You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Mahmoud Mostafa Mohamed and Version 3 by Fanny Huang.
Many disorders of the anterior region of the eye may be efficiently treated via topical administration; however, it is more challenging to target conventional therapeutic doses to the posterior of the eye in this manner. Thus, various nanocarriers have been created and investigated for the transport of drugs and genes to the anterior or the posterior portions of the eyes. Liposomes, nanoparticles, micelles, inserts, implants, hydrogel, and emulsions are some of the most frequently utilized drug delivery systems.
- implants
- ocular delivery
1. Introduction
Many disorders of the anterior region of the eye may be efficiently treated via topical administration; however, it is more challenging to target conventional therapeutic doses to the posterior of the eye in this manner. Thus, various nanocarriers have been created and investigated for the transport of drugs and genes to the anterior or the posterior portions of the eyes. The most popular nano-drug delivery systems are depicted in Figure 1, and these can be utilized to increase the activity and bioavailability, and/or lessen the toxicity of the active pharmaceutical ingredients used. Liposomes, nanoparticles, micelles, inserts, implants, hydrogel, and emulsions are some of the most frequently utilized drug delivery systems.
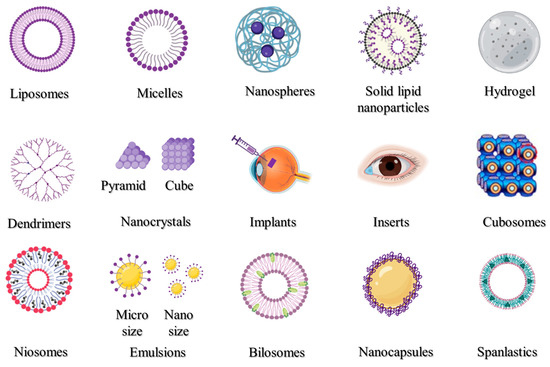
Figure 1. Nanocarrier systems investigated for ophthalmic uses.
2. Liposomes
Liposomes (Figure 1) are closed vesicles made of a phospholipid bilayer that can contain both drugs that are soluble in fat [1][313] and those that are soluble in water [2][314]. Due to their biodegradability, biocompatibility, and capacity to serve as drug carriers, liposomes have been thoroughly investigated for topical ocular administration (Table 1). Liposomes are particularly useful for large molecular weight and inadequately water-soluble drugs because they promote drug permeation through ocular tissues by virtue of their superior spreading ability and rheological properties that enable prolonging the drug availability on the surface of the eye [3][4][86,315]. Liposomes’ amphiphilic lipids form a tear compound-interacting sublayer when they make contact with tear lipid components. Polar heads and tails face the polar and non-polar tear lipid components, respectively, and help distribute the medication throughout the ocular surface [5][316]. Extensive research has examined the merits of liposomes for ocular use, minimizing potential drug toxicity and improving their absorption and bioavailability compared to an unencapsulated drug. These medications include vancomycin, tobramycin, ganciclovir, fluconazole, brinzolamide, triamcinolone acetonide, and cyclosporine A. Drug-loaded liposomal formulations injected intravitreally have a number of benefits. Some benefits include lengthening the half-life of drugs [6][317], safeguarding labile compounds [7][318], and prolonging the time that liposomes spend in the tissues of the eye [8][319].
3. Polymeric Micelles
Amphiphilic polymers can self-assemble into different structures, known as micelles (Figure 1). The formation of micelles within the nanometer range can efficiently improve the aqueous stability and enhance cell permeability. Prior research has demonstrated that the use of a nanomicelle formulation increased medication absorption in the eye [9][10][320,321]. Nanomicelle formulations are primarily used to improve the solubility of medications with low solubility and subsequently improve their bioavailability. Hesperidin, Sirolimus, Voriconazole, and Sunitinib are only a few of the medications that were made into polymeric nanomicelles with better solubility and therapeutic efficacy (Table 1).
4. Polymeric Nanoparticles
Polymeric nanoparticles (Figure 1) could be produced by the use of naturally occurring or synthetically produced polymers. Chitosan, hyaluronic acid, carboxymethylcellulose sodium, albumin, and sodium alginate are some examples of natural polymers, whereas poly(lactic-co-glycolic acid), poly(-caprolactone), and poly(ethylene glycol) are examples of synthetic polymers [11][322]. Some retinal drugs are currently not performing as expected due to the physical and chemical properties of the medications, as well as the distinctive anatomical structure of the eye. The bioavailability of these drugs was significantly increased [12][323], their toxicity was reduced [13][324], invasive procedures could be avoided [14][325], and pharmacokinetic modulation was achieved [15][326] via incorporation into polymeric nanoparticles (Table 1). These drugs include dexamethasone, cyclosporin, latanoprost, voriconazole, and ganciclovir. Hyaluronic acid, polyethylene glycol, and chitosan are examples of mucoadhesive polymers that may be employed to alter nanoparticles to lengthen their pericorneal residence duration [16][327]. Moreover, mucus penetrating nanoparticles, which possess low surface tension, low viscosity, and higher hydration water content, can enhance the penetration of therapeutic medicines through the cornea, increasing their bioavailability and resulting in better pharmacologic results. Consequently, mucus penetrating nanoparticles may significantly improve the treatment of posterior ocular problems, which include posterior uveitis, CMV, and retinal disorders [17][328].
5. Solid Lipid Nanoparticles
Lipids have been used to ameliorate the limited water solubility of several lipophilic drugs and adapt them as a drug delivery system [18][329]. Müller and Lucks initially developed solid lipid nanoparticles (SLNs, Figure 1) in 1996, which received the attention of scientists as a popular, stable, safe, and effective nanoscale drug delivery device. A surfactant layer that surrounds a solid lipid core in SLNs stabilizes and holds the medication [19][330]. Drug molecules can be found mostly in the center of particles or molecularly scattered throughout the matrix, depending on the drug solubility and the drug/lipid ratio [20][331]. SLNs are considered an efficient system intended for ocular drug delivery. SLNs can improve corneal drug absorptivity, enhance ocular tissue penetration and bioavailability, prolong residence time, and provide extended drug release properties [21][332]. SLNs were efficiently used to improve the delivery of bimatoprost [22][185], ofloxacin [23][333], and dorzolamide [24][334], as shown in Table 1.
6. Hydrogels
Hydrogels (Figure 1) are produced when polymeric solutions are crosslinked to form a network. The hydrogel complexation is formed on the basis of hydrophilic interactions between the polymer tail and water molecules [25][335]. Hydrogels are widely employed to provide ocularly applied or injectable dosage forms to a variety of eye regions. For ocular application, there are various hydrogel formulations that have FDA approval. A hydrogel sealant called ReSure® has been authorized for use in the non-operative treatment of clear corneal incisions. Hydrogels were also used to formulate and enhance the therapeutic activity of ocularly applied drugs, such as dexamethasone [26][336], bevacizumab [27][337], and timolol [28][338], as shown in Table 1.
7. Dendrimers
Dendrimers (Figure 1) are globular, negatively, positively, or neutrally branch-like nanostructured polymers. They derive their net charge from the functional groups, which are located at the ends of their branches [29][339]. These molecules consist of a fundamental unit called the “core”, which comprises the major component, and side chain units called “dendrons” [30][340]. Drugs may be conjugated to the ligands on the dendrimer surface or may be retained in the dendrimer core. Dendrimer manufacturing, generation, surface characteristics, and conjugation technique all have an impact on the drug-loading and drug-release kinetics of dendrimers [31][341]. As a result of their ability to selectively target inflammatory cells while causing no harm to healthy tissue, dendrimers have proven to be a viable drug delivery vehicle for the treatment of inflammatory eye conditions. The capacity to lower medication toxicity off-target is the key advantage of dendrimers’ targeting abilities [32][153]. Utilizing dendrimers effectively can increase the therapeutic effectiveness of various active pharmaceutical compounds (Table 1), including pilocarpine [33][241], tropicamide [33][241], dexamethasone [34][342], brimonidine, and timolol [35][343].
8. Nanocrystals
Nanocrystals (Figure 1) are crystals of therapeutic drugs with particle sizes as small as a few hundred nanometers, where pure drug crystals may occasionally be stabilized by the addition of surface active agents or polymeric solutions [36][344]. The benefits of nanocrystals over conventional nanocarriers, such as their high drug payload and comparative ease of manufacture, make them appealing candidates for the delivery of medications that are not readily water soluble [37][38][345,346]. The preparation of therapeutic drugs in the form of nanocrystals for ocular administration has various advantages. These advantages include better tolerability, increased ocular absorption, providing intermediated and prolonged release of drugs in the eye, and improved ocular permeation [39][347]. They also include improved ocular safety, increased formulation retention in cul-de-sac, and enhanced ocular permeation [40][152]. A number of medications used ocularly have been transformed into nanocrystals (Table 1) with enhanced properties, and these include dexamethasone [41][348], itraconazole [42][349], tedizolid [43][350], and brinzolamide [44][227]. Moreover, Novartis Pharmaceutical Corporation’s formulation of nepafenac nanocrystals received approval for commercial release (FDA, 2012) under the brand name Ilevro®.
9. Cubosomes
Cubosomes (Figure 1) are made up of two inner aqueous pathways that are separated into two arched interpenetrating lipid bilayers, which are structured in three dimensions resembling honeycombs [45][351]. These pathways can be occupied by a variety of bioactive molecules, including natural bioactives, chemical pharmaceuticals, peptides, polypeptides, and proteins [46][352]. Cubosomes are thought to be promising delivery systems because of their special characteristics, including thermodynamic stability, bioadhesion, the capacity to encapsulate different types of drugs, and their potential to control drug release [47][353]. Active medicines and macromolecules can successfully be applied topically to the posterior portion of the eye using cubosomes (Table 1). These drugs include beclomethasone [46][352], flurbiprofen [48][354], timolol [49][199], and brimonidine [50][355].
10. Niosomes
Niosomes, which are a type of vesicular system that includes a non-ionic surfactant, are closed bilayer structures produced once the nonionic surfactants self-assemble in an aqueous media to create nanocarriers (Figure 1). Researchers have begun using niosomal systems to treat severe inflammatory diseases and conditions, such various malignancies, because of their potential to boost the bioavailability and efficiency of the encapsulated therapeutics [51][356]. Niosomes are being investigated more and more for improving drug delivery to both segments of the eye, anterior and posterior, as well as promoting drug penetration and retention in ocular tissues. As a consequence, niosomes showed a considerable increase in the absorption and transcorneal permeability of topically applied drugs at the ocular surface (Table 1). These drugs include cyclopentolate [52][357], voriconazole [53][358], acetazolamide [54][359], gentamicin [55][360], brinzolamide [56][361], pilocarpine [57][362], and tacrolimus [58][363]. Additionally, niosomes, particularly charged vesicles, have been effectively used to transfer genes by subretinal or intravitreal injection to the retinal area [59][364].
11. Emulsions
An emulsion (Figure 1) is a uniform dispersion system that is formed upon mixing two or more immiscible liquids under certain circumstances [60][365]. Lipid-based emulsions have become a potential vehicle for ocular medication administration. The emulsions enhance ocular delivery using one of two major strategies, either by improving ocular permeability or by lengthening the period the formulation is retained on the ocular surface [61][366]. Both hydrophilic and lipophilic drug types may be loaded into emulsions [62][63][367,368]. Emulsions have been successfully used to create more effective formulations for several medications used intraocularly that have increased absorption and therapeutic effectiveness. These drugs include cyclosporine A [64][369], coumarin-6 [65][370], azithromycin, and disulfiram [66][371].
12. Bilosomes
One type of vesicular drug delivery system is the bilosome (Figure 1), which is made up of non-ionic amphiphilic compounds with integrated bile salt molecules. The negatively charged bile salts serve to maintain the bilosomal structure [67][372]. In comparison to niosomes and liposomes, these drug carriers are more stable and can effectively increase drug absorption through biological membranes [68][373]. Moreover, bilosomes can enhance the permeability of polysaccharides, proteins, and polypeptides, which are poorly transported through mucosal epithelial cells [69][374]. Previous research studies have assessed the effectiveness of bilosomes in the administration of ocular drugs (Table 1) and found that bilosomes are well tolerated by corneal tissues [70][236]. These drugs include terconazole [71][375], acetazolamide [70][236], ciprofloxacin [72][376], ciprofloxacin [72][376], agomelatine [73][377], and betaxolol [74][211].
13. Nanocapsules
Nanocapsules (Figure 1) are a subtype of nanoparticles that are comparable to vesicular systems, in which a medicine is contained in a hollow vessel with an inner liquid core encircled by a polymeric coating [75][378]. Nanocapsules are well-known to be retained in the cornea for a prolonged time and to enhance penetration throughout the deep ocular tissues [40][152]. Thus, the development of topically applied drug-loaded nanocapsules could reduce uncomfortable intravitreal injections and systemic delivery, which have serious side effects [40][152]. The therapeutic action of several medications was effectively potentiated via formulation in the form of nanocapsules (Table 1). These drugs include bevacizumab [76][379], prednisolone [77][165], tacrolimus [40][152], brinzolamide [44][227], and cyclosporine [78][380].
14. Spanlastics
Elastic niosomes, also known as spanlastics (Figure 1), are a subtype of vesicular drug delivery systems that are relatively new to the market. They resemble niosomes (non-ionic surfactant vesicles), except they contain an edge activator. They were first described as systems for ocular drug delivery [79][381], but since then, they have been used to deliver medications to a variety of bodily organs. The spanlastics’ bilayers become more elastic and deformable when an edge activator is present, which improves drug absorption across biological membranes. Spanlastics were efficiently used to payload hydrophilic, hydrophobic, and amphiphilic therapeutic pharmaceuticals for ocular use, especially the delivery to the posterior segment (Table 1). These drugs include ketoconazole [79][381], cyclosporine A [80][382], clotrimazole [81][383], and vanillic acid [82][384].
Table 1.
Chronic eye conditions, available therapies, and drug delivery systems and their merits.
| Disease | Treatment | Drug | Delivery System Platform | Advantages of Delivery Systems In Vivo | Refs. | |
|---|---|---|---|---|---|---|
| Dry eye syndrome | Tear substitutes | Hypromellose | Solution | [83][126] | ||
| Methylcellulose and derivatives | Solution | [84][127] | ||||
| hyaluronic acid | Solution | [85][128] | ||||
| Aqueous secretagogues | Diquafosol sodium | Solution | [86][129] | |||
| Punctal plugs | Collagen and atelocollagen | In situ hydrogel | Prolonged activity | [87][88][89][49,50,130] | ||
| methacrylate-modified silk fibroin | In situ hydrogel | Prolonged activity | [90][54] | |||
| Mucin secretagogues | Rebamipide | Nanoparticles | Sustained release | [91][131] | ||
| Liposomes | Improved activity | [92][132] | ||||
| Micelles | Improved penetration | [93][133] | ||||
| Anti-inflammatory and immunomodulatory drugs | Cyclosporine | Micelles | Improved activity | [94][134] | ||
| Self-nanoemulsifying | Improved efficacy | [95][135] | ||||
| Liposomes | Improved activity | [96][136] | ||||
| Nanoparticles | Improved activity | [97][137] | ||||
| Nano-emulsion | Improved penetration | [98][138] | ||||
| Solid lipid nanoparticles | Controlled release | [99][139] | ||||
| In situ hydrogel | Improved activity | [94][134] | ||||
| Epigallocatechin gallate | Nanoparticles | Extended activity | [100][140] | |||
| In situ gels | Enhanced efficacy | [101][141] | ||||
| Lactoferrin | Nanoparticles | Enhanced efficacy | [102][142] | |||
| Nanocapsules | Controlled release | [103][143] | ||||
| Liposomes | Reduced irritation | [104][144] | ||||
| Nanostructured lipid carriers | Controlled release | [105][145] | ||||
| Vitamin A | Liposomes | Improved activity | [106][146] | |||
| Tacrolimus | Nanoparticles | Improved penetration | [107][147] | |||
| Progylcosomes | Improved activity | [108][148] | ||||
| Microcrystals | Improved efficacy | [109][149] | ||||
| Liposomes | Improved retention time | [110][150] | ||||
| Micelles | Prolonged activity | [111][151] | ||||
| Nanocapsules | Improved activity | [40][152] | ||||
| Corticosteroids | Dexamethasone | Dendrimer | Improved activity | [32][153] | ||
| Nano-wafer | Improved activity | [112][154] | ||||
| Nanostructured lipid carriers | Improved activity | [113][155] | ||||
| Nanoparticles | Improved penetration | [114][156] | ||||
| Micelles | Release modulation | [115][157] | ||||
| Nanosuspension | Prolonged activity | [116][158] | ||||
| Nano emulsion | Improved activity | [117][159] | ||||
| Nanosponges | Improved permeability | [118][160] | ||||
| Fluorometholone | Nanoparticles | Improved activity | [119][161] | |||
| Triamcinolone acetonide | Micelles | Release modulation | [120][60] | |||
| Nanoparticles | Improved activity | [121][162] | ||||
| Hydrocortisone | Nanosuspension | Prolonged activity | [116][158] | |||
| Micelles | Improved targeting | [122][163] | ||||
| Nanoparticles | Improved penetration | [122][163] | ||||
| Nanosuspension | Prolonged activity | [116][158] | ||||
| Prednisolone | Nanoparticles | Prolonged activity | [123][164] | |||
| Nano capsules | Reduced toxicity | [77][165] | ||||
| Lotep rednol etabonate |
Nanoparticles | Improved penetration | [124][166] | |||
| Non-steroidal anti-inflammatory drugs | Diclofenac sodium | Nanoparticles | Improved bioavailability | [125][167] | ||
| Nanosuspension | Prolonged activity | [126][168] | ||||
| Pranoprofen | Nanosuspension | Improved activity | [127][169] | |||
| Nanoparticles | Improved activity | [127][128][169,170] | ||||
| Bromfenac sodium | Liposomes | Extended release | [129][171] | |||
| Nanoparticles | Improved permeation | [130][172] | ||||
| Cubosomes | Improved bioavailability | [131][61] | ||||
| Ketorolac | Nanoparticles | Improved delivery | [132][173] | |||
| lymphocyte function-associated antigen-1 antagonists | Lifitegrast | Solution | [133][134][174,175] | |||
| Glaucoma | Prostaglandin analogues | Latanoprost | Nanoparticles | Controlled release | [135][176] | |
| PEGylated solid lipid | Improved permeability | [136][177] | ||||
| Micelles | Extended release | [137][178] | ||||
| Cubosomes | Sustained release | [138][179] | ||||
| Nanoparticles | Improved permeability | [139][180] | ||||
| Travoprost | Gold nanoparticles | Improved stability | [140][181] | |||
| Liposomes | Sustained release | [141][182] | ||||
| Spanlastics | Prolonged activity | [142][183] | ||||
| Nanoemulsion | Improved pharmacokinetics | [143][75] | ||||
| Implant | Controlled release | [144][184] | ||||
| Bimatoprost | Nanoparticles | Improved therapeutic activity | [22][185] | |||
| Gold nanoparticles | Controlled release | [145][186] | ||||
| Nanoparticle hydrogel | Controlled release | [146][187] | ||||
| Microemulsion | Improved permeability | [147][188] | ||||
| Graphene oxide-laden | Controlled release | [148][189] | ||||
| Implants | Sustained release | [149][190] | ||||
| Nanovesicular systems | Sustained release | [150][191] | ||||
| Inserts | Extended release | [151][192] | ||||
| Unoprostone | Transscleral device | Sustained release | [152][193] | |||
| Rho kinase inhibitors | Fasudil | Liposomes | Enhanced bioavailability | [153][194] | ||
| Microspheres | Sustained release | [154][195] | ||||
| Ripasudil | Solution | [155][196] | ||||
| Netarsudil | Solution | [156][197] | ||||
| β-adrenergic blockers | Timolol | Nanoparticles | Extended release | [157][198] | ||
| Micelles | Extended release | [137][178] | ||||
| Cubosomes | Improved bioavailability | [49][199] | ||||
| Nanogel | Sustained release | [158][200] | ||||
| Gelatinized core liposomes | Improved encapsulation | [159][201] | ||||
| Microemulsion | Improved bioavailability | [160][202] | ||||
| Levobunolol | Nanoparticles | Extended release | [161][203] | |||
| Microparticles | Sustained release | [162][76] | ||||
| Carteolol | Nanocapsules | Improved activity | [163][204] | |||
| Nanoparticles | Improved activity | [164][205] | ||||
| Chitosomes | Improved penetration | [165][206] | ||||
| Metipranolol | Nanocapsules | Reduced systemic side effects | [166][207] | |||
| Betaxolol | Liposomes | Extended activity | [167][208] | |||
| Nanoparticles | Controlled release | [168][209] | ||||
| Niosomes | Improved bioavailability | [169][210] | ||||
| Bilosomes | Improved transcorneal permeation | [74][211] | ||||
| α-adrenergic agonists | Brimonidine | Nanoparticles | Sustained release | [170][212] | ||
| Inserts | Controlled release | [171][213] | ||||
| Niosomes | Sustained release | [172][214] | ||||
| Microspheres | Sustained release | [173][215] | ||||
| Liposomes | Improved effectiveness | [174][216] | ||||
| Implant | Sustained release | [175][217] | ||||
| Gelatin-core liposomes | Improved drug loading | [176][77] | ||||
| Carbonic anhydrase inhibitors | Dorzolamide | Nanoparticles | Improved activity | [177][218] | ||
| Nanoemulsion | Enhanced ocular delivery | [178][219] | ||||
| Liposomes | Prolonged action | [179][78] | ||||
| Microparticles | Sustained release | [180][220] | ||||
| Niosomes | Improved activity | [181][221] | ||||
| Implant | Extended drug delivery | [182][222] | ||||
| Inserts | Improved activity | [183][223] | ||||
| Brinzolamide | Nanoparticles | Improved therapeutic activity | [184][224] | |||
| Nanocrystals | Improved penetration | [185][225] | ||||
| Liposomes | Sustained release | [186][226] | ||||
| Nanocapsules | Improved bioavailability | [44][227] | ||||
| Nanoemulsion | Improved therapeutic efficacy | [187][228] | ||||
| Nanofibers | Improved patient compliance | [188][229] | ||||
| Implant | Sustained release | [189][230] | ||||
| Acetazolamide | Cubosomes | Improved therapeutic efficacy | [190][231] | |||
| Spanlastics | Enhanced ocular delivery | [191][232] | ||||
| Transgelosomes | Enhanced ocular delivery | [192][233] | ||||
| Implants | Sustained release | [193][234] | ||||
| Niosomes | Improved permeability | [194][235] | ||||
| Bilosomes | Improved permeability | [70][236] | ||||
| Microsponges | Improved therapeutic efficacy | [195][237] | ||||
| Dendrimers | Sustained release | [196][238] | ||||
| Cholinergic agonists | Pilocarpine | Nanoparticles | Sustained release | [197][239] | ||
| Nanocapsules | Improved bioavailability | [198][240] | ||||
| Dendrimers | Prolonged residence time | [33][241] | ||||
| Uveitis | Corticosteroids | Fluocinolone acetonide | Implant (Retisert®) | Sustained release | [199][242] | |
| Nanoparticles | Improved bioavailability | [200][243] | ||||
| Difluprednate | Microneedles | Sustained release | [201][244] | |||
| Fluormetholone | Nanoparticles | Improved penetration | [202][245] | |||
| Nanocrystals | Improved sustained activity | [203][246] | ||||
| Triamcinolone acetonide | Nano lipid carriers | Improved penetration | [204][247] | |||
| Immunomodulator drugs | Adalimumab | Hydrogel | Improved permeability | [205][248] | ||
| Infliximab | Liposomes | Prolonged activity | [206][249] | |||
| Methotrexate | Implant | Sustained release | [207][250] | |||
| Sirolimus (Rapamycin) | Implant | Extended release | [208][251] | |||
| Micelles | Sustained release | [209][252] | ||||
| Exosomes | Improved therapeutic activity | [210][253] | ||||
| Liposomes | Improved therapeutic activity | [3][86] | ||||
| Endophthalmitis | Antimicrobials | Daptomycin | Nanoparticles | Noninvasive and improved activity | [211][254] | |
| Vancomycin | Nanostructured lipid carriers | Improved permeability and activity | [212][255] | |||
| Nanoparticles | Sustained release | [213][256] | ||||
| Thermoresponsive hydrogels | Controlled release | [214][257] | ||||
| Liposomes | Improved permeability | [215][258] | ||||
| Implant | Controlled release | [216][259] | ||||
| Niosomes | Improved permeability | [217][260] | ||||
| Ceftazidime | Nanoparticles | Improved activity and permeability | [218][261] | |||
| Antifungals | Amphotericin B | Liposomes | Improved activity-reduce toxicity | [219][262] | ||
| Voriconazole | Thermo-sensitive in situ gel | Sustained release | [220][263] | |||
| Nanoparticles | Improved permeability | [221][264] | ||||
| Microemulsion | Controlled release | [222][265] | ||||
| Elastosomes | Improved activity and reduced toxicity | [223][266] | ||||
| Micelles | Improved stability | [223][266] | ||||
| Liposomes | Improved permeability | [224][267] | ||||
| Antivirals | Cidofovir | Micelles | Prolonged activity | [225][268] | ||
| Liposomes | Prolonged activity | [226][269] | ||||
| Foscarnet | Liposomes | Improved activity and permeability | [227][270] | |||
| Ganciclovir | Nanoparticles | Sustained release | [228][271] | |||
| Glycerosomes | Sustained release | [229][272] | ||||
| Microemulsion | Improved permeability | [230][273] | ||||
| Vitrasert | Prolonged activity | [231][274] | ||||
| Minitablets | Sustained release | [232][275] | ||||
| Retinal diseases | Age-related macular degeneration | Anti-VEGF Agents | Ranibizumab | Nanoparticles | Improved activity | [233][276] |
| (Antibody fragment) | Microparticles | Improved intravitreal delivery | [234][277] | |||
| Liposomes | Increased encapsulation-release | [235][278] | ||||
| Quantum dots | Sustained release | [236][279] | ||||
| Implant | Sustained release | [237][280] | ||||
| Bevacizumab | Nanoparticles | Sustained delivery | [238][281] | |||
| (Monoclonal antibody) | Bi-layered capsule | Sustained delivery | [239][282] | |||
| Nanocapsules | Improved bioavailability | [240][283] | ||||
| Implant | Sustained release | [241][284] | ||||
| Microparticles | Sustained release | [242][285] | ||||
| Liposomes | Sustained release | [243][286] | ||||
| Aflibercept (VEGF-Trap) | Nanoparticles | Sustained drug release | [244][287] | |||
| Microspheres | Extended release | [245][288] | ||||
| Sunitinib | Nanoparticles | Superior prolonged activity | [246][289] | |||
| Micelles | Extended release | [247][290] | ||||
| Axitinib | Nanoparticles | Superior activity | [248][291] | |||
| Pegaptanib | PEGylated aptamer | Prolonged activity | [249][113] | |||
| Gene therapy | VEGF-siRNA | Liposomes | Improved activity-stability | [250][292] | ||
| Nanoball | Improved activity-targeting | [251][293] | ||||
| Nanoparticles | Improved therapeutic activity | [252][294] | ||||
| Integrin antagonists | C16Y peptide | Nanoparticles | Sustained release | [253][295] | ||
| Antioxidants | Serine-threonine-tyrosine peptide | Nanoparticles | Targeting | [254][296] | ||
| Resveratrol | Nanoparticles | Sustained release | [255][297] | |||
| Curcumin | Liposomes | Improved activity | [256][298] | |||
| Astragaloside | Nanocapsules | Improved activity | [257][299] | |||
| Diabetic retinopathy | Antiangiogenics | Anti-Flt1 peptide | Nanoparticles | Sustained release | [258][300] | |
| Micropump implant | On-demand targeting | [259][301] | ||||
| Fenofibrate | Nanoparticles | Controlled release | [260][302] | |||
| Pioglitazone | Nanoparticles | Controlled/improved activity | [261][303] | |||
| Apatinib | Nanoparticles | Improved activity | [262][304] | |||
| Silicate | Nanoparticles | Improved activity | [263][305] | |||
| Tacrolimus | Nanoparticles | Improved activity | [264][306] | |||
| Sorafenib tosylate | Nanoparticles | Improved activity | [265][307] | |||
| Octreotide | Nanoparticles | Improved activity-targeting | [266][308] | |||
| Anti-inflammatory and antioxidants | p-Coumaric acid | Nanoparticles | Improved activity | [267][309] | ||
| Connexin43 mimetic peptide | Nanoparticles | Targeting | [268][310] | |||
| Inulin D α-tocopherol succinate | Nanomicelles | Improved activity | [269][311] | |||
| Citicoline | Liposomes | Improved permeation | [270][312] | |||
| Melatonin | Nanoparticles | Controlled release and enhanced tolerability | [270][312] | |||
