Recent studies show that searching for new possibilities of phytotherapy using compounds isolated from Centaureinae plants is worth the effort. Treatment based on active substances from plants of the Centaureinae subtribe is often effective and does not cause side effects, as was demonstrated on an example of antifungal infections and SD and melasma and lentigo solaris treatment.
- Centaureinae,
- sesquiterpene lactones
- coumarins
- phytoecdysones
- arbutin
1. Introduction
For a long time, pharmacologists have been analyzing natural compounds, such as sesquiterpene lactones, coumarins, phytoecdysones and phenol glucosides, isolated from the plants of the Asteraceae family.
Special interest has been given to sesquiterpene lactones because of their strong pharmacological properties
[1]
. Sesquiterpene lactones isolated from
Matricariae flos
,
Arnicae flos
and
Millefoli herba
have anti-inflammatory effects
[2]
. It has been established that sesquiterpene lactones can inhibit DTH (Delayed-Type Hypersensitivity test), especially contact dermatitis induced by intratracheal administration of
hapten
[3]
. Guaianolides and germacranolides are the two types of sesquiterpene lactones most commonly found in species of Centaureinae subtribe, Asteraceae family.
It is also interesting to note that the presence of those two types of compounds occurring in the subtribe Centaureinae (Asteraceae) plants is related to the morphological structure of those plants' flowers.
Stizolophus balsamita
(Lam.) K. Koch, is a plant which flowers have appendages (not covering the bracts) with a stout apical spine. There are germacranolides in plants with this morphological feature.
Psephellus bellus
(Trautv.) Wagenitz, is a plant were the appendages of the flowers they are not tipped with a sharp spike. This feature determines the biosynthesis of guaianolides in this Centaureinae plant. The absence of sesquiterpene lactones in some species from Centaureinae plants might be reflected their morphological features (flowers with bracts without appendages) what may be observed in the species of the
Serratula
L genus (
)
[4]
.
Figure 1. Stizolophus balsamita, Psephellus bellus, Serratula coronata
Natural steroids (phytoecdysones) are a class of natural compounds found together with b-arbutin in species of the genus
Serratula
(Centaureinae).
Coumarins, on the other hand, often accompany guaianolides in species of the genus
Psephellus
Cass. (Centaureinae).
Coumarin compounds isolated from Centaureinae plants may be treated as chemotaxonomy markers for the
Psephellus
genus
[4]
. 7-hydroxycoumarin (umbelliferone) can absorb UV light in the range of 280–315 nm, and it is therefore used for anti-UV cosmetics production
[5]
, and it is one of the active substances appearing in the root and herb of
Hieracium pilosella
L., a plant with proven antifungal properties
[6]
.
2. Selected Centaureinae (Asteraceae) Plant Materials in Phytotherapy
2.1. Phytotherapy Possibilities for Treating Fungal Infections
Fungal infections affect about 40% of the world′s population and may be viewed as an epidemiological, therapeutic and social problem. Over a billion people are directly affected by mycoses globally, 150 million of whom have a serious or life-threatening infection
[7]
. Fungal infections can cause serious illnesses, several of which may be fatal if left untreated. Commonly used antibiotics change human microflora and consequently increase the number of people with impaired immunity
[8]
.
Such factors as the development of industry, agriculture, technology and life extension make the population more susceptible to infections
[9]
. The number and variety of fungi causing infections are increasing all over the world. Not only in different parts of the world but sometimes even within one country, there are differences in fungal flora, and species of fungi can be identified with variable frequency of occurrence. Among most commonly occurring fungi infections are
aspergillosis
,
coccidioidomycosis
,
candidosis
,
cryptococcosis
,
mycetomas
,
histoplasmosis
,
mucormycosis
, and
paracoccidio-idomycosis
[10]
.
Conventional antifungal treatment is based on polyene agents, flucitosine and azole agents, or more recently, on virulence factor inhibitors and immunomodulators. This has led to the production of new and improved azoles and polyene formulations, as well as a new family of drugs, the echinocandins
[11]
.
The measurements were taken for three days from
Candida
cultures and 14 days from the dermatophytes and mold fungus (
Scopulariopsis brevicaulis
). The assessment of compounds′ antifungal activity on
Candida albicans
was only possible in the case of cebellins
2
–
4
mixture and cebellin A (
11
) due to the difficulties with growing these fungi strain cultures. Other strains turned out to be easier to grow, which is why the antifungal activity of all of the studied compounds could have been observed
[12]
.
The most significant number of fungi (among them
Candida albicans, Microsporum canis
and
Rhodotorula rubra
) strains turned out to be very susceptible to cebellins
1
–
4
from the
P. bellus
herb (
). These compounds are the most lipophilic of the studied lactones.
[13]
.
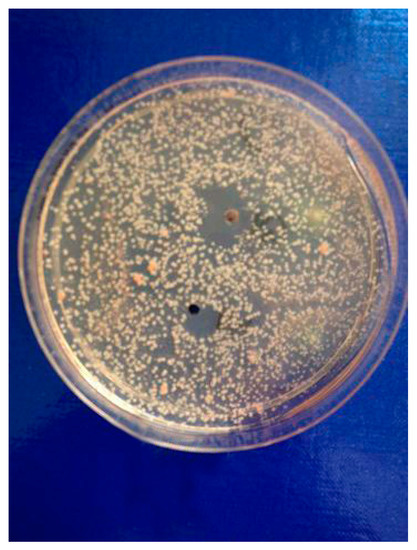
Figure 2. The results of susceptibility assessment of Rhodotorula rubra to the mixture of C-2 ester guaianolides (compound 1 inhibition zone diameter = 12, compounds 2–4 inhibition zone diameter = 22 mm).
Yeast-like fungi
Candida famata
and
C. glabrata,
as well as dermatophytes from the
Trichophyton
genus,
T.
rubrum
and
T. mentagrophytes var. interdigitale,
were the most susceptible to the analyzed compounds. The highest potency of the
P. bellus
herb extract was shown (inhibition zone′s diameter reached 34 mm)
[12]
.
2.2. Possibilities for Phytotherapy in Serotonin Inhibition
Chronic migraine headaches are an important health problem. A headache in a migraine episode is described as hemicranial, pulsating and so intense that it strongly interferes with the patient’s everyday life
[14]
. One explanation of the origin of migraines is the “serotonin theory”, confirmed by the increased excretion of serotonin metabolites in urine during the headache episode. Serotonin (5-HT) is released from platelets, which causes contraction of the smooth muscle of the blood vessels
[15]
. Afterward, as a result of biochemical changes, the serotonin level decreases, causing vasodilation and an increase in vascular permeability, allowing the flow of substances able to lower the sensitivity threshold of perivascular space nociceptor
[16]
.
Parthenolide (
31
) is a sesquiterpene lactone derived from the leaves of Feverfew (
Tanacetum parthenium
) and is considered the main bioactive component of this herb
[17]
. Feverfew is used orally or as an infusion for the treatment of migraine, arthritis, fever and stomachache
[18]
. Parthenolide (
31
) reduces the cellular level of GSH in cancer cells, followed by ROS accumulation and apoptosis
[19]
. Parthenolide’s ability to induce cell death, mainly in cancer cells, while sparing healthy cells is unique and may be linked to the presence of 4,5-epoxide, lactone ring and an exo-methylene
[20]
. The compound also protects normal cells from UVB and oxidative stress, and it seems to have the potential to target some cancer stem cells
[21]
.
2.3. Phytotherapy’s Possibilities in Treating Seborrheic Dermatitis
The bothersome symptoms of seborrheic dermatitis (SD) are difficult to control. SD is a chronic dermatitis characterized by erythema and skin flaking, which occur most often on the face, scalp, ears, chest and body folds—in other words, places with a high concentration of sebaceous glands
[22]
.
20-hydroxyecdysone derivatives found in plants of the
Serratula
genus, when applied to the skin, restore dermis and strengthen protective functions of the epidermis, making the skin more hydrated and resilient. Phytoecdysones may therefore be used in dry and very dry skin care and eases such symptoms as ichtyosis and psoriatic conditions
[23]
. Phytoecdysones are able to activate keratinocytes and increase their amount and differentiation, which is why those compounds are used in projects of creating artificial skin
[24]
.
It is postulated that phytoecdysones may have a significant impact on the reduction of inflammation, probably through their immunomodulatory function and the modulation of proinflammatory cytokines level (e.g., IL-6, TNF-α)
[25]
. Moreover, it has been reported that phytoecdysteroids improve skin quality by accelerating the healing process of wounds and burns
[26]
. Several studies
[27]
have claimed associations between
Malassezia restricta
lipase and seborrheic dermatitis. In light of this, ecdysteroids, especially 20-hydroxyecdysone (
41
), probably enhance antifungal immunity, resulting in the reduction of disease symptoms
[28]
.
Ecdysteroids from plants of the Centaureinae subtribe are characterized by two OH groups on C2 and C3. Some, though only those of vegetable origin, have extra hydroxyl groups on C1, C5 and C11 (
). The presence of those additional groups seems to translate into increased safety in use
[29]
.
Table 1. Pharmacological properties of the compounds and the extracts from selected plants of Centaureinae subtribe.
| Source | Compound | Structure | Properties/Uses |
|---|---|---|---|
| Psephellus bellus herb | Cebellin L (1) Budesinsky et al. (1994) [30] | 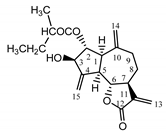 |
anti-inflammatory/antifungal |
| Cebellin O (2) Daniewski and Nowak (1993) [31] | 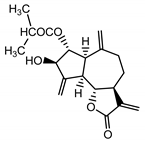 |
anti-inflammatory/antifungal | |
| Cebellin K (3) Budesinsky et al. (1994) [30] |
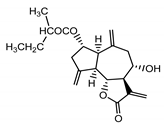 |
anti-inflammatory/antifungal | |
| Cebellin N (4) Daniewski and Nowak (1993) [31] | 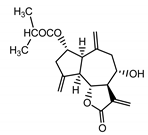 |
anti-inflammatory/antifungal | |
| 19-deoxychloro-janerin (5) El-Dahmy et al. (1985) [32] | 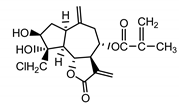 |
anti-inflammatory/antifungal (in extract) | |
| 17,18-epoxy-19-deoxy-chlorojanerin (6) Budesinsky et al. (1994) [31] | 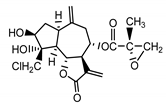 |
anti-inflammatory/antifungal (in extract) | |
| Cebellin M (7) Budesinsky et al. (1994) [31] | 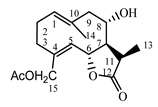 |
anti-inflammatory/antifungal (in extract) | |
| 8-desacylo-8α-(2′-methyl-acryloxy)-subluteolide (8) Bohlmann and Ziesche (1980) [33] | 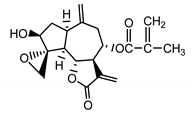 |
anti-inflammatory/antifungal (in extract) | |
| Repin (9) Gonzales et al. (1977) [34] | 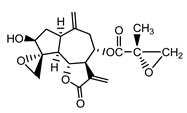 |
anti-inflammatory/antifungal (in extract) | |
| Centaurepensin (10) Gonzales et al. (1974) [35] | 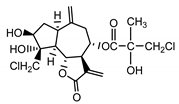 |
anti-inflammatory/antifungal (in extract) | |
| Cebellin A (11) Nowak et al. (1986a) [36] | 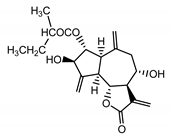 |
anti-inflammatory/antifungal | |
| Cebellin B (12) Nowak et al. (1986a) [36] | 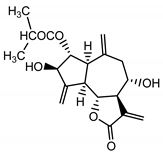 |
anti-inflammatory/antifungal | |
| Acroptilin (13) Estratova et al. (1967) [37] | 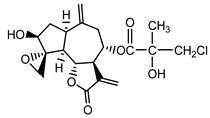 |
anti-inflammatory/antifungal (in extract) | |
| Cynaropicrin (14) Samek et al. (1971) [38] | 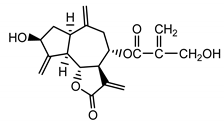 |
anti-inflammatory/antifungal (in extract) | |
| Cebellin F (15) Nowak et al. (1986a) [36] | 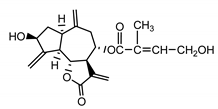 |
anti-inflammatory/antifungal (in extract) | |
| 15-deoxyrepin (16) Nowak et al. (1986b) [39] | 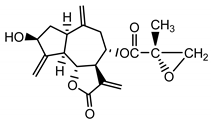 |
anti-inflammatory/antifungal (in extract) | |
| Chlorojanerin (17) Stevens (1982) [40] | 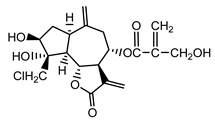 |
anti-inflammatory/antifungal (in extract) | |
| 8-desacetyl-centaurepensin-8-O-(4′-hydroxy)-tiglate (18) Stevens (1982) [40] | 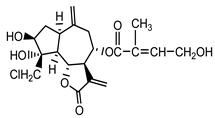 |
anti-inflammatory/antifungal (in extract) | |
| Repensolide (19) Jakupovic et al. (1986) [41] | 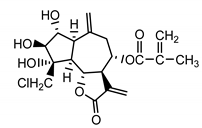 |
anti-inflammatory/antifungal (in extract) | |
| Janerin (20) Gonzales et al. (1977) [34] | 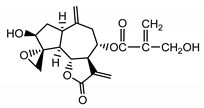 |
anti-inflamatory/antifungal (in extract) | |
| 8-4′-tiglinate-8-desacetyl-subluteolide (21) Budesinsky et al. (1994) [31] | 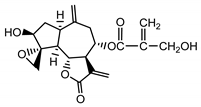 |
anti-inflamatory/antifungal (in extract) | |
| Cebellin G (22) Nowak et al. (1986a) [36] | 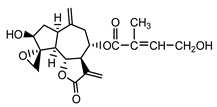 |
anti-inflamatory/antifungal (in extract) | |
| Cebellin H (23) Nowak et al. (1986a) [36] | 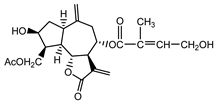 |
anti-inflamatory/antifungal (in extract) | |
| Cebellin I (24) Nowak et al. (1986a) [36] | 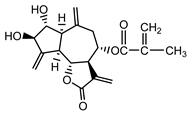 |
anti-inflammatory/antifungal (in extract) | |
| Repdiolide (25) Bohlmann et al. (1982) [42] | 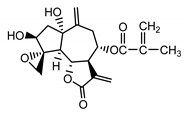 |
anti-inflammatory/antifungal (in extract) | |
| Cebellin J (26) Budesinky et al. (1994) [31] | 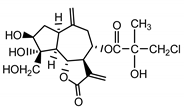 |
anti-inflammatory/antifungal (in extract) | |
| Psephellus sibiricus leaf | Coumarin (27) Dean (1952) [43] | 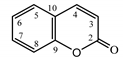 |
anti-inflammatory/antifungal (in extract) |
| Scoparone (28) Ma et al. (2006) [44] | 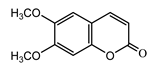 |
anti-inflammatory/antifungal (in extract) | |
| Scopoletin (29) Tsukamoto et al. (1984) [45] | 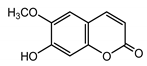 |
anti-inflammatory/antifungal | |
| Umbelliferone (30) Hulting (1967) [46] | 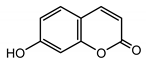 |
anti-inflammatory/antifungal (in extract) | |
| Tanacetum parthenium herb | Parthenolide (31) Hevlett et al. (1996) [47] | 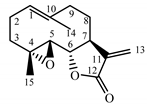 |
anti-inflammatory/antimigraine/ Anticancer |
| Stizolophus balsamita leaf | Balsamin (32) Rybalko et al. (1969) [48] | 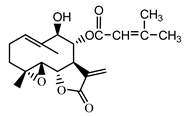 |
anti-inflammatory/antiserotonin (in extract) |
| Izospiciformin (33) Nowak et al. (1989) [49] | 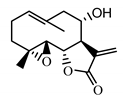 |
anti-inflammatory/antiserotonin (in extract) | |
| Stizolin (34) Mukametzhnov et al. (1971) [50] | 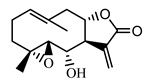 |
anti-inflammatory/antiserotonin (in extract) | |
| 9α-hydroxy-parthenolide (35) Tyson et al. (1981) [51] | 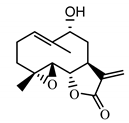 |
anti-inflammatory/antiserotonin (in extract) | |
| 8-E-(4′-hydrohy)-senecioyloxy-9α-hydroxyparthe-nolide (36) Oksuz and Ayyildiz (1986) [52] | 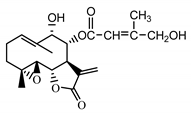 |
anti-inflammatory/antiserotonin (in extract) | |
| 11βH,13-dihydro-stizolicin (37) Nawrot et al. (2019) [4] | 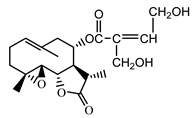 |
anti-inflammatory/antiserotonin (in extract) | |
| Stizolicin (38) Mukametzhanov et al. (1971) [50] | 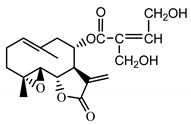 |
anti-inflammatory/antiserotonin | |
| Serratula coronata herb | Ajugasterone C (39) Imai et al. (1969) [53] | 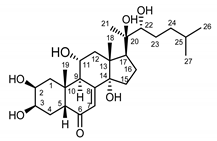 |
anti-Malassesia restricta Seborrheic dermatitis (in extract) |
| Polypodine B (40) Jizba et al. (1967) [54] | 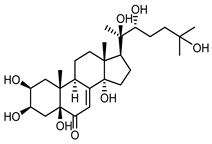 |
anti-Malassesia restricta Seborrheic dermatitis (in extract) | |
| 20-hydroxyecdysone (41) Hocks and Wiechert (1996) [55] | 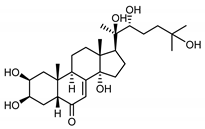 |
anti-Malassesia restricta Seborrheic dermatitis (in extract) | |
| Serratula quinquefolia leaf | β-arbutin (42) Nycz et al. (2010) [56] |
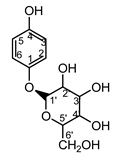 |
hyperpigmentations (in extract) |
2.4. Phytotherapy’s Possibilities in Treating Skin Discoloration
Skin hyperpigmentation is a common cause of patients’ visits to beauty shops and dermatological clinics. Usually located on the exposed parts of the body (face, neck, neckline, forearms and backs of the hands), these changes may be the result of inflammation, endocrine disorders and systemic diseases, as well as UV radiation, phototoxic or photoallergic substances contained in medicines, herbs and cosmetics. They occur due to the disturbance of melanin synthesis and abnormal distribution of melanin in the skin
.
Lighter skin tones have long been associated with youth and beauty among a variety of Asian cultures. Investment in skin-whitening agents, boosted by markets in Asian countries, especially those in China, India and Japan, is increasing annually. Skin color is influenced by a number of intrinsic factors, including skin types and genetic background, and extrinsic factors, including the degree of sunlight exposure and environmental pollution
.
Cosmeceuticals are commonly used for hyperpigmentation. These disorders are generally difficult to treat, hence the need for skin-lightening agents. Arbutin in cream is used as a first choice in treating hyperpigmentation
.
β-arbutin, phenol glucoside, is a compound with anti-inflammatory effect. Moreover, its mechanism of action is based on inhibiting the activity of tyrosinase, a vital enzyme in the process of melanin synthesis
. As it was possible to isolate an unexpectedly large quantity of β-arbutin (
42
) from
St. quinquefolia
plant material without methylarbutin and hydroquinone (
S7)
, water extract from the leaves of
S. quinquefolia
was used for biological studies. The extract was tested during the clinical trial for their efficiency in skin discoloration, specifically melasma and lentigo solaris treatment. A cream containing 2.51% of active compound was applied to the discolored place twice a day—in the morning and the evening (one application of 100 mg = 2.5 mg of the active substance)—for eight weeks
.
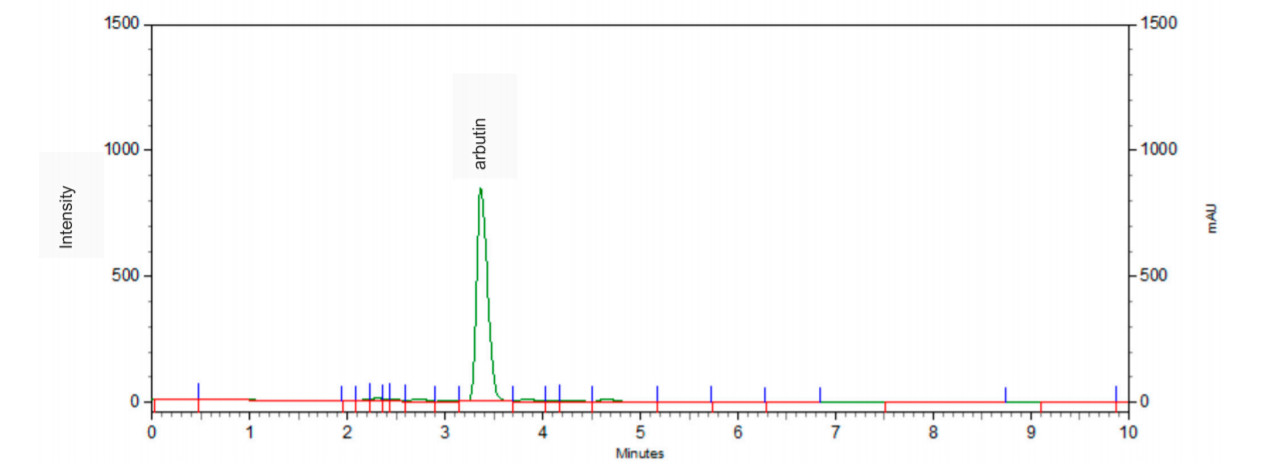
Figure 3. The HPLC chromatogram of water extract from S. quinquefolia leaf.
The cream with this extract decreased melanin level in the skin pigmentation spots. The clinical effect, in the form of lightening and evening skin tone on the discolored side, was observed in 75.86% of the female patients with melasma and 56.00% of the female patients with lentigo solaris. The cream with the aqueous extract from the leaf of five-leaf
The cream with this extract decreased melanin level in the skin pigmentation spots. The clinical effect, in the form of lightening and evening skin tone on the discolored side, was observed in 75.86% of the female patients with melasma and 56.00% of the female patients with lentigo solaris. The cream with the aqueous extract from the leaf of five-leaf
Serratula
proved to be an effective and safe preparation for lightening skin discoloration (66.67% of the female patients in the study group)
.
3. Conclusions
Recent studies show that searching for new possibilities of phytotherapy using compounds isolated from Centaureinae plants is worth the effort. Treatment based on active substances from plants of the Centaureinae subtribe is often effective and does not cause side effects, as was demonstrated on an example of antifungal infections and SD and melasma and lentigo solaris treatment.
There seem to be a correlation between the chemical structure of compounds and their pharmacological properties, which may be helpful, e.g., in selecting the right biological studies for specific compounds. Further research is needed on this issue.
References
- Bruno, M.; Bancheva, S.; Roselli, S.; Maggio, A. sesquiterpenoidsin subtribe Centaureinae (Cass) Dumort (tribe Cardueae, Asteraceae): Distribution, 13C NMR spectra data and biological properties. Phytochemistry 2013, 95, 19–93.
- Lyß, G.; Glasl, S.; Jurenitsch, J.; Pahl, H.L.; Merfort, I. A sesquiterpene and sesquiterpene lactones from Achillea millefolium group possess anti-inflammatory properties but do not inhibit the transcription factor NF-κ B. Pharm. Pharmacol. Lett. 2000, 10, 13–15.
- Matsuda, K.; Kagerura, T.; Toguchida, I.; Ueda, H.; Morikawa, T.; Yoshikawa, M. Inhibitory Effects of Sesquiterpenes from Bay-lef on Nitric Oxide Production in Lipopolisaccharide-actived Macrophages: Structure Requirement and Role of Heat-shock Protein Induction. Life Sci. 2000, 66, 2151–2157.
- Nawrot, J.; Budzianowski, J.; Nowak, G. Phytochemical profiles of leaves of Stizolophus balsamita and Psephellus sibiricus and their chemotaxonomic implications. Phytochemistry 2019, 159, 172–178.
- Malinowska, M.; Bielawska, K. Metabolism and antioxidant properties of coumarins. Bromat. Chem. Toksykol. 2013, 3, 393–403.
- European Medicines Agency. 2015EMA/HMPC/680374/2013 Committee on Herbal Medicinal Products (HMPC) European Union Herbal Monograph on Hieracium pilosella L.; herba cum radice. 2015. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-hieracium-pilosella-l-herba-cum-radice_en.pdf (accessed on 9 November 2020).
- Ghosh, P.N.; Fisher, M.C.; Bates, K.A. Diagnosing Emerging Fungal Threats: A One Health Perspective. Front. Genet. 2018, 54.
- Harsha, M.V.; Venkatachalam, S.; Pooja, M.; Paranjothy, M. Emerging fungal pathogens: A major threat to human life. Int. J. Pharm. Sci. Res. 2017, 8, 1923–1934.
- Rosseeuv, D. Achilles foot screening project: Preliminary results of patients screened by dermatologists. J. Eur. Acad. Derm. Venerol. 1999, 12, 6–9.
- Badiee, P.; Hashenmizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204. [PubMed]
- Segal, E.; Elad, D. Special Issue: Treatments for Fungal Infections. J. Fungi 2018, 4, 135.
- Kamińska, B. Antifungal Activity of Selected Compounds and Extracts of Some Species Centaurea L. Genus. Ph.D. Thesis, Department of Medicinal and Cosmetic Natural Products, Poznan University of Medical Sciences, Poznań, Poland, 2017.
- Barrero, A.F.; Oltra, J.E.; Alvarez, M.; Raslan, D.S.; Saude, D.A.; Aksira, M. New sources and antifungal activity of sesquiterpene lactone. Fitoterapia 2000, 71, 60–64.
- Deen, M.; Christensen, C.E.; Hougaard, A.; Hansen, H.D.; Knudsen, G.M.; Ashina, M. Serotonergic mechanisms in the migraine brain—A systematic review. Cephalalgia 2017, 37, 251–264.
- Bogrdorff, P.; Tangelder, G.J. Migraine: Possible Role of Shear-Induced Platelet Aggregation with Serotonin Release. Headache 2012, 52, 1298–1318.
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Anderson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targetting TRPA1 chanel. Pain 2013, 154, 2750–2758.
- European Scientific Cooperative on Phytotherapy (ESCOP). ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products, 2nd ed.; Thieme: New York, NY, USA, 2003; pp. 492–498.
- Tassorelli, C.; Greco, R.; Morazzoni, P.; Riva, A.; Sandrini, G.; Nappi, G. Parthenolide is the component of Tanacetum parthenium that inhibits nitroglycerin-induced Fos activation: Studies in an animal model of migraine. Cephalalgia 2005, 25, 612–621.
- Lesiak, K.; Koprowska, K.; Zalesna, I.; Nejc, D.; Duchler, M.; Czyź, M. Parthenolide, a sesquiterpene lactone from the medical herb feverfew, shows anti-cancer activity against human melanoma cells in vitro. Melanoma Res. 2010, 20, 21–34.
- Wyrebska, A.; Gach, K.; Janecka, A. Combined effect of parthenolide and various anti-cancer drugs or anticancer candidate substances on malignant cells in vitro and in vivo. Mini Rev. Med. Chem. 2014, 14, 222–228.
- Won, J.K.; Ong, C.N.; Shi, X.; Shen, H.M. Chemopreventive activity of parthenolide against UVB-induced skin cancer and its mechanisms. Carcinogenesis 2004, 25, 1449–1458.
- Dinan, L. Phytoecdysteroids biological aspects. Phytochemistry 2001, 57, 325–339.
- Clark, G.W.; Pope, S.M.; Jaboori, K.A. Diagnosis and treatment of seborrheic dermatitis. Am. Fam. Physician 2015, 91, 185–190.
- Dinan, L.; Lafont, R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J. Endocrinol. 2006, 191, 1–8.
- Patel, S.S.; Savjani, J.K. Systematic review of plant steroids as potential anti-inflammatory agents: Current status and future perspectives. J. Phytopharm. 2015, 4, 121–125.
- Meybeck, A.; Bonté, F. Ecdysteroid-containing liposomes for wound healing and skin regeneration. Chem. Abstr. 1990, 114, 30138.
- Ali, S.; Khan, F.I.; Mohammad, T.; Lan, D.; Hassan, M.I.; Wang, Y. Identification and Evaluation of Inhibitors of Lipase from Malassezia restricta using Virtual High-Throughput Screening and Molecular Dynamics Studies. Int. J. Mol. Sci. 2019, 20, 884.
- Han, P.; Han, J.; Zhang, M. 20-Hydroxyecdysone enhances Immulectin-1ediated immune response against entomogenous fungus in Locusta migratoria. Pest Manag. Sci. 2020, 76, 304–313.
- Dermar, M.; Dumas, M.; Bonte, F. Effect of ecdysterone on the differentiation of normal keratinocytes in vitro. Eur. J. Derm. 1994, 4, 558–569.
- Budesinsky, M.; Nowak, G.; Rychlewska, U.; Hodgson, D.J.; Saman, D.; Daniewski, W.M.; Holub, M. Structure of sesquiterpene lactones of some species of subtribe Centaureinae. Collect. Czech. Chem. Commun. 1994, 59, 1175–1201.Perez-Bernal, A.; Munoz-Perez, M.A.; Camacho, F. Management of facial hyperpigmentation. Am. J. Clin. Derm. 2007, 6, 195–202.
- Daniewski, W.M.; Nowak, G. Further sesquiterpene lactones of Centaurea bella. Phytochemistry 1993, 32, 204–205.Qian, W.; Liu, W.; Zhu, D. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Med. 2020, 20, 173–185.
- El-Dahmy, S.; Bohlmann, F.; Sarg, T.M.; Ateya, A.; Farrag, N. New guaianolides from Centaurea aegyptica. Planta Med. 1985, 51, 176–177.Sarkar, R.; Pooja Arora, P.; Garg, K.V.J. Cosmeceuticals for Hyperpigmentation: What is Available? Cutan. Aesthet. Surg. 2013, 6, 4–11.
- Bohlmann, F.; Ziesche, J. Naturally occurring terpene derivatives. Part 253. New guaianolides and acetyleniccompounds from Ptilostemon species. Phytochemistry 1980, 19, 692–696.Balkrishnan, R.; Kelly, A.P.; Mc Michael, A.; Torok, H. Improved quality of life with effective treatment of facial melasma: The pigment trial. J. Drugs Derm. 2004, 3, 377–381.
- Gonzalez, A.G.; Bermejo, J.; Massanet, G.M. Aportacion al estudio quimiotaxonomico del genero Centaurea. Rev. Lat. Quim. 1977, 8, 176–181.Morąg, M.; Nowak, G.; Michalak, M. The leaves of Serratula quinquefolia M.B. as a new arbutin source. Post. Fitoter. 2013, 1, 17–21.
- González, A.G.; Bermejo, J.L.J.; Bretón, J.L.; Massanet, G.M.; Triana, J. Chlorohyssopifolin C, D, E and vahlenin, four new sesquiterpene lactones from Centaurea hyssopifolia. Phytochemistry 1974, 13, 1193–1197.Morag, M.; Nawrot, J.; Siatkowski, I.; Adamski, Z.; Fedorowicz, T.; Dawid-Pać, R.; Urbańska, M.; Nowak, G. A double-blind, placebo-controlled randomized trial of Serratulae quinquefoliae folium, a new source of β-arbutin, in selected skin hyperpigmentations. J. Cosmet. Derm. 2015, 14, 185–190.
- Nowak, G.; Drozdz, B.; Holub, M.; Budesinsky, M.; Saman, D. Sesquiterpene lactones. XXXI. New guaianolides in Centaurea bella Trautv. and Centaurea adjarica Alb. Acta Soc. Bot. Pol. 1986, 55, 227–231.
- Estratova, R.J.; Rybalko, K.S.; Rzasade, R.Y. Acroptilin—A new sesquiterpene lactone from Acroptilon repens. Khim. Prir. Soed. 1967, 4, 284–286.
- Samek, F.C.; Holub, M.; Drozdz, B.; Jomni, G.; Corbella, A.; Gariboldi, P. Sesquiterpene lactones of Cynara scolymus L. species. Tetrahedrom Lett. 1971, 50, 4775–4778.
- Nowak, G.; Drozdz, B.; Holub, M.; Lagodzinska, A. Sesquiterpene XXXIII. Guaianolides in the subgenus Psephellus (Cass.) Schmalh. genus Centaurea L. Acta Soc. Bot. Pol. 1986, 55, 629–637.
- Stevens, K.I. Sesquiterpene lactones from Centaurea repens. Phytochemistry 1982, 21, 1093–1098.
- Jakupovic, J.; Jia, Y.; Pathak, V.P.; King, R.M. Bisabolone derivatives and sesquiterpene lactones from Centaurea species. Planta Med. 1986, 52, 399–401.
- Bohlmann, F.; Singh, P.; King, R.M.; Robinson, H.E. New guaianolides from Pseudostifftia kingii. Phytochemistry 1982, 21, 1171–1172.
- Dean, F.M. Naturally occurring coumarins. Chem. Org. Nat. 1952, 9, 225–291.
- Morag, M.; Nawrot, J.; Siatkowski, I.; Adamski, Z.; Fedorowicz, T.; Dawid-Pać, R.; Urbańska, M.; Nowak, G. A double-blind, placebo-controlled randomized trial of Serratulae quinquefoliae folium, a new source of β-arbutin, in selected skin hyperpigmentations. J. Cosmet. Derm. 2015, 14, 185–190.
- Tsukamoto, H.; Hisada, S.; Nishibe, S.; Roux, D.G.; Rourke, J.P. Phenolic glucosides from Olea europea subsp. africana. Phytochemistry 1984, 23, 2839–2841.
- Huitink, G.M. Substituted Coumarins as Metallofluorochromic Indicators. Retrospective Theses and Dissertations, Iowa State University, Ames, IA, USA, 1967. Paper 3942.
- Hewlett, M.J.; Begley, M.J.; Groenewegen, W.A.; Heptinstall, S.; Knight, D.W.; May, J.; Salan, U.; Toplis, D. Sesquiterpene lactones from feverfew, Tanacetum parthenium: Isolation, structural revision, activity against human blood platelet function and implications for migraine therapy. J. Chem. Soc. Perkin Trans. 1996, 16, 1979–1986.
- Rybalko, K.S.; Mukametzhanov, M.N.; Sheinchenko, V.I.; Konovalova, O.A. Sesquiterpene lactones of Stizolophus balsamita. Khim. Prir. Soedin. 1976, 12, 467–468.
- Nowak, G.; Drozdz, B.; Budesinsky, M.; Holub, M. Sesquiterpene lactones. XXXVII. Germacranolides in the genus Stizolophus Cass. Acta Soc. Bot. Pol. 1989, 58, 247–251.
- Mukametzhanov, M.N.; Sheinchenko, V.I.; Bankowskii, A.I.; Rybalko, K.S. A sesquiterpene lactone from Stizolophus balsamita. Khim.Prir. Soedin. 1971, 7, 405–406.
- Tyson, R.L.; Chang, C.J.; Mc Laughlin, J.L.; Aynehchi, Y.; Cassady, J.M. 9-hydroxyparthenolide a novel antitumor sesquiterpene lactone from Anvilea garcini (Burm.) DC. Experientia 1981, 37, 441–442.
- Oksuz, S.; Ayyidiz, H. Sesqiuterpene lactones from Stizolophus coronopifolia. Phytochemistry 1986, 25, 535–537.
- Imai, S.; Murta, S.; Koreeda, M. Structure of ajugasterone C, a phytoecdysone with an 11-hydroxy-group. J. Chem. Soc. D. 1969, 10, 546–547.
- Jizba, J.; Herout, V.; Sorm, F. Polypodine B—A novel ecdysones-like substance from plant material. Tedrahedrom Lett. 1967, 18, 1689–1691.
- Hocks, P.; Wiechert, R. 20-hydroxyecdysone isoliert aus insekten. Tetrahedron Lett. 1996, 26, 2089–2993.
- Nycz, E.J.; Małecki, G.J.; Morag, M.; Nowak, G.; Ponikiewski, L.; Switlicka, A.; Kusz, J. Arbutin: Isolation, X-ray structure and computional studies. J. Mol. Struct. 2010, 980, 13–17.
- Ma, C.H.; Ke, W.; Sun, Z.L.; Peng, J.Y.; Li, Z.H.; Zhou, X. Large scale isolation and purification of scoparone from Herba Artemisiae scopariae by high speed counter-current chromatography. Chromatographia 2006, 64, 81–87.
- Perez-Bernal, A.; Munoz-Perez, M.A.; Camacho, F. Management of facial hyperpigmentation. Am. J. Clin. Derm. 2007, 6, 195–202.
- Qian, W.; Liu, W.; Zhu, D. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Med. 2020, 20, 173–185.
- Sarkar, R.; Pooja Arora, P.; Garg, K.V.J. Cosmeceuticals for Hyperpigmentation: What is Available? Cutan. Aesthet. Surg. 2013, 6, 4–11.
- Balkrishnan, R.; Kelly, A.P.; Mc Michael, A.; Torok, H. Improved quality of life with effective treatment of facial melasma: The pigment trial. J. Drugs Derm. 2004, 3, 377–381.
- Morąg, M.; Nowak, G.; Michalak, M. The leaves of Serratula quinquefolia M.B. as a new arbutin source. Post. Fitoter. 2013, 1, 17–21.