Electrochemical energy storage and conversion systems such as electrochemical capacitors, batteries and fuel cells are considered as the most important technologies proposing environmentally friendly and sustainable solutions to address rapidly growing global energy demands and environmental concerns. Their commercial applications individually or in combination of two or more devices are based on their distinguishing properties e.g., energy/power densities, cyclability and efficiencies.
- Electrochemical energy storage and conversion syst
- electrochemical capacitors
- batteries
- fuel cells
Dear author, the following contents are excerpts from your papers. They are editable.
(Due to the lack of relevant professional knowledge, our editors cannot complete a complete entry by summarizing your paper, so if you are interested in this work. you may need to write some contents by yourself. A good entry will better present your ideas, research and results to other scholars. Readers will also be able to access your paper directly through entries.)
1. Introduction
Comprehensive classification of electrochemical energy storage, conversion systems is shown in Figure 1, explain their basic working principles, and technical characteristics, highlight the distinctive properties of each system, and discuss their fields of application. A diverse range of energy storage and conversion devices is shown in Figure 1 based on their energy delivery time varying with the type of mechanism involved in energy storage or conversion systems. For example, electrochemical capacitors are considered as high power density devices and their delivery time is in the range of few seconds to minutes since these devices utilise only the material on the electrode surface unlike batteries or fuel cells where bulk of the material is involved in energy storage and conversion respectively. Other characteristics of these devices vary as well due to the fundamental difference in the mode of energy storage or conversion (physical/electrochemical). In case of electrochemical capacitors, most of the commercially used devices use electric double layer charge storage phenomenon, which results in inferior energy densities as compared to other electrochemical energy storage or conversion devices shown in Figure 2.
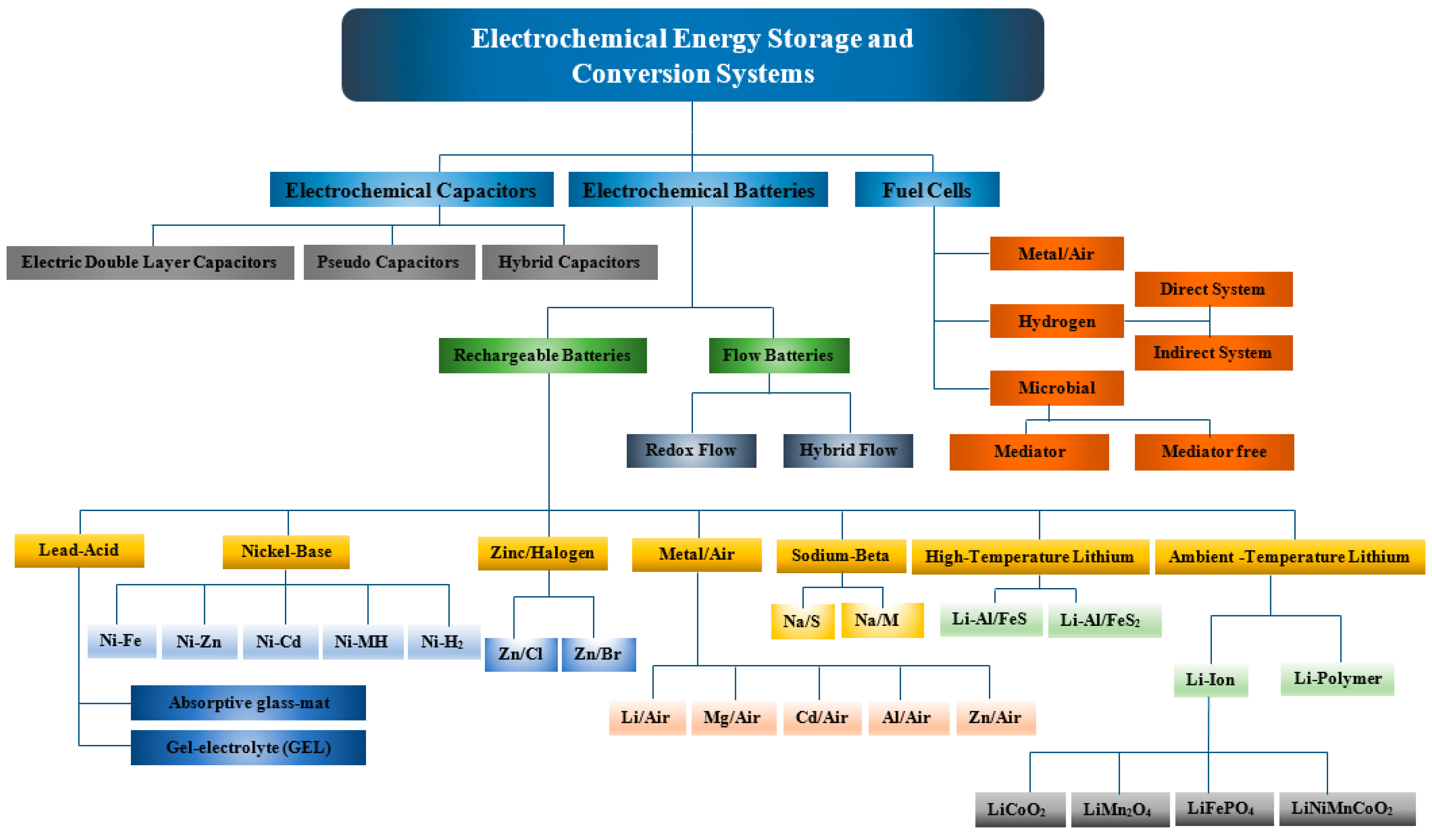
Electrochemical capacitors/batteries and fuel cells are key electrochemical energy storage and conversion technologies respectively, used in commercial applications with their particular selection dependent on performance limitations such as energy densities, power densities, and cycle life.
Electrochemical batteries and fuel cells are considered as high energy density devices with typical gravimetric energy densities in the range of 100–200 Wh kg−1 and 600–1200 Wh kg−1 respectively, whereas current ECs have significantly lower energy densities with typical values typically between 0.05–30 Wh kg−1 [12][1]. However, ECs are considered as high-power density devices with very short charge/discharge times (of the order of seconds) which is difficult to achieve by other electrochemical energy storage and conversion devices. Figure 2 shows a comparison of specific energy, specific power and their delivery timescale for different energy storage and conversion devices. At present, none of these devices has the capability to meet the wide spectrum of requirements demanded by the diverse range of renewable energy sources such as wind, tidal and solar. However, they can respond to a broad rang requirements such as fast charge/discharge, peak power demands and high energy storage needs over a longer period of time when used in a combination of two or more.
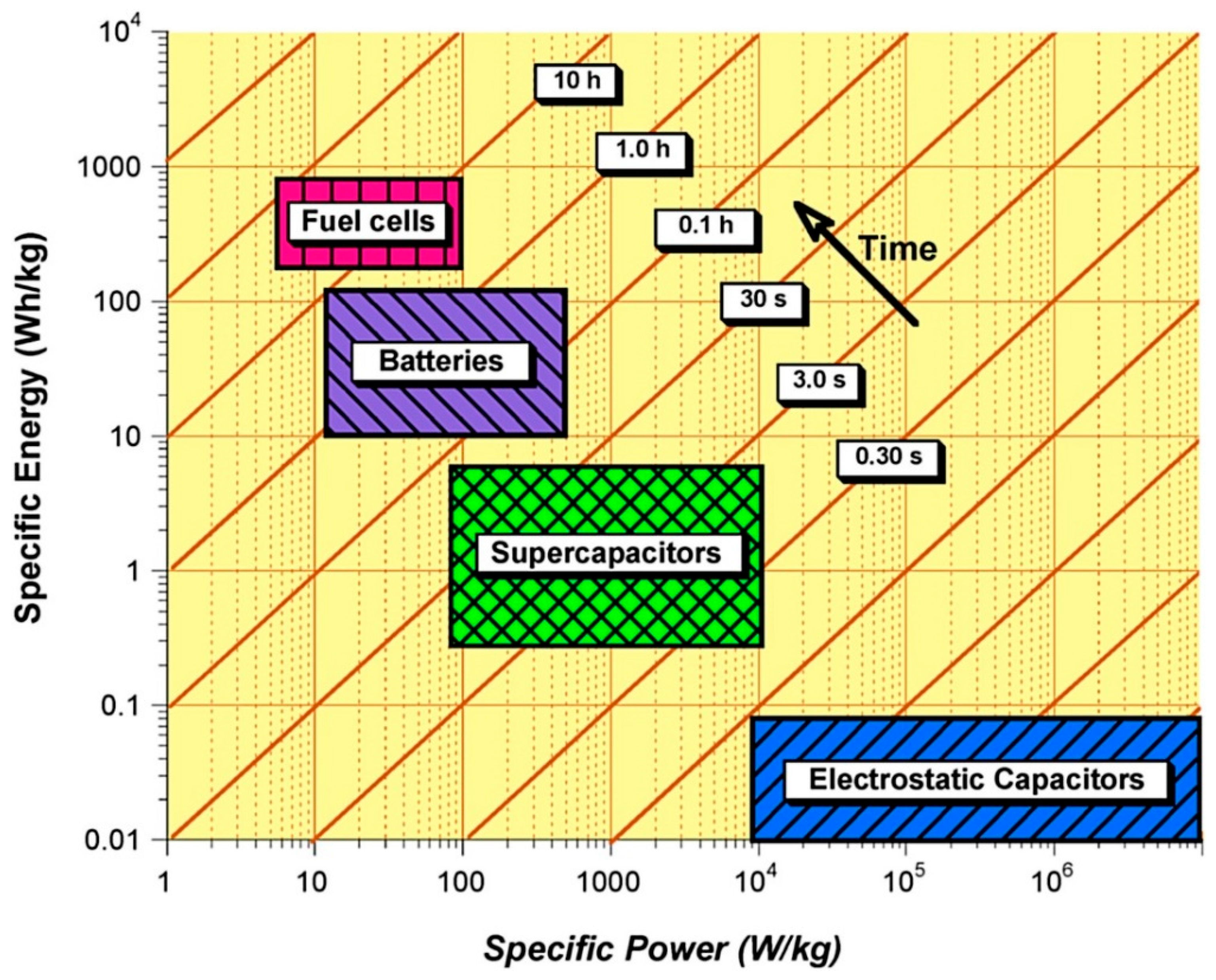
2. Comparison of Different Electrochemical Energy Storage and Conversion Systems
Energy storage/conversion devices perform two important tasks through time shifting bulk energy from renewables production to time of energy demand (supplied by batteries + fuel cells) and by production of clean, stable power and frequency, avoiding voltage spikes whish are important for digital economy by supercapacitors and high power batteries. Table 1 compares a number of fundamental characteristics of different electrochemical energy storage and conversion devices, such as electrochemical capacitors, batteries and fuel cells. It can be seen that electrochemical batteries and fuel cells are high energy density devices with typical gravimetric energy densities in the range of 75–200 Wh kg-1 and 800–1000 Wh kg-1 respectively. Conversely, electrochemical capacitors possess much lower energy densities, ranging from 10–230 Wh kg−1, particularly in commercialised electric double layer capacitors where energy densities are in the range of 5–10 Wh kg−1. Nevertheless, for high power density applications (i.e., power fluctuations, load shifting and short-term storage requiring fast charge/discharge). Furthermore, ECs are beneficial in applications requiring long cycle life and high efficiencies including backup power, safety and low maintenance applications such as uninterruptible power supplies (UPS) which results in improvements in power quality. However, the higher level of self-discharge in electrochemical capacitors when compared with other electrochemical devices is one of the major drawbacks which restricts their wider applications. On the contrary, electrochemical batteries and fuel cells are predominantly useful power sources for high energy density applications where the delivery is needed over longer period of time. Electrochemical batteries are more desirable in transportation and portable electronics applications since the technology is fully grown compared to FCs and ECs whereas other applications such as micro-grids, medical and power source for remote military installations where high power delivery is required over a sustained period with long cycle life is very vital makes FCs more desirable choice. Other benefits using fuel cells is the use of secondary heat generated during their operations in applications such for combined heat and power (CHP) when driving micro gas turbines. Even though FCs are extremely useful power devices, they are still in testing phase and require R&D efforts to bring them in line with electrochemical batteries commercially. Electrochemical batteries are more useful and still maintain the highest market share in applications such as portable electronics, electric and hybrid electric vehicles due to scalability and the maturity of the technology [148,165,166,167][3][4][5][6].
Table 1. Technical characteristics of key energy storage and conversion technologies.
| Technology | Specific Energy (Wh kg−1) |
Specific Power (Wkg−1) |
Life-Time (Years) |
Cycle-Ability (Cycles) |
Cyclic Efficiency (%) |
Daily Discharge (%) |
|---|---|---|---|---|---|---|
| Different operating parameters of electrochemical capacitors | ||||||
| EDLCs | 6.8–12 [7] | 65–10,200 [8] | <30 [9][10] | Up to 500,000 [11] | 60–100 [12] | ~25 [13] |
| PCs | 23–67 [8] | 21,000–220 [8] | 5–9 [14] | Up to 5000 [12] | 52–96 [15][16] | |
| HCs | 132–231 [17] | 2800–57 [17] | <10 [18] | 12,000 [19] | 80–95 [20][21] | |
| Different operating parameters of Batteries | ||||||
| Lead-acid | 25–50 [22] | 10–400 [23] | 5–15 [24] | 200–1800 [25] | 63–90 [26] | 0.1–0.3 [23] |
| Li-ion | 75–200 [23] | 500–2000 [24] | 14–16 [27] | Up to 20,000 [28] | 75–90 [29] | 1–5 [25] |
| NiCd | 45–80 [30] | 150–300 [23] | 10–20 [23] | Up to 3500 [31] | 60–83 [26] | 0.2–0.6 [23] |
| NiMH | 145–152 [32][33] | 390–2000 [32][34] | <15 [35] | 40,000 [35] | 88–98 [32] | ~1 [36] |
| VRB | 10–30 [23] | 166 [37] | 5–20 [38] | ≤12,000 [23] | 75–85 [23][39] | Very low [25] |
| ZnBr | 30–80 [23] | 45–100 [40][41] | ~10 [27] | ≤2000 [60][23] | 66–80 [83][26] | Small [42] |
| PSB | 97–165 [43][44] | 77–83 [45][46] | 10–15 [23] | ≤2000 [47] | 93–95 [48] | ~Zero [38] |
| Different operating parameters of Fuel cells | ||||||
| SOFC | 800–1000 [49] | 200–1000 [50] | ~4.5 [51] | 50–1000 [52][53] | 35–45 [54] | |
| PEMFC | 500–1000 [55][56] | 90–1000 [57][58] | ~5 [59] | Up to 9000 [60] | 53–58 [54] | |
| DMFC | Up to 1500 [61] | ~1000 [62] | ~10 [63] | 300–10,000 [64][65] | ~40 [54] | ~Zero [66] |
| Technology | Specific Energy (Wh kg−1) |
Specific Power (Wkg−1) |
Life-Time (Years) |
Cycle-Ability (Cycles) |
Cyclic Efficiency (%) |
Daily Discharge (%) |
|---|---|---|---|---|---|---|
| Different operating parameters of electrochemical capacitors | ||||||
| EDLCs | 6.8–12 [40] | 65–10,200 [41] | <30 [168,169] | Up to 500,000 [170] | 60–100 [44] | ~25 [171] |
| PCs | 23–67 [41] | 21,000–220 [41] | 5–9 [172] | Up to 5000 [44] | 52–96 [49,51] | |
| HCs | 132–231 [42] | 2800–57 [42] | <10 [173] | 12,000 [46] | 80–95 [52,53] | |
| Different operating parameters of Batteries | ||||||
| Lead-acid | 25–50 [174] | 10–400 [60] | 5–15 [84] | 200–1800 [61] | 63–90 [83] | 0.1–0.3 [60] |
| Li-ion | 75–200 [60] | 500–2000 [84] | 14–16 [175] | Up to 20,000 [176] | 75–90 [89] | 1–5 [61] |
| NiCd | 45–80 [177] | 150–300 [60] | 10–20 [60] | Up to 3500 [178] | 60–83 [83] | 0.2–0.6 [60] |
| NiMH | 145–152 [179,180] | 390–2000 [179,181] | <15 [182] | 40,000 [182] | 88–98 [179] | ~1 [183] |
| VRB | 10–30 [60] | 166 [184] | 5–20 [185] | ≤12,000 [60] | 75–85 [60,186] | Very low [61] |
| ZnBr | 30–80 [60,187] | 45–100 [187,188] | ~10 [175] | ≤2000 [60] | 66–80 [83] | Small [189] |
| PSB | 97–165 [190,191] | 77–83 [192,193] | 10–15 [60] | ≤2000 [194] | 93–95 [195] | ~Zero [185] |
| Different operating parameters of Fuel cells | ||||||
| SOFC | 800–1000 [196] | 200–1000 [197] | ~4.5 [198] | 50–1000 [199,200] | 35–45 [127] | |
| PEMFC | 500–1000 [201,202] | 90–1000 [203,204] | ~5 [205] | Up to 9000 [206] | 53–58 [127] | |
| DMFC | Up to 1500 [207] | ~1000 [208] | ~10 [209] | 300–10,000 [210,211] | ~40 [127] | ~Zero [212] |
In case of electrochemical batteries short cycle life and inferior efficiencies are the drawbacks requiring attention. None of these technologies has the capability to fulfil all the requirements for very broad range of applications alone, a problem which can be overcome by using hybrid systems consisting of combination of two or more of these devices, with ability to respond to applications with more complex requirements (e.g., in EVs/HEVs where high energy density batteries or fuel cells are coupled with high power capable ECs). Such combinations will result in longer overall system life, performance improvements and design flexibility, since batteries or fuel cells will not be pushed to the limits of their power capabilities and edge of their stabilities and response time Comprehensive details of technical characteristics of different electrochemical energy storage and conversion devices are listed in Table 1 above.
References
- Ferreira, H.L.; Garde, R.; Fulli, G.; Kling, W.; Lopes, J.P. Characterisation of electrical energy storage technologies. Energy 2013, 53, 288–298.
- Mirzaeian, M.; Abbas, Q.; Ogwu, A.; Hall, P.; Goldin, M.; Mirzaeian, M.; Jirandehi, H.F. Electrode and electrolyte materials for electrochemical capacitors. Int. J. Hydrogen Energy 2017, 42, 25565–25587.
- Ong, B.; Kamarudin, S.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrogen Energy 2017, 42, 10142–10157.
- Wang, C.; Nehrir, M.H. Distributed Generation Applications of Fuel Cells. In Proceedings of the 2006 Power Systems Conference: Advanced Metering, Protection, Control, Communication, and Distributed Resources, Clemson, SC, USA, 14–17 March 2006; Institute of Electrical and Electronics Engineers (IEEE): Piscataway Township, NJ, USA, 2006; pp. 244–248.
- Ozawa, K. Lithium Ion Rechargeable Batteries: Materials, Technology, and New Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–329. ISBN 978-3-527.31983-1.
- Conway, B.E. Electrochemical Supercapacitors; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1999; pp. 1–698.
- Wei, J.-S.; Wan, S.; Zhang, P.; Ding, H.; Chen, X.-B.; Xiong, H.-M.; Gao, S.; Wei, X. Preparation of porous carbon electrodes from semen cassiae for high-performance electric double-layer capacitors. New J. Chem. 2018, 42, 6763–6769.
- Wang, G.; Oswald, S.; Löffler, M.; Müllen, K.; Feng, X. Beyond Activated Carbon: Graphite-Cathode-Derived Li-Ion Pseudocapacitors with High Energy and High Power Densities. Adv. Mater. 2019, 31, e1807712.
- Bohlen, O.; Kowal, J.; Sauer, D.U. Ageing behaviour of electrochemical double layer capacitors. J. Power Sour. 2007, 173, 626–632.
- Simon, P.; Brousse, T.; Favier, F. Supercapacitors Based on Carbon or Pseudocapacitive Materials; Wiley: New York, NY, USA, 2017.
- Miller, J.R.; Simon, P. Materials Science: Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652.
- Gao, H.; Li, J.; Miller, J.R.; Outlaw, R.A.; Butler, S.; Lian, K. Solid-state electric double layer capacitors for ac line-filtering. Energy Storage Mater. 2016, 4, 66–70.
- Ricketts, B.; Ton-That, C. Self-discharge of carbon-based supercapacitors with organic electrolytes. J. Power Sour. 2000, 89, 64–69.
- Zhang, Y.; Zhao, Y.; An, W.; Xing, L.; Gao, Y.; Liu, J. Heteroelement Y-doped α-Ni(OH)2 nanosheets with excellent pseudocapacitive performance. J. Mater. Chem. A 2017, 5, 10039–10047.
- Yang, H.-Z.; Zou, J.-P. Controllable preparation of hierarchical NiO hollow microspheres with high pseudo-capacitance. Trans. Nonferrous Met. Soc. China 2018, 28, 1808–1818.
- Cheng, Z.; Tan, G.; Qiu, Y.; Guo, B.; Cheng, F.; Fan, H. High performance electrochemical capacitors based on MnO2/activated-carbon-paper. J. Mater. Chem C 2015, 3, 6166–6171.
- Ahn, W.; Lee, D.U.; Li, G.; Feng, K.; Wang, X.; Yu, A.; Lui, G.; Chen, Z. Highly Oriented Graphene Sponge Electrode for Ultra High Energy Density Lithium Ion Hybrid Capacitors. ACS Appl. Mater. Interfaces 2016, 8, 25297–25305.
- Sasaki, M.; Araki, S.; Miyata, T.; Kawaji, T. Development of capacitor hybrid system for urban buses. JSAE Rev. 2002, 23, 451–457.
- Attias, R.; Hana, O.; Sharon, D.; Malka, D.; Hirshberg, D.; Luski, S.; Aurbach, D. Solid state synthesis of Li0.33MnO2 as positive electrode material for highly stable 2V aqueous hybrid supercapacitors. Electrochim. Acta 2017, 254, 155–164.
- Wang, P.; Wang, R.; Lang, J.; Zhang, X.; Chen, Z.; Yan, X. Porous niobium nitride as a capacitive anode material for advanced Li-ion hybrid capacitors with superior cycling stability. J. Mater. Chem. A 2016, 4, 9760–9766.
- Zeng, Z.; Wang, D.; Zhu, J.; Xiao, F.; Li, Y. NiCo2S4 nanoparticles//activated balsam pear pulp for asymmetric hybrid capacitors. CrystEngComm 2016, 18, 2363–2374.
- Martín, J.S.; Zamora, I.; Martín, J.S.; Aperribay, V.; Eguia, P. Energy storage technologies for electric applications. Renew. Energy Power Qual. J. 2011, 593–598.
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312.
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522.
- Díaz-González, F.; Sumper, A.; Gomis-Bellmunt, O.; Villafáfila-Robles, R. A review of energy storage technologies for wind power applications. Renew. Sustain. Energy Rev. 2012, 16, 2154–2171.
- Beaudin, M.; Zareipour, H.; Schellenberglabe, A.; Rosehart, W. Energy storage for mitigating the variability of renewable electricity sources: An updated review. Energy Sustain. Dev. 2010, 14, 302–314.
- Rydh, C.J.; Sandén, B.A. Energy analysis of batteries in photovoltaic systems. Part II: Energy return factors and overall battery efficiencies. Energy Convers. Manag. 2005, 46, 1980–2000.
- Author, N. Review of electrical energy storage technologies and systems and of their potential for the UK. EA Technol. 2004, 1, 34.
- Rastler, D. Electricity Energy Storage Technology Options: A White Paper Primer on Applications, Costs, and Options; Electric Power Research Institute (EPRI): Palo Alto, CA, USA, 2010; Available online: http://large.stanford.edu/courses/2012/ph240/doshay1/docs/EPRI.pdf (accessed on 17 October 2020).
- Baker, J. New technology and possible advances in energy storage. Energy Policy 2008, 36, 4368–4373.
- McDowall, J. Integrating energy storage with wind power in weak electricity grids. J. Power Sour. 2006, 162, 959–964.
- Ren, Z.; Yu, J.; Li, Y.; Zhi, C. Tunable Free-Standing Ultrathin Porous Nickel Film for High Performance Flexible Nickel-Metal Hydride Batteries. Adv. Energy Mater. 2018, 8, 1702467.
- Kong, L.; Li, X.; Liao, X.; Young, K.-H. A BCC-C14 alloy suitable for EV application of Ni/MH battery. Int. J. Hydrogen Energy 2019, 44, 29338–29343.
- Meng, T.; Young, K.-H.; Hu, C.; Reichman, B. Effects of Alkaline Pre-Etching to Metal Hydride Alloys. Batteries 2017, 3, 30.
- Shen, Y.; Noréus, D.; Starborg, S. Increasing NiMH battery cycle life with oxygen. Int. J. Hydrogen Energy 2018, 43, 18626–18631.
- Idowu, I.A. Feasibility Study on the Use of Recycled NIMH Batteries for Emergency Power during a Natural Disaster; ProQuest Dissertations Publishing LLC: Morgan State University, MD, USA, 2018.
- Barote, L.; Weissbach, R.; Teodorescu, R.; Marinescu, C.; Cîrstea, M. Stand-alone wind system with Vanadium Redox Battery energy storage. In Proceedings of the 2008 11th International Conference on Optimization of Electrical and Electronic Equipment, Brasov, Romania, 22–24 May 2008; Institute of Electrical and Electronics Engineers (IEEE): Berlin/Heidelberg, 2008; pp. 407–412.
- De León, C.P.; Ferrer Ángel, F.; García, J.G.; Szánto, D.; Walsh, F.C. Redox flow cells for energy conversion. J. Power Sour. 2006, 160, 716–732.
- Amodeo, S.J.; Chiacchiarini, H.; Solsona, J.; Busada, C.A. High-performance sensorless nonlinear power control of a flywheel energy storage system. Energy Convers. Manag. 2009, 50, 1722–1729.
- Pistoia, G. Electric and Hybrid. Vehicles; Elsevier BV: Amsterdam, The Netherlands, 2010.
- Li, P. Energy storage is the core of renewable technologies. IEEE Nanotechnol. Mag. 2008, 2, 13–18.
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613.
- Zhang, S.; Guo, W.; Yang, F.; Zheng, P.; Qiao, R.; Li, Z. Recent Progress in Polysulfide Redox-Flow Batteries. Batter. Supercaps 2019, 2, 627–637.
- Zhang, C.; Ding, Y.; Zhang, L.; Wang, X.; Zhao, Y.; Zhang, X.; Yu, G. A Sustainable Redox-Flow Battery with an Aluminum-Based, Deep-Eutectic-Solvent Anolyte. Angew. Chem. Int. Ed. 2017, 56, 7454–7459.
- Cheng, Y.; Zhang, H.; Lai, Q.; Li, X.; Shi, D.; Zhang, L. A high power density single flow zinc–nickel battery with three-dimensional porous negative electrode. J. Power Sour. 2013, 241, 196–202.
- Zhang, L.; Lai, Q.; Zhang, J.; Zhang, H. A High-Energy-Density Redox Flow Battery based on Zinc/Polyhalide Chemistry. ChemSusChem 2012, 5, 867–869.
- Yang, Y.; Zheng, G.; Cui, Y. A Membrane-Free Lithium/Polysulfide Semi-Liquid Battery for Large-Scale Energy Storage. ECS Meet. Abstr. 2013, 6, 1552–1558.
- Li, Z.; Weng, G.; Zou, Q.; Cong, G.; Lu, Y.-C. A high-energy and low-cost polysulfide/iodide redox flow battery. Nano Energy 2016, 30, 283–292.
- Mah, J.C.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic interconnects for solid oxide fuel cell: A review on protective coating and deposition techniques. Int. J. Hydrogen Energy 2017, 42, 9219–9229.
- Cable, T.L.; Sofie, S.W. A symmetrical, planar SOFC design for NASA’s high specific power density requirements. J. Power Sour. 2007, 174, 221–227.
- Yang, Z. Recent advances in metallic interconnects for solid oxide fuel cells. Int. Mater. Rev. 2008, 53, 39–54.
- Huang, Y.-H.; Dass, R.I.; Xing, Z.-L.; Goodenough, J.B. Double Perovskites as Anode Materials for Solid-Oxide Fuel Cells. Chemin 2006, 37, 254–257.
- Chou, Y.; Stevenson, J. Long-term thermal cycling of Phlogopite mica-based compressive seals for solid oxide fuel cells. J. Power Sour. 2005, 140, 340–345.
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989.
- Guida, D.; Minutillo, M. Design methodology for a PEM fuel cell power system in a more electrical aircraft. Appl. Energy 2017, 192, 446–456.
- Gang, B.G.; Kwon, S. All-in-one portable electric power plant using proton exchange membrane fuel cells for mobile applications. Int. J. Hydrogen Energy 2018, 43, 6331–6339.
- Farnes, J.; Bokach, D.; Hoopen, S.T.; Skåtun, K.; Geneste, X.; Vik, A.; Schautz, M. Optimized High Temperature PEM Fuel Cell & High Pressure PEM Electrolyser for Regenerative Fuel Cell Systems in GEO Telecommunication Satellites. In Proceedings of the E3S Web of Conferences, EDP Sciences, Porto Palace Thessaloniki, Greece, 3–7 October 2017; Volume 16, p. 10004.
- Cano, Z.P.; Banhamd, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289.
- Barbir, F. Efficiency and economics of proton exchange membrane (PEM) fuel cells. Int. J. Hydrogen Energy 1997, 22, 1027–1037.
- Shaneeth, M.; Basu, S.; Aravamuthan, S.; Suddhasatwa, B. PEM fuel cell cathode catalyst layer durability: An electrochemical spectroscopic investigation. Chem. Eng. Sci. 2016, 154, 72–80.
- Yamaguchi, T.; Zhou, H.; Nakazawa, S.; Hara, N. An Extremely Low Methanol Crossover and Highly Durable Aromatic Pore-Filling Electrolyte Membrane for Direct Methanol Fuel Cells. Adv. Mater. 2007, 19, 592–596.
- Kimiaie, N.; Wedlich, K.; Hehemann, M.; Lambertz, R.; Müller, M.; Korte, C.; Stolten, D. Results of a 20,000 h lifetime test of a 7 kW direct methanol fuel cell (DMFC) hybrid system—Degradation of the DMFC stack and the energy storage. Energy Env. Sci. 2014, 7, 3013–3025.
- Müller, M.; Kimiaie, N.; Glüsen, A. Direct methanol fuel cell systems for backup power—Influence of the standby procedure on the lifetime. Int. J. Hydrogen Energy 2014, 39, 21739–21745.
- Haldorai, Y.; Arreaga-Salas, D.; Rak, C.S.; Huh, Y.S.; Han, Y.-K.; Voit, W. Platinized titanium nitride/graphene ternary hybrids for direct methanol fuel cells and titanium nitride/graphene composites for high performance supercapacitors. Electrochim. Acta 2016, 220, 465–474.
- Pawar, S.M.; Kim, J.; Inamdar, A.I.; Woo, H.; Jo, Y.; Pawar, B.S.; Cho, S.; Kim, H.; Im, H. Multi-functional reactively-sputtered copper oxide electrodes for supercapacitor and electro-catalyst in direct methanol fuel cell applications. Sci. Rep. 2016, 6, 21310.
- Grigoriev, A.S. A Hybrid Power Plant Based on Renewables and Electrochemical Energy Storage and Generation Systems for Decentralized Electricity Supply of the Northern Territories. Int. J. Electrochem. Sci. 2018, 13, 1822–1830.
