Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Michał Musiał and Version 2 by Conner Chen.
The main factors determining the efficiency of heat storage systems are their cost, the efficiency of their heat storage and distribution, and, indirectly, their environmental impact. The important factors determining the efficiency of the heat accumulator are enthalpy of the phase change, heat transferability, chemical neutrality, low flammability, and low toxicity.
- autonomous buildings
- composite thermal energy storage
- sorption storage
1. Introduction
The development of modern energy-efficient construction engineering has been determined by changes occurring during building design. A logical choice is to increase the use of thermal energy from renewable sources for building heating, to reduce the heat losses, and, ultimately, to reduce the building’s demand for heat or cold. In this context, a satisfactory solution in terms of architectural and construction design of a building is to achieve a state where the direct and indirect solar radiation gains and internal gains from the building’s operation make up for the heat losses due to penetration and building ventilation. Nevertheless, when striving to achieve low-emission buildings, their functioning across their entire life needs to be considered.
In addition to their obvious energy efficiency, autonomous buildings are powered with energy from renewable sources and, characteristically, are as independent as possible from an outside energy and matter supply throughout their lives. Such buildings are also analysed from the perspective of the construction materials used and the way that they relate to closed-loop systems; these analyses use the life cycle cost (LCC), life cycle assessment (LCA), and life sustainability cost analysis (LSCA) methods [1][2][3][4][5][6][1,2,3,4,5,6]. In this context, autonomous buildings fulfil the criteria of sustainable growth [7][8][9][10][11][7,8,9,10,11] with regard to reductions in the consumption of non-renewable resources and their replacement with renewable resources, reductions in waste production and pollution, and the consumption of renewable resources no faster than they can be replenished.
1.1. Thermal Energy Acquisition and Distribution Methods from Renewable Sources
Renewable energy sources are naturally renewed during their use. These are supplies that are replenished as quickly as they are consumed. By definition, renewable energy (electricity or heat) is produced from solar, wind, water, geothermal energy, biomass, biofuels, biogas, or hydrogen obtained from renewable sources.- Solar energy. Most renewable sources are directly or indirectly dependent on the sun. Most of the direct gains are absorbed at latitudes around the equator, but this energy is then dispersed across the planet in the form of winds and ocean currents.
- Wind energy. Air currents can be captured and used to drive wind turbines. Wind energy shows the fastest growth among all renewable sources.
- Hydropower. We can also obtain energy from water, based on either its movement or its temperature differences.
- Geothermal energy. This is obtained by capturing the heat of the earth itself, usually from depths of up to several kilometres below its surface. It is an expensive source of renewable energy.
- Biomass. We know different forms of solid biomass: wood fuel, organic components of municipal waste, or unused parts of agricultural crops. Most types of biomass contain usable energy.
- Biofuels. Liquid biofuels are generally bioalcohols (e.g., bioethanol) or biooils (biodiesel or pure vegetable oils). Their biggest advantage is the lower emissions.
- Biogas. This can easily be produced from biologically active waste substances that arise, for example, from the production of paper or sugar and from sewage, animal waste, and other substances. These various wastes must be allowed to settle together and to undergo natural fermentation to produce methane.
-
Solar energy. Most renewable sources are directly or indirectly dependent on the sun. Most of the direct gains are absorbed at latitudes around the equator, but this energy is then dispersed across the planet in the form of winds and ocean currents.
-
Wind energy. Air currents can be captured and used to drive wind turbines. Wind energy shows the fastest growth among all renewable sources.
-
Hydropower. We can also obtain energy from water, based on either its movement or its temperature differences.
-
Geothermal energy. This is obtained by capturing the heat of the earth itself, usually from depths of up to several kilometres below its surface. It is an expensive source of renewable energy.
-
Biomass. We know different forms of solid biomass: wood fuel, organic components of municipal waste, or unused parts of agricultural crops. Most types of biomass contain usable energy.
-
Biofuels. Liquid biofuels are generally bioalcohols (e.g., bioethanol) or biooils (biodiesel or pure vegetable oils). Their biggest advantage is the lower emissions.
-
Biogas. This can easily be produced from biologically active waste substances that arise, for example, from the production of paper or sugar and from sewage, animal waste, and other substances. These various wastes must be allowed to settle together and to undergo natural fermentation to produce methane.
1.2. ZeroEnergy and Autonomous Buildings
Zeroenergy buildings (ZBs) are designed and built to use as little energy as possible. When renewable energy is added to these buildings, they are able to produce enough energy to meet or exceed their operating requirements. The idea of zeroenergy buildings is based on ensuring that the heat gains are equal to the heat losses generated during their operation. This is achieved by reducing heat losses through the thermal enclosure and ventilation. This should be accompanied by maximum heat gains from solar radiation and the profits from building equipment and users. Autonomous buildings (ABs) are designed to be energy self-sufficient. They require the unique modelling and engineering of a forward-looking building with renewable technology integration, clean energy storage, and demand reduction as focal points. Moreover, the AB is not only a zero carbon and zero energy building, but also has zero grid connection and zero energy bills (ZZZZ)—engineering zero. These are original and innovative approaches, leading to practical solutions to national environmental challenges, such as climate change, air quality, green energy production, and local management. The study found that ABs are more expensive than traditional buildings because they embody the integration of renewable technologies and highly energy-efficient materials; yet, they offer the best engineering services and products, which can raise the bar for Kuwaiti villas and provide multiple solutions: increased housing, increases in green energy production, increases in air quality, and significant reductions in CO2 emissions, along with the saving of money and the built environment. An extension of the idea of zeroenergy buildings is the concept of netzeroenergy buildings (NZEB). This is based on the aim of increasing the energy self-sufficiency of a building. The NZEB concept includes the achievement of a zero-heat balance for the building as well as the full renewability of the energy sources required to power the auxiliary equipment for the heating system and everyday appliances. The construction of such buildings relies on an extensivephotovoltaic (PV) system and small wind turbines. Legal acts specifying the energy performance building design (EPBD) take into consideration only the energy demand for the heating, cooling, and, sometimes, lighting of buildings. Such acts omit the energy needed to power home appliances and the aspects related to the temporary thermal comfort of building users. Unfortunately, the temporary sense of satisfaction with the thermal conditions inside the building determines the actual demand for electricity, heat, and cold, such as by opening the window when the ventilation system is temporarily inefficient. Furthermore, an important factor that is currently not considered when establishing the building energy performance is the formingof building heat balances on an average monthly, not hourly, basis, which neglects the effects of the demand for heat and cooling that vary over the day. The above deficiencies in the procedures for assessing the energy efficiency of buildings lead to major discrepancies between the theoretical and the actual performance. Therefore, heat storage in the building structure can reduce the temporary demand for heat and cooling by the building users. Such actions significantly contribute to reducing the actual energy demand of the building by improving the thermal comfort of its users.2. Conventional Possibilities, Advantages, and Disadvantages of Heat Storage in Building Elements
The main factors determining the efficiency of heat storage systems are their cost, the efficiency of their heat storage and distribution, and, indirectly, their environmental impact. The important factors determining the efficiency of the heat accumulator are enthalpy of the phase change, heat transferability, chemical neutrality, low flammability, and low toxicity. The most important property is the heat capacity of the substance, which directly affects the amount of accumulated heat in the substance in relation to the volume. The higher the heat capacity, the smaller the volume of the substance needed to accumulate a certain amount of heat. Thermal conductivity is also an important property; it affects the transfer of heat at the interface between the substance that accumulates heat and the heat-carrying substance that distributes the heat from the accumulation system to the point of consumption. Higher thermal conductivity ensures better heat transfer and increased efficiency. Last, but not least, the reversibility of the substance is also important, i.e., the ability to heat/cool repeatedly without degrading the material. This property is extremely important for substances that change states in the heating or cooling process. In the accumulation of sensible heat, thermal energy is stored during the heating of a substance that has suitable properties for these purposes. Most often, water, which has a high heat capacity (4.18 kJ/(kg·K)), is used to accumulate thermal energy in the form of sensible heat. The advantages of this system mainly include low investment costs, the non-toxicity of the heat storage substance, and the available range of hot water storage tanks. The fact that the thermal capacity of the accumulated sensible heat is limited can be considered a negative. In order to store a large amount of energy, a large storage volume is theoretically required, which reduces the efficiency in terms of heat loss. It is also true that increasing the temperature of the substance in order to store more heat increases the heat loss at the interface of the material with the surrounding environment, which reduces the efficiency of the process. Heat storage using phase changes is a method frequently discussed in the scientific literature. The advantages of this method are high heat storage density, almost isothermal storage, and the capability to enable wide application in the building structure. Nevertheless, the most commonly used groups of phase change materials are often characterised by a limited heat transfer capacity when they are in the solid state, with a thermal conductivity of 0.2–0.7 W/m·K. This problem has been addressed by adding thermal conductors, such as copper and aluminium alloys or carbon fibres with a thermal conductivity of 200, 370, or 470 W/m·K, respectively. There are various heat storage materials. With each method of heat accumulation, it is advisable to use different materials and to consider their physical and chemical properties. Water, oils (with the ability to accumulate higher temperatures), and solid substances (aggregates and concrete) are mainly used to accumulate sensible heat. The advantages of using solid substances are the elimination of the risk of liquid substance leakage and their affordability, although their heat capacity is significantly lower compared to that of liquids. The accumulation of latent heat mainly involves the use of organic (paraffins, fatty acids) and inorganic substances (inorganic salts). The melting point of paraffins is in the range of 12–71 °C, while inorganic salts are in the range of 30–120 °C. The disadvantages of organic substances are their flammability and low thermal conductivity, which limits their use. For the accumulation of thermal energy bound in a chemical reaction, many organic and inorganic substances can be used which primarily meet the perfect reversibility of the chemical reaction. Currently, there are many systems that use accumulated solar energy. Its use finds application in heating applications (hot water heating, radiant heating), but also for technological purposes and electricity production. In practice, the most widespread is the heating of drinking water and the accumulation of energy in the form of sensible heat in hot water tanks. However, for the needs of heating and seasonal accumulation, the systems are still limited in terms of efficiency and financial return. Inorganic salts, which are able to accumulate thermal energy, appear to be an advantageous solution, but their use is limited due to their low thermal conductivity. To optimize this method, it is necessary to use measures that increase the thermal conductivity and thus the overall efficiency of the heat accumulation system. Currently, the accumulation of heat in the form of group heat can mainly be solved experimentally, although putting it into practice still requires further research. Similarly, the method of heat accumulation in the form of a chemical reaction is currently the subject of experiments, and so far, it appears to be a highly advantageous process in terms of the efficient storage of a large amount of heat in a small volume with minimal heat loss. The literature describes methods to better systematise the options for thermal energy storage in the structures of different materials; Figure 12 shows a diagram of examples.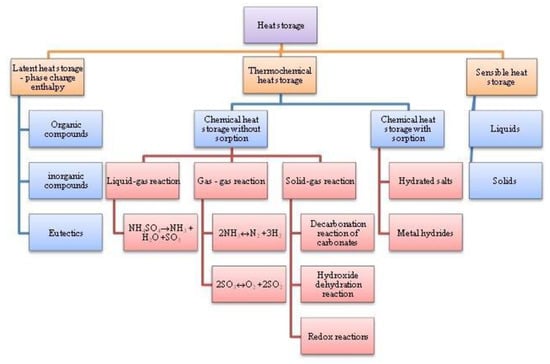
Figure 12.
Summary of thermal energy storage systems in terms of material structures and chemical compounds.
