |
KOR % stim. |
||||||
| DOR | KOR | K | i Ratio MOR/DOR/KOR |
|||||||||||
| 14-OMO (1) | 0.10 | 4.80 | 10.2 | 1/48/102 | 3.83 | 97 | 37.3 | 106 | 116 | 77 | 0.48 | |||
| HS-730 (2a) | α-Gly | 0.89 | 15.4 | 43.2 | 1/7/49 | 1.16 | 99 | 9.61 | 103 | 399 | 87 | −3.35 | ||
| HS-731 (2b) | β-Gly | 0.83 | 7.86 | 44.8 | 1/9.5/54 | 3.78 | ||||||||
| 3.16 | ||||||||||||||
| 3.91 | ||||||||||||||
| 325 | ||||||||||||||
| POMO (23) | 0.073 | 0.13 | 0.30 | 1/1.8/4.1 | 2.89 | |||||||||
| 98 | 7.92 | 103 | 361 | 82 | −3.35 | |||||||||
| HS-935 (3a) | α-L-Ala | 0.77 | 26.9 | 142 | 1/35/184 | 1.34 | 97 | 9.55 | 93 | 214 | 51 | −2.81 | ||
| HS-936 (3b) | β-L-Ala | 1.90 | 7.71 | 63.7 | 1/4.1/34 | 6.24 | 87 | 5.20 | 104 | 392 | 64 | −2.81 | ||
| HS-937 (4a) | α-L-Phe | 0.95 | 3.67 | 28.5 | 1/3.9/30 | 0.38 | 93 | 0.39 | 102 | 219 | 39 | −1.13 | ||
| HS-938 (4b) | β-L-Phe | 2.58 | 1.03 | 151 | 1/0.4/59 | 6.76 | 99 | 0.48 | 94 | 1172 | 81 | −1.13 | ||
| 5a | α-L-Ser | 2.21 | 5.32 | 196 | 1/2.4/89 | 1.60 | 87 | 13.9 | 101 | 1213 | 44 | −3.89 | ||
| 5b | β-L-Ser | 2.14 | 5.29 | 152 | ||||||||||
| 1/1.2/103 | 10.5 | 95 | 33.8 | 91 | 462 | 51 | −1.94 | |||||||
| 6b | β-L-Val | 3.04 | 3.52 | 305 | 1/1.2/100 | 11.7 | 84 | 5.73 | 96 | 1117 | 68 | −1.94 | ||
| 7a | α-L-Lys | 0.19 | 1.27 | 12.6 | 1/6.7/66 | 2.25 | 9 | 152 | 106 | 118 | 79 | −5.57 | ||
| 7b | β-L-Lys | 0.53 | 3.34 | 33.7 | 1/6.3/64 | 6.85 | 90 | 45.1 | 93 | 525 | 62 | −5.57 | ||
| 8a | α-L-Tyr | 0.83 | 2.18 | 39.5 | 1/2.6/48 | 1.87 | 92 | 1.76 | 88 | 100 | 62 | −1.41 | ||
| 8b | β-L-Tyr | 3.20 | 3.89 | 186 | 1/1.2/58 | 24.7 | 93 | 6.23 | 95 | 774 | 60 | −1.41 | ||
| 9a | α-L-Trp | 0.36 | 1.02 | 25.1 | 1/2.8/70 | 0.51 | 93 | 2.52 | 102 | 70.1 | 61 | −1.03 | ||
| 9b | β-L-Trp | 0.65 | 1.19 | 8.66 | 1/1.8/13 | 1.64 | 101 | 2.18 | 96 | 181 | 87 | −1.03 | ||
| 10a | α-L-Asn | 1.17 | 3.37 | 74.0 | 1/2.9/63 | 0.83 | 99 | 9.78 | 106 | 81.7 | 67 | −4.29 | ||
| 10b | β-L-Asn | 1.26 | 2.25 | 103 | 1/1.8/82 | 2.04 | 96 | 3.18 | 88 | 923 | 71 | −4.29 | ||
| 11a | α-L-Gln | 3.24 | 5.13 | 351 | 1/1.6/108 | 2.27 | 90 | 7.80 | 104 | 185 | 70 | −4.04 | ||
| 11b | β-L-Gln | 2.48 | 4.87 | 290 | 1/2.0/117 | 9.54 | 98 | 3.96 | 103 | 1410 | 63 | −4.04 | ||
| 12a | α-L-Asp | 1.36 | 14.6 | 50.2 | 1/11/37 | 4.10 | 90 | 10.1 | 97 | 2991 | 83 | −5.64 | ||
| 12b | β-L-Asp | 3.42 | 22.6 | 351 | 1/6.6/103 | 1.45 | 74 | 11.8 | 101 | 753 | 49 | −5.64 | ||
| 13a | α-L-Glu | 1.45 | 9.03 | 87.2 | 1/6.2/60 | 3.11 | 105 | 10.8 | 98 | 1167 | 68 | −5.39 | ||
| 13b | β-L-Glu | 11.6 | 7.64 | 1252 | ||||||||||
| 17b | ||||||||||||||
| β-L-Chg | ||||||||||||||
| 1.66 | ||||||||||||||
| 24a | α-Gly | 0.19 | 0.22 | 0.73 | 1/1.2/3.8 | −0.85 | ||||||||
| 1.30 | 118 | |||||||||||||
| 24b | β-Gly | 0.16 | 0.19 | 0.81 | 1/1.2/5.1 | −0.85 | 1/2.5/71 | 3.56 | 101 | 6.98 | 98 | 201 | 88 | −3.89 |
| 6a | α-L-Val | 1/0.7/108 | 12.7 | 98 | 4.60 | 101 | 2233 | 76 | −5.39 | |||||
| 14a | α-D-Ala | 0.69 | 10.4 | 71.5 | 1/15/104 | 1.44 | 100 | 24.3 | 106 | 254 | 67 | −2.81 | ||
| 14b | β-D-Ala | 1.48 | 11.3 | 142 | 1/7.6/96 | 15.4 | 102 | 5.46 | 106 | 1001 | 86 | −2.81 | ||
| 15a | α-D-Val | 1.70 | 1.93 | 202 | 1/1.1/119 | 4.51 | 105 | 1.12 | 93 | 2218 | 96 | −1.94 | ||
| 15b | β-D-Val | 1.02 | 1.68 | 159 | 1/1.6/156 | 2.38 | 101 | 1.30 | 99 | 1278 | 98 | −1.94 | ||
| 16a | α-D-Phe | 0.61 | 3.69 | 76.4 | 1/0.8/71 | 5.33 | 86 | 3.57 | 96 | 282 | 59 | −1.26 | ||
| 18a | α-L-Abu | 0.76 | 37.5 | 144 | 1/49/189 | 5.12 | 88 | 98.2 | 104 | 942 | 61 | −2.34 | ||
| 18b | β-L-Abu | 1.83 | 1.30 | 201 | 1/0.7/110 | 5.47 | 83 | 2.22 | ||||||
| Compound | Amino Acid Substitution at Position 6 |
Radiant Heat Tail-Flick Test (Rat) ED50 (nmol/kg, s.c.) |
Writhing Assay (Mouse) ED50 (µg/kg, s.c.) |
Formalin Test (Rat) ED50 (nmol/kg, s.c.) |
|
|---|---|---|---|---|---|
| Phase I | Phase II | ||||
| 1/6.0/125 |
| 0.77 |
| 96 |
| 8.36 |
| 95 |
| 215 |
| 80 |
| −1.13 |
| 16b |
| β-D-Phe |
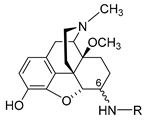
| Compound | R, Amino Acid Substitution at Position 6 |
Radiant Heat Tail-Flick Test (Rat), ED50 | Ratio ED50 (nmol/kg, s.c.)/ ED50 (nmol/rat, i.c.v.). |
|
|---|---|---|---|---|
| s.c. (nmol/kg) | i.c.v. (nmol/rat) | |||
| Morphine | 6053 | 35.1 | 172 | |
| Fentanyl | 38.6 | 1.66 | 23 | |
| 14-OMO (1) | 14.9 | 0.172 | 87 | |
| HS-730 (2a) | α-Gly | 58.5 | 0.031 | 1887 |
| HS-731 (2b) | β-Gly | 29.0 | 0.030 | 967 |
| HS-935 (3a) | α-L-Ala | 68.9 | 0.121 | 569 |
| HS-936 (3b) | β-L-Ala | 53.4 | 0.082 | 651 |
| HS-937 (4a) | α-L-Phe | 315 | 0.063 | 5000 |
| HS-938 (4b) | β-L-Phe | >3600 | 0.776 | >4600 |
2.2.2. 6-O-Sulfate Esters of Morphine and Codeine
Further chemical strategies to limit the penetration of morphinans from the periphery to the CNS following systemic administration include 6-O-sulfation. Sulfate conjugation generally leads to an increase in the water solubility of the compounds (fully ionized at neutral pH) yet is a biotransformation step in humans to facilitate the excretion of xenobiotics, including opioids [49]. Therefore, 6-O-sulfate esters of morphine and codeine were synthesized, such as morphine-6-O-sulfate (M6SU) [50][51][50,51], codeine-6-O-sulfate (C6SU) [50][51][52][50,51,52], 14-methoxymorphine-6-O-sulfate (14-O-MeM6SU) [53], 14-methoxycodeine-6-O-sulfate (14-O-MeC6SU) [53] and others [51][54][55][51,54,55]. For the synthesis of sulfate esters, a number of procedures have been reported and reviewed [56][57][56,57]. The most common methods are the reaction of phenol or alcohol with chlorosulfonic acid in pyridine, and with trimethylamine-SO3 complex or pyridine-SO3 complex in DMF, 1,4-dioxane or pyridine. The early synthesis of M6SU and C6SU was based on employing chlorosulfonic acid as a sulfonating reagent [50]. Other synthetical procedures of sulfate esters using pyridine-SO3 complex were reported for morphine derivatives [51][55][51,55]. For example, preparation of 6-O-sulfate esters of morphine and codeine, M6SU and C6SU, respectively, was achieved by means of the sulfation of the C-6 hydroxyl function with pyridine-SO3 complex (Scheme 5) [51].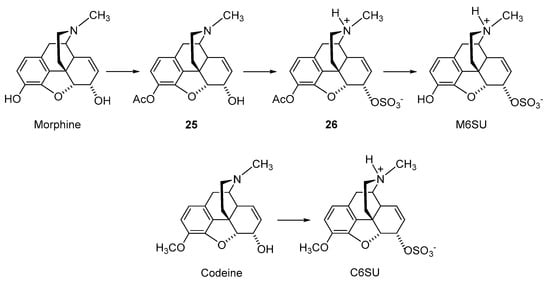
| Compound | Opioid Receptor Binding, Ki (nM) a | |||
|---|---|---|---|---|
| MOR | DOR | KOR | Ki Ratio MOR/DOR/KOR |
|
| Morphine | 4.37 | 2951 | 113 | 1/675/26 |
| Codeine | 737 | - b | - | - |
| M6SU | 11.5 | 525 | 275 | 1/46/24 |
| C6SU | 96.9 | 968 b | - | 1/10/- |
| 14-O-MeM6SU | 1.12 | 10.2 | 295 | 1/9/263 |
| 14-O-MeC6SU | 3.37 | 346 | 246 | 1/103/73 |
| Compound | MVD Bioassay | [35S]GTPγS Binding Assay | ||
|---|---|---|---|---|
| EC50 (nM) | Tissue | EC50 (nM) | Emax (%) | |
| Morphine | 347 | Rat brain Guinea-pig brain |
250 462 |
129 119 |
| Codeine | >1000 | Rat brain Guinea-pig brain |
- a - |
110 104 |
| M6SU | 103 | Rat brain Guinea-pig brain |
105 - |
133 - |
| C6SU | >1000 | Rat brain Guinea-pig brain |
>10,000 - |
121 102 |
| 14-O-MeM6SU | 4.38 | Rat brain Guinea-pig brain |
19.1 - |
201 - |
| 14-O-MeC6SU | 238 | Rat brain Guinea-pig brain |
301 >1000 |
128 130 |
| Compound | Pain Model (Species) | ED50 (Systemic Administration) | ED50 (Central Administration) | Reference |
|---|---|---|---|---|
| Morphine | hot-plate test (mouse) | 4.5 mg/kg, s.c. | [50] | |
| hot water tail-flick test (rat) | 3.41 mg/kg, i.p. | [64] | ||
| radiant heat tail-flick test (mouse) | 5.5 nmol/mouse, i.c.v. | [59] | ||
| radiant heat tail-flick test (mouse) | 314 ng/mouse, i.c.v. | [52] | ||
| radiant heat tail-flick test (rat) | 6221 nmol/kg, s.c. | 38.6 nmol/rat, i.c.v | [58] | |
| radiant heat tail-flick test (rat) | 2.68 mg/kg, i.p. | 3.51 µg/rat, i.t. | [60] | |
| radiant heat tail-flick test (rat) | 9.1 mg/kg, p.o. | [60] | ||
| [ | ||||
| 53 | ||||
| ] | ||||
| M6SU | hot-plate test (mouse) | 1.7 mg/kg, s.c. | [50] | |
| hot water tail-flick (rat) | 0.82 mg/kg, i.p. | [63] |
| Compound | Pain Model (Species) | ED50 Ratio Peripheral/Central Administration |
Reference | ||||
|---|---|---|---|---|---|---|---|
| Fentanyl | radiant heat tail-flick test (rat) | 23 | [50] | ||||
| acetic-acid-induced writhing assay (mouse) | |||||||
| 238.6 nmol/kg, i.p. | |||||||
| 2.02 nmol/mouse, i.c.v. | |||||||
| [ | 61 | ] | |||||
| formalin-induced inflammatory pain (rat) | Phase II: 0.259 mg/kg, i.p. | [61] | |||||
| formalin-induced inflammatory pain (rat) | |||||||
| radiant heat tail-flick test (mouse) | |||||||
| 0.19 nmol/mouse, i.c.v. | |||||||
| [ | 59 | ] | |||||
| radiant heat tail-flick test (mouse) | 10.6 ng/mouse, i.c.v. | [52] | |||||
| radiant heat tail-flick test (rat) | |||||||
| Morphine | radiant heat tail-flick test (rat) | 172 | [40] | ||||
| 159 | [58 | 9305 nmol/kg, s.c. | 0.356 nmol/rat, i.c.v. | [58] | |||
| radiant heat tail-flick test (rat) | 0.54 mg/kg i.p. | 0.29 μg/rat, i.t. | [60] | ||||
| ] | radiant heat tail-flick test (rat) | 4.97 mg/kg, p.o. | [60] | ||||
| paw pressure threshold test (rat) | 2.3 mg/kg, i.p. | [64] | |||||
| acetic-acid-induced writhing assay (mouse) | 1993 nmol/kg, s.c. | 9 pmol/mouse, i.c.v. | [62] | ||||
| formalin-induced inflammatory pain (rat) | Phase II: 0.094 mg/kg, i.p. | [60] | |||||
| CFA-induced inflammatory pain—paw pressure test (rat) | 292 nmol/kg, s.c. | [62] | |||||
| neuropathic pain, CCI (rat) hyperalgesia allodynia |
0.40 mg/kg, i.p. 0.19 mg/kg, i.p. |
[60] | |||||
| STZ-induced diabetic neuropathic pain—tail withdrawal test (rat) | 0.35 mg/kg, i.p. | [63] | |||||
| C6SU | radiant heat tail-flick test (mouse) | 200 ng, i.c.v b | [52] | ||||
| radiant heat tail-flick test (rat) | weak effect (<20%), s.c. | [53] | |||||
| CFA-induced inflammatory pain—paw pressure test (rat) | 6.6 and 13.2 µmol/kg, s.c. a | [53] | |||||
| 14-O-MeM6SU | radiant heat tail-flick test (rat) | 182.4 nmol/kg, s.c. | 0.0157 nmol/rat, i.c.v. | [58] | |||
| acetic-acid-induced writhing assay (mouse) | 87 nmol/kg, s.c. | 1.7 nmol/mouse, i.c.v. | [62] | ||||
| 125 | [53] | ||||||
| acetic-acid-induced writhing assay (rat) | 118 | [61] | |||||
| M6SU | radiant heat tail-flick test (rat) | 25,493 | [58] | ||||
| acetic-acid-induced writhing assay (mouse) | 221,444 | [62] | |||||
| 14-O-MeM6SU | radiant heat tail-flick test (rat) | 11,615 | [58] | ||||
| acetic-acid-induced writhing assay (mouse) | 51,177 | [62] | Phase I and II: 3884, 7769, 15,538, 31,075 nmol/kg, s.c. a | [65] | |||
| 14-O-MeC6SU | radiant heat tail-flick test (rat) | 314 | [53] | neuropathic pain, CCI (rat) hyperalgesia allodynia |
2.65 mg/kg, i.p. 1.45 mg/kg, i.p. |
[60] | |
| STZ-induced diabetic neuropathic pain— tail withdrawal test (rat) |
6.47 mg/kg, i.p. | [63] | |||||
| STZ-induced diabetic neuropathic pain— paw withdrawal test (rat) |
40,000 nmol/kg, s.c. a | [66] | |||||
| Codeine | radiant heat tail-flick test (rat) | 54.01 µmol/kg | formalin-induced inflammatory pain (rat) | Phase I: 3506 and 1012 nmol/kg, s.c. a Phase II: 253, 506 and 1012 nmol/kg, s.c. a |
[65] | ||
| CFA-induced inflammatory pain—paw pressure test (rat) | 45 nmol/kg, s.c. | [62] | |||||
| STZ-induced diabetic neuropathic pain—paw withdrawal test (rat) | 253, 506 and 1012 nmol/kg, s.c. a | [66] | |||||
| 14-O-MeC6SU | radiant heat tail-flick test (rat) | 5.34 µmol/kg, s.c. | 0.017 µmol/animal, i.c.v. | [53] | |||
| CFA-induced inflammatory pain—paw pressure test (rat) | 6.1 and 12.2 µmol/kg, s.c. a | [53] |
| 1.28 | ||||||||||||
| 1.19 | ||||||||||||
| 139 | ||||||||||||
| 1/0.9/109 | ||||||||||||
| 0.68 | ||||||||||||
| 78 | ||||||||||||
| 1.71 | ||||||||||||
| 96 | ||||||||||||
| 611 | ||||||||||||
| 92 | ||||||||||||
| −1.13 | ||||||||||||
| 17a | ||||||||||||
| α-L-Chg | ||||||||||||
| 1.23 | ||||||||||||
| 14.3 | ||||||||||||
| 177 | ||||||||||||
| 1/12/144 | 2.88 | 86 | 20.5 | 101 | 250 | 52 | −1.26 | |||||
| 100 | ||||||||||||
| 572 | 72 | −2.34 | ||||||||||
| 19a | α-β-Ala | 1.30 | 60.0 | 182 | 1/46/140 | 3.52 | 99 | 96.4 | 97 | 186 | 78 | −3.18 |
| 19b | β-β-Ala | 1.04 | 13.9 | 71.4 | 1/13/69 | 5.74 | 78 | 20.1 | 99 | 622 | 67 | −3.18 |
| 20a | α-GABA | 0.77 | 12.5 | 45.6 | 1/16/59 | 2.88 | 103 | 34.4 | 103 | 2034 | 104 | −2.93 |
| 20b | β-GABA | 1.41 | 6.61 | 147 | 1/4.7/104 | 12.3 | 86 | 6.06 | 103 | 3396 | 74 | −2.93 |
| 21a | α-L-Val-L-Tyr | 0.82 | 1.19 | 69.0 | 1/1.5/84 | 0.89 | 84 | 1.16 | 88 | 330 | 50 | −1.02 |
| 21b | β-L-Val-L-Tyr | 0.44 | 1.38 | 390 | 1/3.1/886 | 0.16 | 73 | 1.56 | 89 | 1884 | 63 | −1.02 |
| 22a | β-Gly-Gly | 4.62 | 7.52 | 203 | 1/1.6/44 | 4.39 | 85 | 2.84 | 102 | 885 | 75 | −4.27 |
2.2.3. Morphine-6-glucuronide
Peripheral restriction can also be achieved by O-glucuronidation at position 6 in the morphinan skeleton. A prominent example is morphine-6-glucuronide (M6G) (Figure 45), the active metabolite of morphine and a potent agonist to the MOR [47][71][47,71]. Approximately 10% of morphine is metabolized to M6G [72]. M6G contributes to the clinical analgesic effect of morphine, showing equivalent analgesia but with an improved side effect profile compared to that of morphine [73][74][75][73,74,75].
2.3. Nanocarrier-Based Approaches of Drug Delivery
A promising strategy to alter the pharmacokinetic profile and improve therapeutic effects of drugs is nanotechnology, that is, the use of biocompatible nanocarriers, including nanoparticles, liposomes, nanocapsules, micelles, dendrimers and nanotubes that may carry different therapeutic agents (for reviews, see [83][84][85][86][87][88][89][85,86,87,88,89,90,91]). The advantage of such nanocarriers is the direct delivery of drugs to the region or cells of interest, improved efficacy and decreased risk of negative side effects. Furthermore, carriers should be biologically stable, should protect the drug from degradation and the host body from toxic side effects, and should be able to deliver the loaded drug specifically to the target cell population in vivo (for reviews, see [83][84][85][86][87][88][89][85,86,87,88,89,90,91]). Nanotechnology has been extensively examined for tumor-directed delivery of chemotherapeutics to reduce their off-target toxicity [87][89], and has also been proposed for pain management [88][89][90,91]. Recently, a nanocarrier-based approach was developed that uses hyperbranched, dendritic polyglycerols (PG) to selectively deliver morphine to peripheral inflamed tissue [90][92]. Morphine was covalently bound to PG, via a cleavable ester linker sensitive to esterases and low pH. The rationale was that due to its high molecular weight and hydrophilicity, the i.v. injection of PG-morphine will not cross the BBB, but will selectively extravasate from leaky blood vessels characteristic of inflamed tissue. The local low pH and leukocyte esterases will then trigger the release of morphine from PG-morphine to reduce pain behavior [90][92]. In radioligand binding studies using human embryonic kidney (HEK) 293 cells stably expressing the rat MOR, PG-morphine was 10,000 times less effective than morphine (IC50 of 18.2 µM vs. 0.002 µM, respectively), indicating that PG-M does not bind to the MOR [90][92]. Different to morphine, dosages containing equivalent amounts of morphine-free PG-morphine exclusively activated peripheral opioid receptors following systemic i.v. administration in rats with unilateral hind paw inflammation. PG-M selectively induced antinociception in the inflamed paw in a naloxone-methiodide-dependent manner. Free morphine was only detected in inflamed paw tissue, but not in the contralateral, non-inflamed paw tissue, blood and brain of rats [90][92]. Furthermore, PG-M was reported not to cause sedation and constipation at antinociceptive doses after i.v. injection in rats with inflammatory pain [90][92]. However, the organ toxicity and broader side effect profile, including abuse potential and effects on respiration of PG-M were not reported yet to strengthen the clinical applicability of this strategy, which is able to deliver morphine exclusively in injured tissue, precluding not only CNS side effects but also constipation. Further, morphine-loaded hydrogels have been reported. Preclinical studies demonstrated that peptide-based hydrogels loaded with morphine as new controlled-drug delivery systems produced effective, sustained antinociceptive effects in mice after s.c. administration, and no sedative effects were observed [91][92][93,94].3. Conclusions
In the current context of the ‘opioid crisis’, the development of new opioid analgesics with improved pharmacology (i.e., efficacy in various pain conditions and reduced capability of inducing unwanted side effects) is of crucial clinical and public health attention. Diverse opioid analgesic discovery efforts are therefore made towards identifying effective and well-tolerated opioids that have an improved benefit/risk ratio compared with currently available drugs.
The basis for drug discovery targeting peripheral opioid receptors is supported by the knowledge that opioid receptors are expressed in the CNS, PNS and peripheral tissues. Furthermore, activating opioid receptors in the periphery leads to an effective analgesic response, and the most serious opioid-related adverse effects (i.e., apnea, sedation, physical dependence and addiction) are due to the activation of opioid receptors in the CNS. Thus, peripherally restricted opioids are viewed as viable targets to avoid many of the lethal side effects associated with opioids targeting the CNS.
In this rentry, diview, we discussed different peripheralization strategies applied to opioids are discussed. Emphasis was placed on the morphinan class of opioid ligands represented by morphine and its structurally related analogues that are used extensively not only clinically but also as experimental tools and that are important as scaffolds for the design of new ligands. Broad chemical and pharmacological work was performed on modifications of the morphinan scaffold to reduce the ability to cross the BBB, and substantial achievements have been made in the field, increasing the feasibility for clinical application. ResearchersWe discussed chemical variations on the morphinan skeleton to increase the hydrophilicity, such as quaternization of the morphinan nitrogen (N17), the introduction of polar/ionizable substituents at C-6 position (i.e., amino acid, sulfation and glucuronidation), and nanocarrier-based approaches to selectively deliver morphine to peripheral tissue. Although the available preclinical and clinical data are favorable to the potential use of these compounds, their clinical impact, and the extent to which they will replace existing opioids, needs further investigations.
