1. Background
Wastage and improper disposal of pharmaceuticals is a global issue that impacts every continent on Earth
[1][2][3] and, with medicine usage in the UK alone predicted to double by 2050, is an issue that will only increase in severity
[4]. With an estimated GBP 300 million being spent annually on unused medicines by the UK National Health Service (NHS)
[5], significant quantities of medication being wasted across Europe
[6][7], and a large disparity between patients being unable to afford treatment, while huge quantities of medicines are being discarded in the USA
[8][9], the economic effects of medicine waste can be felt across multiple countries. Pharmaceutical waste, defined as hazardous or in some cases cytotoxic
[10], has been found in wastewater, rivers, and even sources of drinking water
[11][12][13]. This includes every major estuary tested in the UK
[14] and at every point tested along the Thames
[11]. Active Pharmaceutical Ingredients (APIs) have been shown to impact plants and animals in the wild
[15][16][17][18]. This may also affect humans through the consumption of drinking water and produce plants, as the consequences of long-term exposure to multiple APIs at a low concentration is unknown
[19]. If not properly disposed of, unused medicines can be a danger to the public. Through abuse, self-medication without a prescription, or acquisition from an illegitimate source, the misuse of prescription drugs can lead to addiction, long-term health complications, and death
[20][21][22][23]. While there are many papers on waste reducing behaviours
[24][25][26][27], there are relatively few surrounding the reuse of discarded medicines.
Medicine reuse is a solution that can reduce medicine wastage and its impacts worldwide. In the USA, legislation has been passed in 40 states and Guam legalising medicine reuse
[28]. There are 27 individual state-endorsed programs that accept medicines from around the country to redistribute within their area
[29][30][31]. SIRUM and GIVMED are the two existing large-scale systems for medicine reuse
[32][33]. SIRUM operates in the USA. Since its foundation in 2013, SIRUM has aided in USD 89.6 million worth of prescriptions and aims to have helped 70% of the 10 million most in need by 2030
[32]. Since 2016, GIVMED has operated in Greece, receiving over EUR 3.33 million of medicine and healthcare products
[33]. All organisations state that donated medicines must be unopened, unexpired, and stored correctly
[29][30][31][32][33]. Most US states express that medicines must be assessed by a pharmacist, controlled medicines cannot be donated, donors cannot be financially compensated, and donors or recipients are not liable for faulty medicines
[29][30][31].
The concept of a circular economy (CE) has been gaining traction in recent years and could be applied to pharmacy to aid in reducing the amount of excess medicine wastage
[34]. As seen in
Table 1, CE revolves around minimising the amount of waste produced by a system by redefining the process of product design and manufacturing, allowing every opportunity for the product or its components to be reused, and even to the recovery of energy being produced from the incineration of waste. Examples of CE include the phasing out of single-use plastics (with open sourced technology allowing consumers to recycle plastic into 3D printing materials
[35]), encouraging the sharing of rarely used items
[36], and the reuse of items that will be outgrown or outdated
[37].
Table 1.
The 9Rs of a circular economy.
Reverse logistics (RL) is a methodology used by companies when handling products moving backwards through a supply chain
[38]. Through repurposing these products or their components, the system can reduce the number of components needed for the forward supply chain, and can be further utilised to enable a CE. The overseeing of daily operations in the reverse supply chain (i.e., transport, storage, and reimbursement for returns) are typically handled by a third-party logistics company (3PL). Examples of RL can be seen in the return of malfunctioning products to a manufacturer by a customer or retailer for repair, or the redistribution of products unsold due to a surplus.
With the introduction of Industry 4.0, there has been an increase in the application of technologies to CE and RL systems. One example of this includes the use of Blockchain and Internet of Things (IoT) to trace stock (such as asbestos or wine) and throughout a circular economy
[39][40][41]. A further application of this has been the inclusion of machine learning to predict the need of goods or equipment requiring maintenance to enable closer organisation between the reverse and service supply chains
[42]. Research applying Industry 4.0 to the Pharmaceutical Supply Chain (PSC) has included posing the improvement of a supply chain as a decision-making problem and using mathematical and machine learning methods to optimise the solution. This can be seen in studies on optimising a Pharmaceutical Supply Chain Network and designing a reverse supply chain to recycle COVID-19 waste
[43][44].
CE ideals are also being applied within the PSC, with research and campaigns extending into the topics of: maximising patient adherence through altering prescription quantity and frequency
[24][25]; education of the public into medicine waste
[26][45]; the extension of use-by dates on EpiPens during a shortage
[46]; the efficacy of waste reduction measures in pharmacies
[27][47]; the sharing of cancer medications in clinics for maximum stock use
[48]; the recycling of the outer packaging/cartridges for inhalers
[49]; medicine return programs to ensure appropriate disposal of medicines
[50][51]; the recovery of APIs for remanufacturing
[52][53]; and the burning of medicines through pyrolysis to recover any energy from wasted medicines
[54].
2. A Smart Packaging and Returns Assessment System (SPaRAS)
As shown in Table 2, a reliable and efficient system of assessing returned medicines is the vital next step to enable medicine reuse. It will not only ensure the safety of the system, but will also address public and professional concerns surrounding cost efficiency. A practical Smart Packaging and Returns Assessment System (SPaRAS) will ensure medication has been returned before expiry (or a specified threshold), stored in the correct conditions, and unaltered/unadulterated. The inclusion of RFID technology will allow for an effective method of information transfer to reduce the cost and human error in the assessment process.
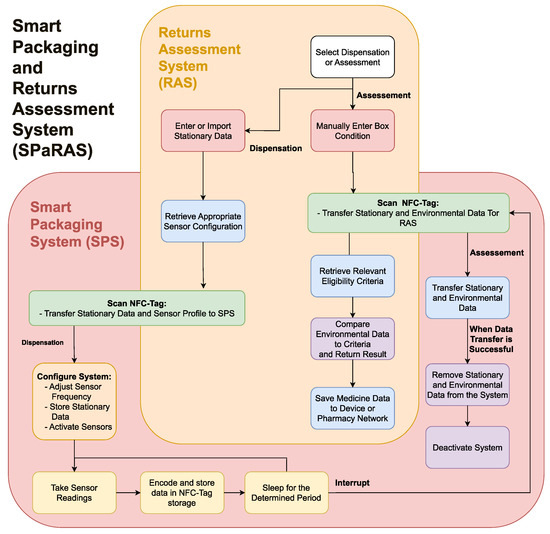
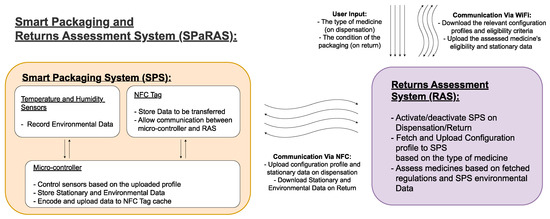
As shown in Figure 1, SPaRAS will be split into two main components: the smart packaging system (SPS) and the returns assessment system (RAS). The SPS will be a device inserted into medicine packaging that will store key information on the assigned medication and record its storage conditions while in patient care. The RAS will be an application on a pharmacy owned device that will assess returns by comparing the data collected from the SPS to current reuse regulations.
Table 2.
The basis and criteria required for a system to enable medicine reuse.
| Criteria |
Relevance |
| System Basis: |
|
| Ensure the safety of redispensed medicines. |
Addresses public and stakeholder concerns over medicine reuse being dangerous. |
| Increase the efficiency of assessing returned medicines. |
Addresses stakeholder concerns of medicine reuse being too expensive. |
| System guidelines: |
|
| Use cheap/reusable components. |
Influences companies by reducing costs. |
| |
Influences the public by not drastically increasing the cost of medicine and by the morality of recycling components. |
| Ensures the system is usable by those with disabilities. |
Allows the whole population to accept the new system. |
| Minimises changes to the exterior and functionality of medicine boxes. |
. This is further broken down to describe the flows of the SPS and RAS in their respective sections. As previously discussed in
Table 2, to ensure public and stakeholder engagement, the system should be cost efficient and reusable, have a minimal change to the exterior of pharmaceutical packaging, and be accessible to those with additional needs. To ensure the system has real world use, stakeholders should be consulted for feedback on the SPS and RAS, while members of the public should be consulted regarding the SPS only. Stakeholders could also be consulted on the feasibility of the potential development of the SPaRAS and the integration of the SPS into a future CPSC.
Figure 2.
A flowchart outlining the functionality of the smart packaging and returns assessment system (SPaRAS).
In order to be successful, the SPS will need to:
| Reduces the chance of public aversion to change. |
Figure 1.
Outline of the proposed smart packaging and returns assessment system (SPaRAS).
As different medicines require different levels of attention, the SPS will be a generic solution that can be tailored to its assigned medicine. When the medicines are assigned, the RAS will activate the sensors and upload vital data on the medicine. However, this system can also be used to upload a profile to the system that will alter the frequency that the environmental conditions are checked. This will allow less stable medicines with a shorter lifespan (i.e., anti-neoplastic drugs) to be checked more regularly with less concern over the power consumption, while longer lasting medicines can be checked less frequently with a longer lifespan and less data to transfer during assessment.
The system will work as an interconnected flow through the two devices, as shown in
Figure 2
-
Efficiently communicate with the assessment system;
-
Fit within a medicine box.
The RAS will need to:
-
Efficiently transfer data with the smart packaging system;
-
Control functionality of the SPS;
-
Accurately compare data from the SPS to determine eligibility;
-
Be easy to use without extensive training.
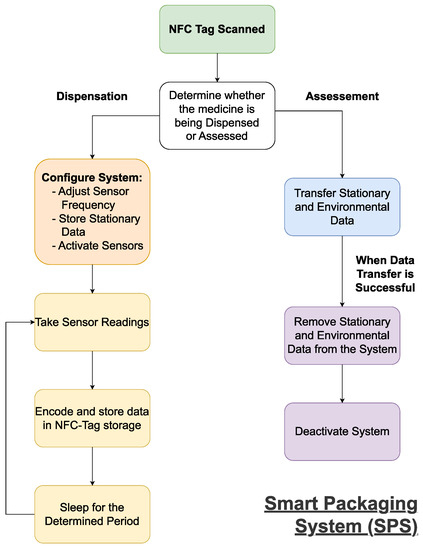
2.1. A Streamlined Smart Pharmaceutical Packaging System
A practical smart packaging system can be developed by using the DTTHI and RFID proposals, as influenced by the literature
[55][56]. The SPS will function as shown in
Figure 3. On dispensation, the SPS will need to receive the stationary data on the medicine (i.e., name, brand, dosage, quantity, and expiry date) from the RAS, as this will be used when the medicines are assessed upon return. The SPS will also need to receive and setup the system using the profile uploaded by the RAS, as this will define the intervals at which environmental readings are taken and could be used to define the eligibility criteria used upon assessment.
Figure 3.
A flowchart outlining the functionality of the smart packaging system (SPS).
Once activated, the system will record the environmental conditions at the preset intervals. The environmental data will need to be encoded to ensure a minimal amount of data is transferred upon assessment. This will be done at regular intervals to ensure all relevant data is ready to be scanned, and to reduce the power consumption. Upon return, the data will be downloaded by the RAS and deleted to enable the system’s reuse.
At the most basic level, the SPS will need a power source, temperature and humidity sensors to record environmental data, a processing unit to store medicine information, control the sensors and manipulate/encode environmental data, and a writable RFID-tag to allow communication with the RAS. Optional additions or future developments to the system are a tamper-proof seal and a physical indicator of the medicine’s eligibility on the exterior of the packaging.
Assuming the devices will not be charged by members of the public, they will need to last until the medicines pass the expiry threshold, which may take years. Once returned to the PSC, it is possible that devices could be recharged wirelessly before being redispensed. Energy harvesting may be considered to simplify the process of recharging a large quantity of devices simultaneously, as this is being developed within the field of wireless sensors
[57]. As the long-term goal is for the system to be used for the majority of medications, the prototype system should be designed with a lifespan of at least one year when the system is configured for more stable medicines. This will require the use of ultra-low power components and simplification of the tasks carried out by the system. The main challenge with selecting a power source will be increasing its longevity while minimizing its size and cost.
The environmental sensors must be able to read the main factors that accelerate medicine degradation (temperature, humidity, and direct light). An ultra-low-power temperature and humidity sensor can be used similar to the DTTHI. However, an electric light sensor may be redundant, as medicines that are exposed to direct light will have a broken tamper-proof seal and will likely be discarded. A physical indicator of medicines’ eligibility (i.e., a small colour indicator on the exterior of the packaging) is unlikely to be included, as this would require a system to self-determine eligibility, requiring more processing power. It could also be considered an unnecessary change by stakeholders and the public.
The DTTHI included a temperature and humidity sensor, power source, micro-controller, WiFi connection, and E-ink display
[55]. A companion cloud database was developed to store the environmental data, which was uploaded in intervals through a WiFi connection. The issues that should be addressed by the SPS include: a battery life (while in active use) of only 6 months, substantial changes to the exterior design and functionality of the packaging, and large/costly components. Although this latter issue could be improved by not using off-the-shelf components and through the creation of an economy of scale, excluding expensive components or those with limited functionality could drastically reduce the cost and power consumption of the SPS.
The visual E-ink display posed several issues. They are relatively expensive, an unnecessary drain on the power and excessively alter the exterior of the box. The display contains a lot of information in various methods that may confuse patients with poor eyesight, learning conditions, or those that are less technically capable. The majority of information displayed is either irrelevant to the general public (e.g., bar graphs displaying the storage conditions and time until expiry, the activation date of the system, and an indicator on the reusability of the medicine) or can be printed on the packaging (the expiry date). The QR code displayed to allow patients to track environmental data in real time can also be printed on the packaging, but is unnecessary as the public do not need specific information to store medicines correctly.
A WiFi connection also presented some problems. The components are expensive, consume relatively high amounts of power, and reduce the functionality of the system. The box would have to be connected to the WiFi to update the server when in the owner’s care. This poses a complicated and time-consuming task and a potential privacy risk. If the user was to travel with the medicines (i.e., on a daily routine or on holiday), or simply did not connect the packages to WiFi within the home, the packages would either not record the data or would have a limit to the amount of data they could record before uploading them to the cloud system. The upload of a large section of the data after being offline could also require large amounts of power. A large portion of the data stored on the database would also be unnecessary, as most medicines would be fully consumed and some medicines would be discarded without being returned. This means that in a large-scale system, the vast majority of the database’s space would be wasted.
RFID technology is a low-cost, low-power alternative method, which would allow efficient transfer or viewing of data by professionals while minimising changes to the appearance or functionality of the packaging. RFID is a technology that has been gaining popularity in recent years, and it is more likely to be developed further, which will more easily allow future developments to increase and expand the functionality of the SPS in the near future
[58]. RFID is used in everyday life without much of a learning curve for the user or a need for additional training to edit the contents of the tag in the case of ID cards and key fobs. RFID tags have been shown to be a reliable method of transferring environmental data when used to record the temperature and humidity of a controlled environment
[59]. An implant to monitor the internal temperature of individual cattle shows the ability for RFID technology to communicate environmental information for an extended period without maintenance
[60].
The use of an RFID tag would reduce the time and human error involved in data transfer as the user would only have to scan the tag and manually approve the condition of the box. A wired connection is less practical when considering the time taken to connect many devices individually as well as the risk of damage while in patient care. RFID is becoming more commonplace in inventory management with its use in the chain Decathlon, allowing a store-wide inventory every 1–4 weeks as opposed to twice a year under the bar-code system and the tracking of stock throughout the supply chain. The benefits of an RFID system far outweighs the costs of its implementation, with Decathlon reporting gains within 5 years of initial testing in 2010
[61]. In the agro-food industry, the use of RFID tags has increased the safety of food products and transparency with the customer, allowing the tracking of pigs, including monitoring the pig’s diet and tracking the meat as it is processed. This allows the customer to scan the tag and read the information gathered (i.e., date, location, and facility) as the meat goes through the supply chain
[62].
The technology could allow a proposed smart packaging system to be used throughout a CPSC in a passive state for efficient inventory management, similar to the method proposed by Kongar et al.
[56].
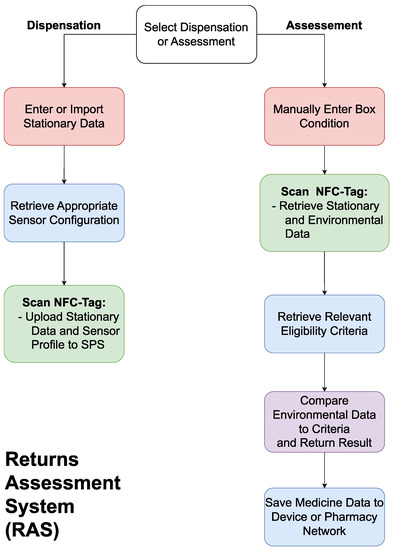
2.2. A Returns Assessment System
The RAS will be an application on a smartphone or tablet with an RFID reader and wireless connection to allow communication with the SPS and the pharmacy’s network. The system will be needed to communicate with the SPS on both the dispensation of the medicines and upon its return, as shown in Figure 4.
Figure 4.
A flowchart outlining the functionality of the returns assessment system (RAS).
On dispensation, the RAS will update the SPS’s stationary data (i.e., name, brand, expiry date, dosage, quantity) based on a user’s input or through scanning the barcode on the medicine packaging, use the stationary information to configure the SPS to the relevant profile based on current regulations, and activate the system. On return, the user will scan the packaging, allowing the RAS to deactivate the system and download the stationary and environmental data. The user will then need to input the condition of the box, as this cannot be measured electronically. The RAS will then fetch the eligibility criteria relevant to the medicine being assessed, compare this to the data from the SPS, and return a pass/fail result. The system will then present the user with the option to save the gathered information for the purposes of inventory management or studying trends in medicine returns. The RAS will require bi-directional communication with the SPS to upload the medicine’s stationary data, configure the SPS for the assigned medicine, and download the environmental data upon return. The connection with the pharmacy’s network is essential to ensure the criteria for reuse eligibility is up to date and to upload the information on the medicine to the pharmacy’s network.
2.3. Potential Applications and Future Developments
The aim of this system is to aid in enabling the reuse of prescription medicines by providing a safe and efficient method for assessing returned medicines. Through the introduction of medicine reuse, SPaRAS could be further developed or used in conjunction with other devices to continue to improve implemented medicine reuse systems. Once a viable SPS is developed and can be implemented, it can be expanded further to carry out other functions. Additional sensors could also be incorporated for medication with specific needs (i.e., those that are light or motion-sensitive). The addition of a wire that breaks with the tamper-proof seal can serve as an electronic method of preventing adulteration of medicines. This would be a useful future addition, as it would cut down on the time an examiner must spend visually assessing each box.
The SPS could later be upgraded to authenticate any returned medicines. With the system involving data transfer, the authentication system can include encrypting the data or adding an authentication code to the data being transferred, but this would fall under a separate project, as the authentication of medicines is currently being heavily researched as a separate topic. The SPS could be further developed to log when medicines are collected or returned by patients to supplement their medical record and a wire mesh could be added to blister packaging to track when medicines are taken. This would allow health services to track adherence in the case of mentally unwell patients and could be used in studies regarding alternative prescription methods. However, this should be limited to areas where consent is given to maintain the public’s right to privacy.
A specialised tool for inventory management would reduce waste at every stage of the pharmaceutical supply chain. With the use of RFID within inventory management becoming more commonplace, the RFID tags in the SPS system could be further developed to integrate with an electronic inventory management database. A centralised inventory management database tailored for a circular pharmaceutical supply chain would need to be developed to minimise waste at all medicine handling facilities. This would entail adjusting the functionality of the system for each separate stage (i.e., a pharmacy would not need the same functionality as a warehouse). At each stage of the CPSC, the database would be able to track the expiry dates of stored medicines, allowing easier stock rotation. If the system’s database was centralised, each facility would be able to share information on quantities of medicines stored throughout the supply chain. This could allow sharing of medicines in times of shortage and redistribution of medicines when there is a surplus to reduce the amount of waste generated by stagnation. The use of RFID not only allows for faster stock management but would also enable the tracking of medicines throughout the PSC. The tracking of flows throughout the supply chain would allow for a more accurate prediction of trends in demand to minimise excess manufacturing.