Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Meng Wang.
Lonicera japonica Thunb. is a widely distributed plant with ornamental, economic, edible, and medicinal values. L. japonica is a phytoantibiotic with broad-spectrum antibacterial activity and a potent therapeutic effect on various infectious diseases. The anti-diabetic, anti-Alzheimer’s disease, anti-depression, antioxidative, immunoregulatory, anti-tumor, anti-inflammatory, anti-allergic, anti-gout, and anti-alcohol-addiction effects of L. japonica can also be explained by bioactive polysaccharides isolated from this plant.
- Lonicera japonica Thunb.
- polysaccharide
- structural characteristic
1. Introduction
Lonicera japonica Thunb. is a common perennial semievergreen vine belonging to the genus Lonicera of the family Caprifoliaceae [1]. Its flower shows two colors, yellow and white, and is commonly called double flower, two flower, or mandarin duck vine (vernacular names). This phenomenon is attributed to the easy oxidation and structural instability of flavonoids, inositols, saponins, and other components, specifically anthocyanin, which gradually turn yellow after light exposure or at flower maturity. L. japonica flower is fragrant and blossoms in summer, and its pistil and style extend out of the corolla. The beginning of the flowering period is indicated by the appearance of two colors of flowers [2]. The flower or bud in this state has edible, nutritional, and medicinal values. A plant image of Lonicera japonica is shown in Figure 1. In addition, L. japonica can adapt and survive in adverse natural environments, including waterlogging. This characteristic allows for the worldwide distribution of the species, and it mainly grows in temperate and tropical regions in Asia, Europe and Africa [3]. L. japonica is a valuable cash crop that is extensively distributed throughout China. The plant has been used for various purposes and has a long history of use as a traditional Chinese medicine (TCM) [4,5,6][4][5][6]. The nutritional and medicinal properties of L. japonica have gained more attention from health-seeking consumers, nutritionists, and natural botanists.
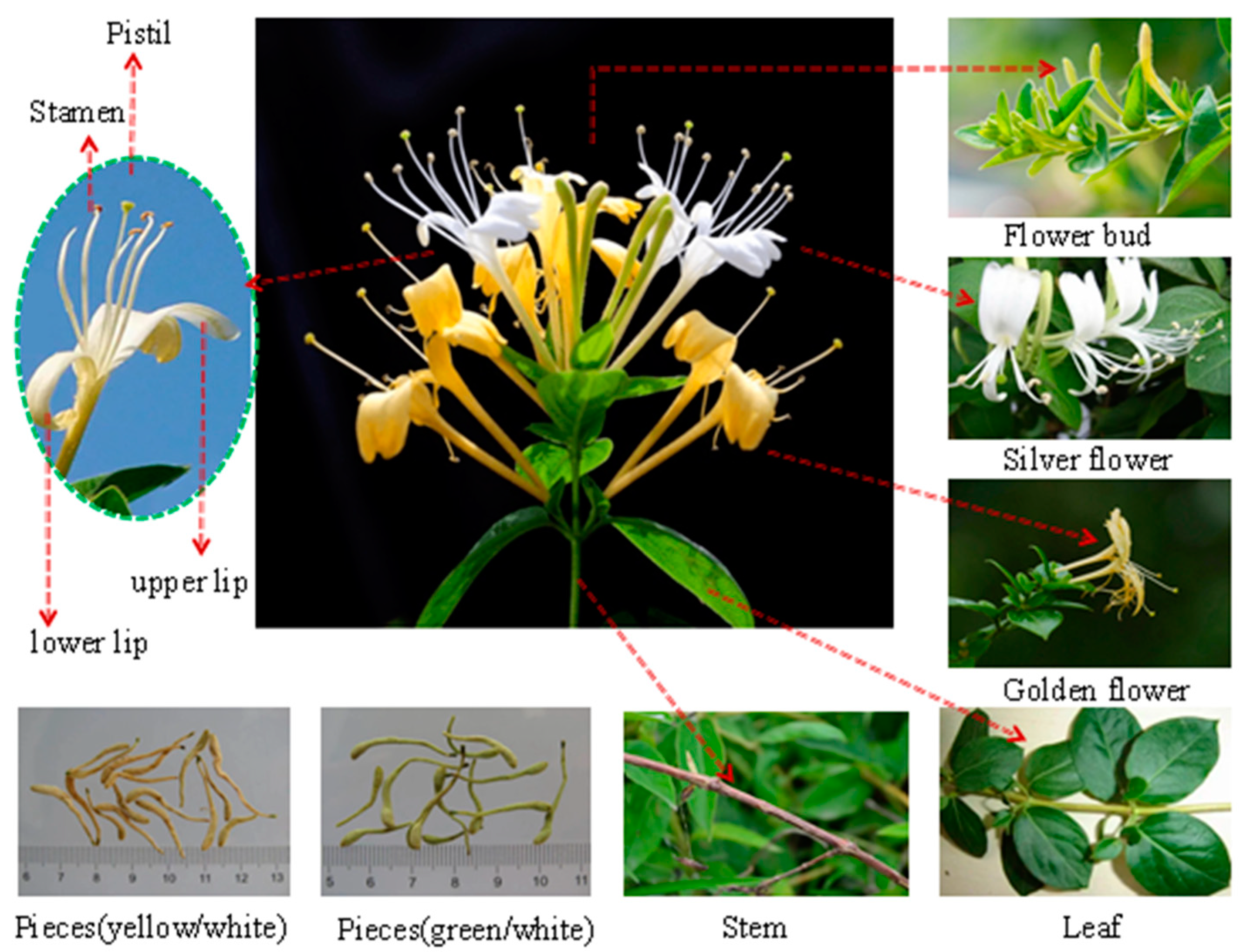
Figure 1. A plant image of Lonicera japonica Thunb. (L. japonica).
L. japonica has been cultivated and consumed for over 1000 years as a nutritional supplement and for treating infections [7]. Notably, it has a broad-spectrum antibacterial activity and is considered a phytoantibiotic for treating infectious diseases [8]. In addition, L. japonica is an important natural antiviral in the empirical medicine system (plant prevention needle for treating viral infections) [9]. L. japonica is the main raw material of the two patented Chinese medicines, Lianhua Qingwen and Shuanghuanglian, which have been approved for the treatment and prevention of COVID-19 worldwide [10,11,12][10][11][12]. The plant has been extensively studied worldwide because of its medicinal uses. L. japonica is a rich source of various bioactive substances, including small-molecule compounds and macromolecular polysaccharides. Several researchers have isolated and purified small-molecule bioactive compounds from L. japonica, including essential oils, saponins, organic acids, iridoids, and flavonoids [13]. The polysaccharides obtained from L. japonica have advanced structures and unique biological properties, including anti-diabetic, anti-Alzheimer’s disease, anti-depression, anti-tumor, anti-inflammatory, anti-allergic, and anti-gout effects [14,15,16][14][15][16]. Moreover, as L. japonica polysaccharides are natural, safe, and minimally toxic with few side effects, they have several prospective uses as a health food, functional food, nutritional supplement, and as medicine.
L. japonica was included in the list of items that are both food and medicine by the National Health Commission of the People’s Republic of China in 2002 [17]. Further, it has been recognized for its edible, nutritional, and pharmaceutical properties with immense health benefits. L. japonica was also included in the Chinese Pharmacopoeia (1963–2020 edition) [18]. Further, L. japonica is an indispensable TCM for the clinical treatment of influenza, fever, headache, and pharyngitis [19]. Conventionally, the bioactive small-molecule compounds in L. japonica were considered active ingredients [20]. However, several polysaccharides have been isolated from L. japonica in recent years, and their diverse structure and unique biological activities are being explored worldwide [21,22][21][22]. L. japonica polysaccharides are a kind of plant polysaccharide, which is mostly extracted by water, enzyme and ultrasonic coenzyme methods. The polysaccharide extracted by these methods has the following advantages: (1) the extracted polysaccharide content is high, (2) there are no of organic solvent residues, and (3) it is safer and more reliable in the development of food, drugs and cosmetics [23,24][23][24]. The safety and efficacy of L. japonica polysaccharides have enabled their use as additives in flower teas, healthy beverages, and throat lozenges. Moreover, they have been used as raw materials for the production of nutritional additives, daily chemical products, cleaning products, and drugs. Overall, the polysaccharide components in L. japonica are being tested for use in food, nutrition, medicine, and other fields because of the high development and economic potential.
L. japonica, as a common TCM with a long history of use, not only has ornamental value but also has medicinal and nutritional value [25]. The polysaccharides obtained from this plant meet the nutritional requirements of the body. There are literature reviews on L. japonica. However, the studies on small-molecule compounds are sorted out, and those on the macromolecular compounds in L. japonica polysaccharides are lacking. With the increase of polysaccharide research in recent years, an increasing quantity of L. japonica polysaccharides has been extracted, so it has become necessary to summarize and extensively examine research on L. japonica polysaccharides. In this review, research on the extraction, purification, chemical structure, health benefits and structure–activity relationship of L. japonica polysaccharides over the past 12 years were examined, and the existing applications and potential of L. japonica polysaccharides in the food, pharmaceutical, and daily chemical field were summarized.
2. Extraction and Purification Methods of L. japonica Polysaccharides
Polysaccharides are polar macromolecules, which are soluble in water and insoluble in ethanol. Therefore, polysaccharides are extracted using polar solvents, such as water (the principle of similar-phase dissolution) [26]. Traditionally, L. japonica polysaccharides are extracted using hot water. Hot water extraction (HWE) is simple to perform, cost-effective, and does not alter the properties of polysaccharides with maximum retention of their activity [27,28,29][27][28][29]. The process is performed multiple times to ensure the complete extraction of the polysaccharides. The plant is soaked in 95% ethanol for 1–2 weeks to remove the lipophilic components and increase the dissolution rate and polysaccharide yield [30,31][30][31]. The yield of L. japonica polysaccharides depends on the extraction time and temperature. The extraction time of the HWE was 33–1500 min, the extraction temperature was 45–100 °C, and the total yield was 3.6–7.6%. Several new extraction methods have been developed for L. japonica polysaccharides. EAE is a polysaccharide extraction method, which has been extensively used for extracting plant polysaccharides. EAE is an environmentally friendly technology with the advantages of having a simple operation, safety, mild reaction conditions, and being non-toxic [32]. Moreover, the use of EAE improves the extraction efficiency, yield, and biological activity of polysaccharides. During this process, the enzymes break down the plant cell wall into small molecules, then intracellular L. japonica polysaccharides are rapidly released in the external medium. However, a combination of multiple methods is preferred to a single extraction method [33,34][33][34]. The Ultrasonic-assisted enzymatic extraction (UAEE) method is a combination of ultrasonic extraction technology and enzymatic hydrolysis, and has a high extraction rate, short extraction time, low extraction temperature, and high efficiency [35,36][35][36]. UAEE has been used to extract polysaccharides from L. japonica leaves. Compared to the HWE process, the UAEE process reduces the extraction time from 8 h to 33 min and increases the yield from 5.3% to 14.76%. After the extraction, the crude L. japonica polysaccharides are precipitated using ethanol. The extracted polysaccharides are still mixed with impurities, including proteins, pigments and small molecules. The Sevag, trichloroacetic acid, and chloroform-n-butanol methods—based on the principle of protein denaturation in organic solvents—are used for removing these impurities [57,58][37][38]. The trichloroacetic acid method is the most frequently used method to remove proteins from L. japonica polysaccharide extraction solutions. The solution is neutralized and centrifuged, and the supernatant is collected and dialyzed to remove small-molecule compounds and then dried to obtain the purified extract. A high-purity polysaccharide extract is the primary condition for the experimental analysis of their structure and biological activity. Further, the purified polysaccharide preparations are used to obtain homogeneous polysaccharides. The most common techniques for purifying polysaccharides is by using an ion-exchange column, gel filtration column, or macroporous adsorption resin chromatography [59,60,61][39][40][41]. The schematic representation of the extraction and purification processes of L. japonica polysaccharides are shown in Figure 2.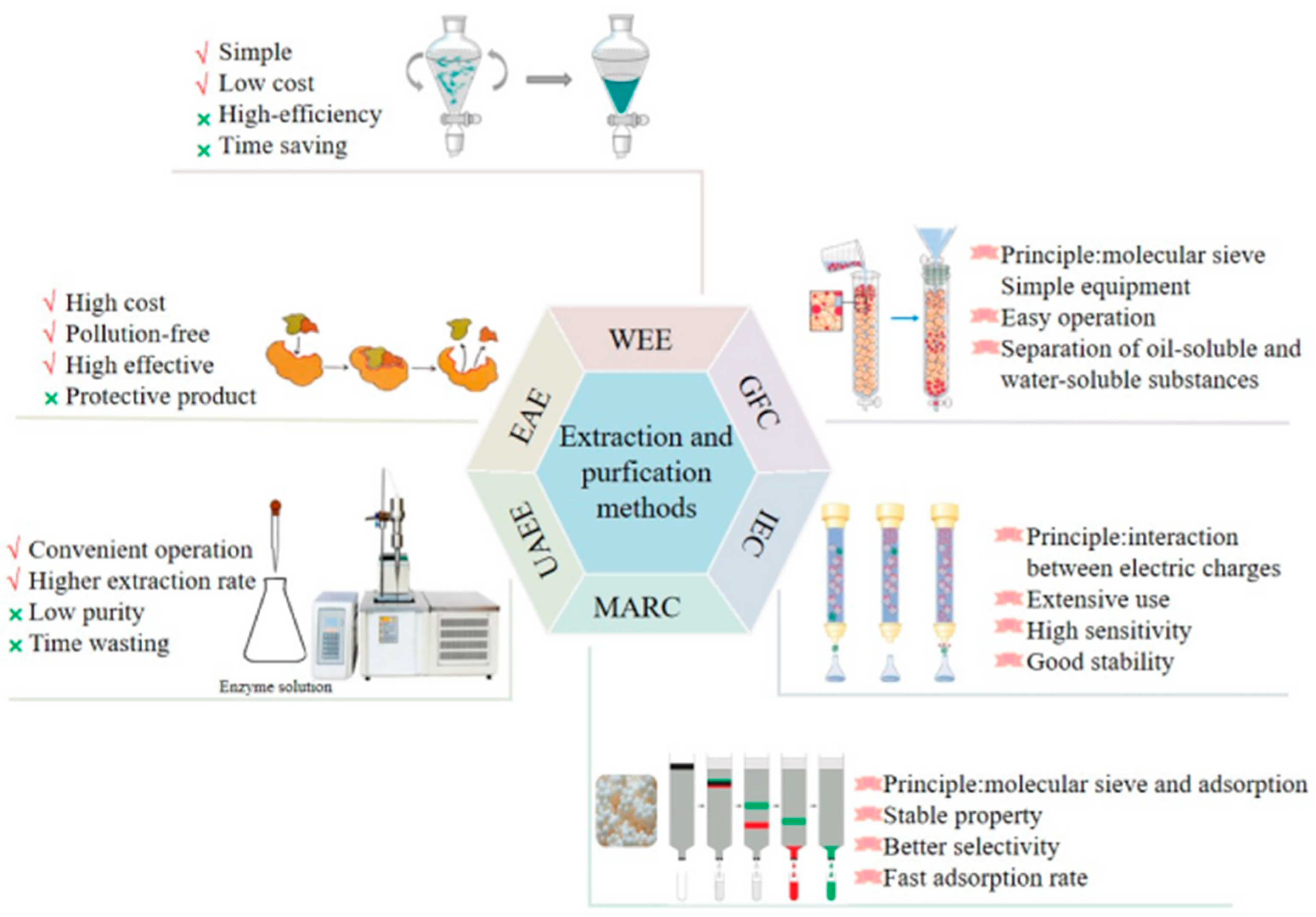
Figure 2. Extraction and purification processes of L. japonica polysaccharides.
3. Structural Characteristics of L. japonica Polysaccharides
Different L. japonica polysaccharides have different structures and biological properties [62][42]. The reported chemical structures of polysaccharides are shown in Figure 3.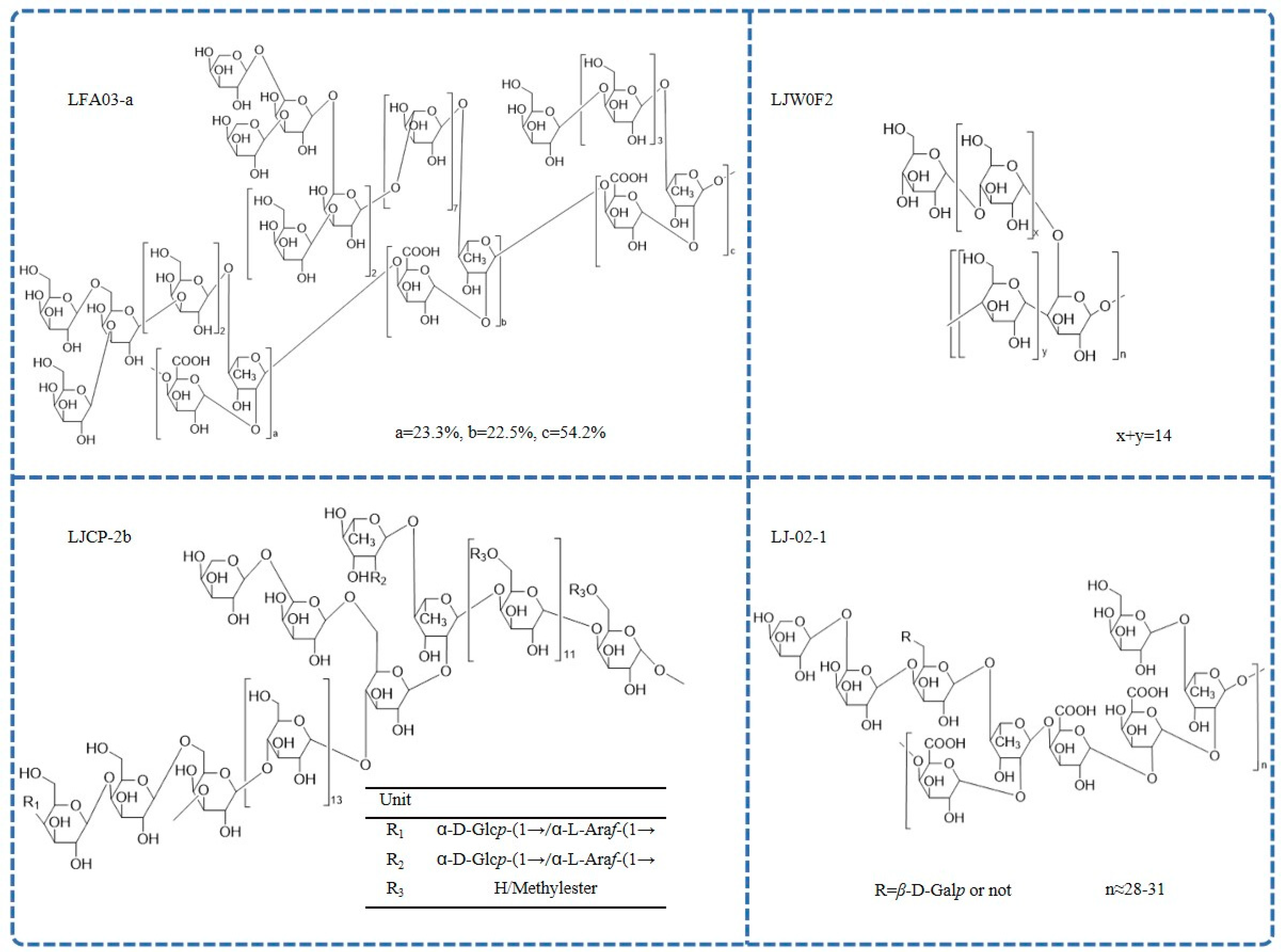
Figure 3. The chemical structure of L. japonica polysaccharides.
3.1. Molecular Weight
The reported average molecular weights (Mw) of L. japonica polysaccharides are variable in different studies because of the variations in plant parts used, extraction methods, purification processes, and analytical methods. For example, the Mw of LJCP-2b extracted from L. japonica caulis using HWE was 7.0 kDa, and the Mw of HEP-4 obtained from L. japonica flowers using the same extraction method was 198 kDa [49,56][43][44]. L. japonica flower polysaccharides have an average molecular weight of approximately <1500 kDa. Pectin polysaccharides have a molecular weight range of 7.2–400 kDa, whereas water-soluble polysaccharides have a range of 3.8–198 kDa. A L. japonica crude polysaccharide (LJP-b) was completely separated into five purified fractions: LJP-N, LJP-A-1, LJP-A-2, LJP-A-3, and LJP-A-4. Their average molecular weight range identified using linear regression analysis was 3.9–383.8 kDa [39][45].3.2. Monosaccharide Composition
The content and ratio of monosaccharides provide the basis of the chemical structure of polysaccharides. The study of their structural characteristics is important because the structure is correlated to the biological activity of polysaccharides. With the use of acidic hydrolysis, derivatization, Fourier-transform infrared spectroscopy (FT-IR), nuclear magnetic resonance (NMR) spectroscopy, gas chromatography (GC), and high-performance liquid chromatography (HPLC), the monosaccharide composition of L. japonica polysaccharides has been investigated and analyzed [63,64,65,66,67][46][47][48][49][50]. The monosaccharide compositions of polysaccharides extracted from the flower, flower bud, stem, branch, and leaf are different, but they are mainly composed of different molar fractions of glucose (Glc), galactose (Gal), rhamnose (Rha), arabinose (Ara), mannose (Man), and xylose (Xyl). Interestingly, ribose (Rib) was detected only in L. japonica leaf polysaccharides (LJLP). LJLP was composed of 32.3%, 20.9%, and 15.2% of Gal, Glc, and Rib, respectively [55][51]. Notably, some L. japonica polysaccharides are homogeneous. A monosaccharide composition analysis indicated that LJW0F2 was composed of 99.7% glucose, and LJW2F2 isolated from L. japonica flowers was composed of 98.2% galacturonic acid [47,48][52][53]. The monosaccharide composition of L. japonica polysaccharides extracted from the stem was Glc:GalA:Ara:Gal:Rha:Xyl:Man:GlcA = (3.89:2.49:1.87:1.51:1.00:0.40:0.21:0.19) [56][44]. In conclusion, the monosaccharide composition of polysaccharide depends partly on the differences in the extraction, purification, and analysis methods.3.3. Chemical Structures
Pectin polysaccharides are the most common type of L. japonica polysaccharides. A pectic polysaccharide named LFA03-a was obtained from the water extract of L. japonica flowers for the first time. The structure of LFA03-a included a rhamnogalacturonan I (RG-I) backbone consisting of repeating units of α-l-1,2-Rhap and α-d-1,4-GalAp disaccharide with a substitution of rhamnose at C4. The side chain was involved with β-d-1,4-Galp; β-d-1,3-Galp; β-d-1,3,6-Galp; and branched α-l-1,5-Araf [21]. Similarly, the structure of another pectin polysaccharide, WLJPA0.2b, was studied using FT-IR spectra, enzymatic hydrolysis, de-esterification, partial acid hydrolysis, NMR spectra, and electrospray ionization-mass spectrometry (ESI-MS) analysis. This polysaccharide consisted of homogalacturonan (HG) along with RG-I and RG-II domains (mass ratio 2.1:0.4:1.0). Highly branched α-l-1,5-arabinan; β-d-1,4-galactan; and type II arabinogalactan side chains were found in the RG-I domain [44][54]. In addition, another pectin polysaccharide, LJ-02-1, was also isolated, and its backbones comprised→4)-α-d-GalpA-(1→2)-α-l-Rhap-(1→, which was substituted partly at the C4 position of rhamnose by side chains, including 1,4,6-linked β-d-Galp; 1,5-linked α-l-Araf; T-linked α-l-Araf; and T-linked β-d-Galp [46][55]. Further, a limited number of reports on the structure of neutral L. japonica polysaccharides are also available. Methylation, HPLC, FT-IR, and extensive 1D- and 2D-NMR were used to examine the detailed structure of a neutral polysaccharide (LJW0F2) from L. japonica flowers. The absorption at 929.2 cm−1 in the infrared spectrum is a typical signal of d-Glc in pyranose. In the 13C NMR spectrum, only one anomeric resonance signal at δ101.00 was present, indicating α-glycosidic linkages. The result showed that the linkages of LJW0F2 consisted of terminal glucose; 1,4-linked glucose; and 1,4,6-linked glucose in the molar ratio of 1:14:1 [47][52]. In addition, there was a neutral-fraction LJP-N that was a starch-like glucan with some arabinogalactan or arabinan domains [37][56]. Another homogeneous heteropolysaccharide, LJCP-2b, mainly consisted of 1,3,6-β-d-Galp; 1,4-α-d-Glcp; 1,4,6-α-d-Glcp; 1,4-β-d-Galp; 1,2,4-α-l-Rhap; and 1,4-α-d-GalpA [56][44]. FT-IR spectrometric analysis showed that LJP-d had typical polysaccharide absorption characteristics at 4000–100 cm−1, which was caused by the vibration of O-H bonds and C-H bonds in sugar compounds and the crystalline water of sugar compounds [42][57].4. Health Benefits of L. japonica Polysaccharides
L. japonica is a medicinal and edible plant with great research and development value [68][58]. As one of its active ingredients, L. japonica polysaccharides have anti-diabetic, anti-Alzheimer’s disease, anti-depression, antioxidation, immunoregulatory, anti-tumor, anti-inflammatory, anti-allergic, anti-gout, and anti-alcohol-addiction activities. Many in vitro and in vivo studies have been conducted on the biological activity and health benefits of L. japonica polysaccharides. The combined health benefits are shown in Figure 4.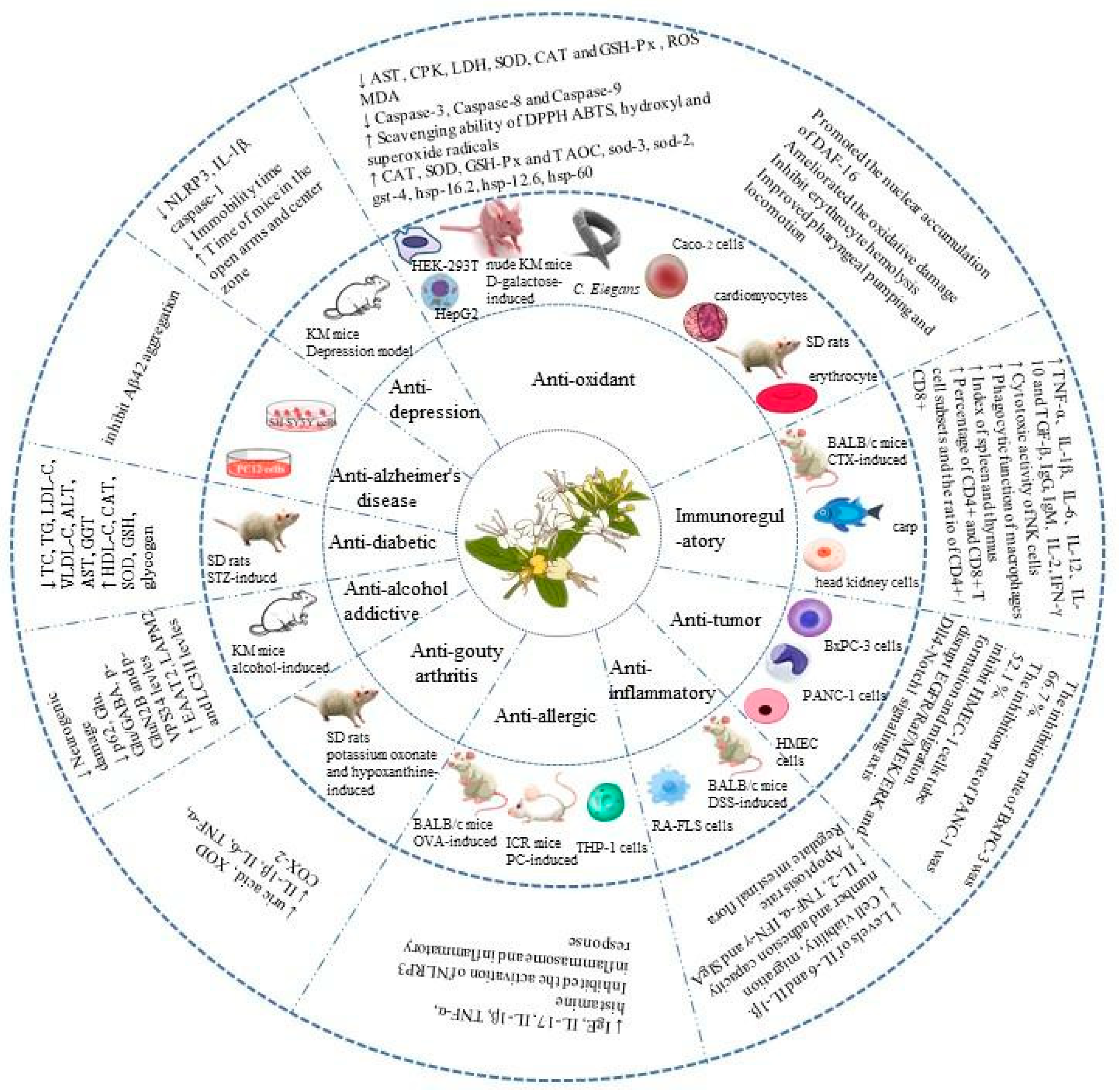
Figure 4. Health benefits of L. japonica polysaccharides.
4.1. Anti-Diabetic Effects
Diabetes mellitus is a global public health problem and is a group of metabolic diseases caused by insulin secretion and utilization disorders, which have multiple causes [69][59]. LJP-w, a crude polysaccharide purified from the water extract of L. japonica, had hypoglycemic and hypolipidemic effects in a rat model of streptozotocin (STZ)-induced diabetes, and it protected the mice from STZ-induced oxidative stress. Compared with the levels in the model group, the oral administration of 200, 400, and 800 mg/kg LJP-w for six weeks markedly reduced fasting blood glucose levels in the treatment groups with a notable reduction in body weight, food consumption, and water intake. In addition, the pyruvate kinase concentrations and hexokinase activity in diabetic rats treated with LJP-w were restored from 37.6 ± 5.8 and 5.0 ± 0.8 U/g protein to 107.70 ± 6.90 and 8.80 ± 0.70 U/g protein, respectively, suggesting that LJP-w alleviated insulin resistance. The levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterin (LDL-C), very-low-density lipoprotein-cholesterin (VLDL-C), and high-density lipoprotein-cholesterin (HDL-C) in serum were detected using commercial enzyme kits. The results indicated that treatment with 800 mg/kg LJP-w significantly decreased the levels of plasma TG, TC, LDL-C, and VLDL-C to 1.60 ± 0.20, 1.70 ± 0.20, 0.47 ± 0.07, and 1.03 ± 0.32 mmol/L, respectively, and significantly increased the HDL-C levels to 0.79 ± 0.07 mmol/L (p < 0.05). These values were similar to those obtained in the normal control group. These findings indicated that LJP-w had an inhibitory effect on the lipid content in the blood. Moreover, LJP-w increased the antioxidant capacity of serum and liver. Oxidative stress is a core pathogenic mechanism in diabetes. The levels of alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyl transpeptidase (GCT) in the sera of diabetic rats treated with 800 mg/kg LJP-w decreased to 48.70 ± 7.7, 81.90 ± 15.10, and 38.30 ± 4.60 IU/L, respectively, whereas the levels of catalase (CAT), superoxide dismutase (SOD), and glutathione (GSH) in the liver increased to 84.50 ± 8.20 U/mg, 300.90 ± 23.00 U/mg, and 9.80 ± 0.70 mg/g protein, respectively. Further, the effects of LJP-w on the activities of α-amylases and α-glucosidases were evaluated in vitro using acarbose as a positive control. These two enzymes hydrolyze polysaccharides, and the inhibition of their activity decreases the production of glucose, thereby controlling postprandial blood sugar levels. The IC50 values of LJP-w for α-amylase and α-glucosidase were 61.2 ± 3.1 and 45.6 ± 1.9 μg/mL, respectively, which were comparable with those of the acarbose treatment group. Taken together, LJP-w exerts anti-diabetic effects by reducing hyperglycemia, decreasing hyperlipidemia, and increasing the antioxidant capacity of serum and liver. Therefore, LJP-w can be incorporated into functional foods for reducing blood glucose levels and may be used for designing novel therapeutic strategies for diabetes [53][60].4.2. Anti-Alzheimer’s Effects
Alzheimer’s disease (AD) is the most common type of senile dementia, which threatens the physical and mental health of the elderly population worldwide. “Amyloid hypothesis” is currently recognized as the main pathogenic mechanism of Alzheimer’s disease. The accumulation of misfolded Aβ peptides in the central nervous system results in plaque formations in the brain and the disruption of neuronal function, and these Aβ aggregates have been extensively studied to develop treatment strategies for AD [70,71][61][62]. Current research focuses on blocking Aβ aggregation and reducing its neurotoxicity for the prevention and treatment of AD [72,73][63][64]. Thioflavin T (ThT) as well as atomic force microscopy (AFM) and fluorescence spectroscopy were used to determine the inhibitory effect of different doses of a glucan (JLW0F2), which was isolated from L. japonica, on Aβ42 aggregation. Compared with that of Aβ42 incubated alone, the aggregation inhibition rate of Aβ42 incubated with 200 g/mL of LJW0F2 was >90% in the ThT spectroscopic assay. The results of the AFM suggested that LJW0F2 delayed the formation of Aβ42 fibrils in a dose-dependent manner. Further, the neurotoxicity of Aβ42 was evaluated by incubating SH-SY5Y cells (human neuroblastoma cells) with Aβ42 alone and with different concentrations of LJW0F2 (0.1, 1, and 10 g/mL) for seven days, and the viable cell count was determined using the CCK-8 assay. Compared to the control treatment, the LJW0F2 treatment attenuated the cytotoxicity of the Aβ42 aggregates on the SH-SY5Y cells [47][52]. Subsequently, the research group determined that a pectin polysaccharide extracted from L. japonica (LFA03-a) also inhibited Aβ42 aggregation. In addition, LFA03-a induced the differentiation of PC12 neuronal cells derived from a transplantable rat pheochromocytoma to promote neuritogenesis. The PC12 cell line was used to determine the degree of neuronal differentiation and neurosecretion after the LFA03-a treatment to investigate the potential effect of LFA03-a on neurite outgrowth, and 25 ng/mL of nerve growth factor (NGF) was used as a positive control. The results showed that the LFA03-a (1 mg/mL) treatment promoted the neurogenesis of PC12 cells [21]. In summary, the polysaccharides LJW0F2 and LFA03-a can inhibit the aggregation of Aβ42, reduce neurotoxicity, and promote neurogenesis. These results suggest that L. japonica polysaccharides may be used for the prevention and treatment of neurodegenerative disorders and nervous system injuries.4.3. Anti-Depressant Effects
Depression is a common disease worldwide with increasing morbidity and mortality, and its main clinical features include low mood, slowed thinking, decreased will, despair for the future, and even suicidal thoughts [74][65]. LJP-l, a polysaccharide from L. japonica, has anti-depressant effects. Seven different methods were used to randomly stimulate mice for 21 days for establishing a depression model. The behavioral tests of mice, including open-field, elevated plus maze, tail suspension, and forced swim tests, were performed after administrating fluoxetine (Flu) and LJP-l. The LJP-l treatment increased the time of opening arms and reduced the resting time of depressed mice. The LJP-l treatment also increased the number of nerve cells and ameliorated the irregular arrangement and deformation of nerve cells in the hippocampus of depressed mice. Western blot analysis showed that LJP-l protected the nerve cells in depressed mice by inhibiting the NLRP3 inflammasome pathway and reducing the production of caspase-1 and IL-1β. However, the effect of LJP-l was more significant than Flu, suggesting that the NLRP3 inflammasome pathway may not be targeted by drugs. Therefore, the NLRP3 inflammasome may be involved in the pathogenesis of depression [52][66]. However, more animal experiments and clinical studies are needed to further confirm the effect of L. japonica polysaccharides for treating depression.4.4. Antioxidant Effects
Oxidative stress is a state of imbalance of free radicals and antioxidants in the body, which results in molecular and cellular damage. Antioxidants protect the body from free radical damage by scavenging the free radicals [75][67]. A water-soluble polysaccharide, HEP-4, extracted from L. japonica exerted antioxidant effects on human hepatoma cells (HepG2). The antioxidant activity of HEP-4 was evaluated using four free-radical scavenging methods, namely 1,1-diphenyl-2-picryl-hydrozyl, (DPPH); hydroxyl (OH); 2,2-azidobisphenol (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS); and superoxide radical scavenging tests. The results showed that the scavenging activities of the DPPH, OH, ABTS, and superoxide radical increased with the increase in the HEP-4 concentration, and the scavenging rates were 69.7%, 40.6%, 92.5%, and 39.8%, respectively, at 1.0 mg/mL of HEP-4. In addition, 800 μg/mL of HEP-4 reduced the concentrations of reactive oxygen species (ROS) and malondialdehyde (MDA) as well as enhanced the activity of CAT and glutathione peroxidase (GSH-Px). All in all, HEP-4 had a protective effect on H2O2-exposed HepG2 cells [49][43]. WLJP-A0.2b, a pectin polysaccharide, was derived from L. japonica, and it was composed of RG-I (WLJP-A0.2b-E1), RG-II (WLJP-A0.2b-E2), and HG domains. Enzymatic hydrolysis of the HG domain produced unesterified and partly methyl-esterified and acetyl-esterified oligogalacturonides (WLJP-A0.2b-E3). The antioxidant activity of different domains was compared, and the results of the DPPH radical scavenging test indicated that the IC50 values for WLJP-A0.2b-E1, -E2, and -E3 were 125.27 ± 0.91, 86.81 ± 0.41, and 27.06 ± 0.53, respectively. The evaluation of HEK-293T cells treated with H2O2 showed that oligogalacturonides protected HEK-293T cells from oxidative damage by inhibiting ROS production [44][54]. Four acidic fractions (LJP-A-1 to LJP-A-4) and a neutral fraction (LJP-N) were obtained from the crude polysaccharide fraction of L. japonica, and their antioxidant potential was determined using six methods [39][45]. In addition, LJLP (heteropolysaccharide) showed antioxidant activity in a mouse model of d-galactose-induced oxidative stress. LJLP decreased the MDA concentrations, increased the activities of CAT, SOD, and GSH-Px, and improved the total antioxidant capacity (TAOC) in the serum and liver of mice. Simultaneously, the scavenging rate of LJLP for superoxide radicals was detected in vitro with ascorbic acid as the standard. The results indicated that the concentration of LJLP was positively correlated with its clearance rate, and LJLP showed a stronger clearance ability when at concentration of 1.0 mg/mL [55][51]. Oxidative stress is one of the important factors leading to aging and disease. Aging is inevitable; however, it is possible to delay aging [76,77][68][69]. Caenorhabditis elegans (C. elegans) and Caco-2 cells were used as models to study the anti-aging and antioxidant effects of crude L. japonica polysaccharide (CLJP) and its purified fraction (LJP-2-1). The anti-aging activity was evaluated by observing the lifespan, exercise capacity, lipofuscin accumulation, and the regulation of daf-16, sod-3, gst-4, and hsp genes of C. elegans before and after administrating the polysaccharides. The results showed that the average lifespans of nematodes in the groups treated with 200 μg/mL of CLJP and LJP-2-1 were prolonged by 13.97% and 11.35%, respectively, and the lipofuscin concentration was lower in the treated groups compared to that in the control group. CLJP did not have any negative effect on the fecundity and body length of nematodes and had a protective effect on oxidative and heat stress in nematodes. The CLJP treatment had an antioxidant effect and increased the activity of antioxidant enzymes in C. elegans. Compared with that of the control group, the SOD activity of the CLJP (400 μg/mL) group increased by 2.81 ± 0.05-fold (p < 0.001), and the CAT activity increased by 2.32 ± 0.07-fold (p < 0.001). The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl terazolium bromide (MTT) assay showed that the CLJP pretreatment notably alleviated the H2O2-induced oxidative damage in Caco-2 cells. In addition, the nuclear localization of HSP-16.2⸬GFP in the CLJP (200 μg/mL) group was 28.97% higher than that in the control group (p < 0.01), and the mRNA expression levels of daf-16, dod-3, skn-1, and daf-12 were significantly higher than those in the control group. Therefore, CLJP affected the lifespan and health of nematodes by mediating DAF-16 [45][70]. An excess of free radicals in the human body can cause atherosclerosis, neurologic diseases, and other diseases. Therefore, antioxidants are crucial in the prevention and management of cardiovascular abnormalities. The L. japonica polysaccharide LJP-z had a protective effect on cardiomyocytes injured by H2O2. The LJP-z-treatment (10, 20, and 40 μg/mL) enhanced the viability of injured cardiomyocytes and reduced cell damage and apoptosis [41][71]. Moreover, a rat model of ischemia/reperfusion was established using the modified middle cerebral artery occlusion (MCAO) method to study the neuroprotective effect of a water-soluble polysaccharide (LJPB2). The polysaccharide reduced the MDA concentration, decreased NO production and enhanced the activity of SOD and GSH-Px. Further, it showed strong DPPH free-radical scavenging ability in vitro [22]. Taken together, L. japonica polysaccharides have excellent antioxidant activity and may be used as effective therapeutic agents for oxidative-stress-related diseases.4.5. Immunoregulatory Effects
Immune regulation is the most important biological activity of polysaccharides. Immunologically active polysaccharides have been extensively studied because of their numerous sources, low price, positive effects, purity, and minimal side effects [78][72]. Several studies have reported the immunomodulatory activity of L. japonica polysaccharides and have compared the immunostimulatory activities of the two L. japonica polysaccharides, LJP-N and LJP-A. A single intraperitoneal injection of cyclophosphamide (CTX) induced immunosuppression in BALB/c mice, then LJP-N (50 mg/kg) and LJP-A (200 mg/kg) were intragastrically administered to these mice. Compared with those in CTX model mice, the spleen and thymus indexes were higher in mice treated with LJP-N and LJP-A, and the spleen index of the LJP-A group was significantly higher than that of the LJP-N group. In addition, the concentrations of interleukin (IL)-2, IL-6, tumor necrosis factor (TNF)-α, immunoglobulin (Ig)G, and IgM in the LJP-A-treatment group were increased by approximately 2.0-, 2.5-, 1.4-, 1.7-, and 1.2-fold compared with those in the CTX model group (p < 0.001), suggesting that LJP-A exerted an immunomodulatory effect by increasing the cytokine secretion and immunoglobulin concentrations. Simultaneously, the phagocytosis of the LJP-N and LJP-A components were determined by injecting India ink into the tail vein of the female BALB/c mice. The phagocytic rate and index in the LJP-A groups were markedly higher at both low and high concentrations (50 and 200 mg/kg, respectively) of polysaccharide, whereas the phagocytosis parameters in the LJP-N group were significantly higher only at a high concentration (200 mg/kg). In addition, both LJP-N and LJP-A enhanced the cytotoxic activity of natural killer (NK) cells. However, the apoptosis rate of YAC-1 cells (the targets of NK cells) in the LJP-A-treated mice was higher than that in the LJP-N-treated mice [37][56]. In a similar study, LJP-d, a polysaccharide extracted from L. japonica using EAE, improved the immune function in CTX-induced immunosuppressed mice. Further, LJP-d increased the CD4+/CD8+ T-cell counts, thereby suggesting that it enhanced the cellular immune response in the immunosuppressed mice [42][57]. The immunomodulatory activity of a water-soluble polysaccharide (HP-02) from L. japonica was evaluated in carp fish. The researchers evaluated the effects of different concentrations of HP-02 (250, 500, and 1000 μg/mL) on the proliferation and phagocytic activity of head kidney cells and the secretion of cytokines in cephalic kidney cells extracted from the common carp. The results showed that the HP-02 treatment increased proliferative and phagocytic activity and stimulated the secretion of TNF-α, IL-1β, IL-6, IL-10, and transforming growth factor-β (TGF-β) in the head kidney cells of carp fish. In addition, the serum concentrations of these cytokines were higher in the HP-02-treated fish than in the untreated fish. Thus, HP-02 showed notable immunoenhancement effects in carp fish [51][73]. Overall, L. japonica polysaccharides can be potentially used as adjuvant immunomodulators in therapeutic drugs and functional foods.4.6. Anti-Tumor Effects
Tumors are solid tissue masses formed by abnormal cells, which are categorized as benign and malignant tumors (cancers). Cancer has become one of the most serious diseases endangering human health, and its incidence and mortality are increasing worldwide [79][74]. Pancreatic cancer is one of the common malignant tumors of the digestive tract (also known as the “king of cancer”). The 5-year survival rate of pancreatic cancer after diagnosis is only 10% with negligible treatment options [80][75]. A homogeneous polysaccharide, LJ-02-1, from the crude polysaccharide of L. japonica was evaluated for anti-pancreatic cancer effects. BxPC-3 and PANC-1 pancreatic cancer cells were treated with different concentrations of LJ-02-1 (0.016, 0.031, 0.063, 0.125, 0.250, 0.500, and 1 mg/mL) for 72 h. The results of the MTT assay showed that the inhibition rates of the proliferation of BxPC-3 and PANC-1 cells treated with 1 mg/mL LJ-02-1 were 66.7% and 52.1%, respectively, in a dose-dependent manner. Moreover, LJ-02-1 was minimally cytotoxic to normal liver cells (LO2) with only a 26.2% inhibition rate [46][55]. Anti-angiogenesis therapy is a new strategy for tumor treatment in recent years and has shown remarkable therapeutic outcomes in several cancers [81,82,83][76][77][78]. A homogeneous polysaccharide, LJW2F2, blocked angiogenesis by affecting the formation of capillary-like tubes and cell migration in HMEC-1 (human microvascular endothelial cells). Epidermal growth factor receptor (EGFR), which is essential for cell proliferation and signal transduction, is converted from monomer to dimer after binding with specific ligands. The autophosphorylation of this dimer guides the phosphorylation of the downstream Raf/MEK/ERK pathway in angiogenesis. LJW2F2 inhibits the phosphorylation of EGFR and its downstream pathways. In addition, LJW2F2 also partially inhibits the Notch1/Dll4 signaling pathway. Therefore, the anti-tumor mechanism of LJW2F2 is associated with the inhibition of the EGFR/Raf/MEK/ERK and Dll4/Notch1 signaling pathways [48][53]. Therefore, L. japonica polysaccharides have potential applications in anti-tumor therapies.References
- Li, Y.; Li, W.; Fu, C.; Song, Y.; Fu, Q. Lonicerae japonicae flos and Lonicerae flos: A systematic review of ethnopharmacology; phytochemistry and pharmacology. Phytochem. Rev. 2020, 19, 1–61.
- Zheng, S.; Liu, S.; Hou, A.; Wang, S.; Na, Y.; Hu, J.; Jiang, H.; Yang, L. Systematic review of Lonicerae Japonicae Flos: A significant food and traditional Chinese medicine. Front. Pharmacol. 2022, 13, 1013992.
- Ge, L.; Xie, Q.; Jiang, Y.; Xiao, L.; Wan, H.; Zhou, B.; Wu, S.; Tian, J.; Zeng, X. Genus Lonicera: New drug discovery from traditional usage to modern chemical and pharmacological research. Phytomedicine 2022, 96, 153889.
- Tang, Y.; Yin, L.; Zhang, Y.; Huang, X.; Zhao, F.; Cui, X.; Shi, L.; Xu, L. Study on anti-inflammatory efficacy and correlative ingredients with pharmacodynamics detected in acute inflammation rat model serum from Caulis Lonicerae japonicae. Phytomedicine 2016, 23, 597–610.
- Zhang, L.; Long, Y.; Fu, C.; Xiang, J.; Gan, J.; Wu, G.; Jia, H.; Yu, L.; Li, M. Different Gene Expression Patterns between Leaves and Flowers in Lonicera japonica Revealed by Transcriptome Analysis. Front. Plant. Sci. 2016, 7, 637.
- Guo, C.; Zhang, X.; Yu, Y.; Wu, Y.; Xie, L.; Chang, C. Lonicerae Japonicae Flos extract and chlorogenic acid attenuates high-fat-diet- induced prediabetes via CTRPs-AdipoRs-AMPK/PPARα axes. Front. Nutr. 2022, 9, 1007679.
- Su, X.; Zhu, Z.H.; Zhang, L.; Wang, Q.; Xu, M.M.; Lu, C.; Zhu, Y.; Zeng, J.; Duan, J.A.; Zhao, M. Anti-inflammatory property and functional substances of Lonicerae Japonicae Caulis. J. Ethnopharmacol. 2021, 267, 113502.
- Li, J.; Ye, C.; Chang, C. Comparative transcriptomics analysis revealing flower trichome development during flower development in two Lonicera japonica Thunb. cultivars using RNA-seq. BMC. Plant. Biol. 2020, 20, 341.
- Xie, X.; Gu, L.; Xu, W.; Yu, X.; Yin, G.; Wang, J.; Jin, Y.; Wang, L.; Wang, B.; Wang, T. Integrating Anti-Influenza Virus Activity and Chemical Pattern Recognition to Explore the Quality Evaluation Method of Lonicerae Japonicae Flos. Molecules 2022, 27, 5789.
- Zhang, B.; Qi, F. Herbal medicines exhibit a high affinity for ACE2 in treating COVID-19. Biosci. Trends. 2023, 17, 14–20.
- Zhang, Q.; Yue, S.; Wang, W.; Chen, Y.; Zhao, C.; Song, Y.; Yan, D.; Zhang, L.; Tang, Y. Potential Role of Gut Microbiota in Traditional Chinese Medicine against COVID-19. Am. J. Chin. Med. 2021, 49, 785–803.
- Su, H.X.; Yao, S.; Zhao, W.F.; Li, M.J.; Liu, J.; Shang, W.J.; Xie, H.; Ke, C.Q.; Hu, H.C.; Gao, M.N.; et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta. Pharmacol. Sin. 2020, 41, 1167–1177.
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential Application of Lonicera japonica Extracts in Animal Production: From the Perspective of Intestinal Health. Front. Microbiol. 2021, 12, 719877.
- Wan, J.; Jiang, C.X.; Tang, Y.; Ma, G.L.; Tong, Y.P.; Jin, Z.X.; Zang, Y.; Osman, E.E.A.; Li, J.; Xiong, J.; et al. Structurally diverse glycosides of secoiridoid; bisiridoid; and triterpene-bisiridoid conjugates from the flower buds of two Caprifoliaceae plants and their ATP-citrate lyase inhibitory activities. Bioorg. Chem. 2022, 120, 105630.
- Hsu, H.F.; Hsiao, P.C.; Kuo, T.C.; Chiang, S.T.; Chen, S.L.; Chiou, S.J.; Ling, X.H.; Liang, M.T.; Cheng, W.Y.; Houng, J.Y. Antioxidant and anti-inflammatory activities of Lonicera japonica Thunb. var. sempervillosa Hayata flower bud extracts prepared by water; ethanol and supercritical fluid extraction techniques. Ind. Crop. Prod. 2016, 89, 543–549.
- Lee, H.L.; Kim, J.M.; Go, M.J.; Kim, T.Y.; Joo, S.G.; Kim, J.H.; Lee, H.S.; Kim, H.J.; Heo, H.J. Protective Effect of Lonicera japonica on PM2.5-Induced Pulmonary Damage in BALB/c Mice via the TGF-β and NF-κB Pathway. Antioxidants 2023, 12, 968.
- Ma, A.; Zou, F.; Zhang, R.; Zhao, X. The effects and underlying mechanisms of medicine and food homologous flowers on the prevention and treatment of related diseases. J. Food. Biochem. 2022, 46, e14430.
- The Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China: Volume 1; Chinese Medical Science and Technology Press: Beijing, China, 2020.
- Kang, M.; Jung, I.; Hur, J.; Kim, S.H.; Lee, J.H.; Kang, J.Y.; Jung, K.C.; Kim, K.S.; Yoo, M.C.; Park, D.S.; et al. The analgesic and anti-inflammatory effect of WIN-34B.; a new herbal formula for osteoarthritis composed of Lonicera japonica Thunb and Anemarrhena asphodeloides BUNGE in vivo. J. Ethnopharmacol. 2010, 131, 485–496.
- Wu, J.Y.; Chen, Y.J.; Bai, L.; Liu, Y.X.; Fu, X.Q.; Zhu, P.L.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Wang, Y.P.; et al. Chrysoeriol ameliorates TPA-induced acute skin inflammation in mice and inhibits NF-κB and STAT3 pathways. Phytomedicine 2020, 68, 153173.
- Liu, Q.; Fang, J.; Wang, P.; Du, Z.; Li, Y.; Wang, S.; Ding, K. Characterization of a pectin from Lonicera japonica Thunb. and its inhibition effect on Aβ42 aggregation and promotion of neuritogenesis. Int. J. Biol. Macromol. 2018, 107, 112–120.
- Su, D.; Li, S.; Zhang, W.; Wang, J.; Wang, J.; Lv, M. Structural elucidation of a polysaccharide from Lonicera japonica flowers.; and its neuroprotective effect on cerebral ischemia-reperfusion injury in rat. Int. J. Biol. Macromol. 2017, 99, 350–357.
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules 2022, 27, 266.
- Wang, S.; He, F.; Wu, H.; Xiang, F.; Zheng, H.; Wu, W.; Li, S. Health-Promoting Activities and Associated Mechanisms of Polygonati Rhizoma Polysaccharides. Molecules 2023, 28, 1350.
- Lai, K.H.; Chen, Y.L.; Lin, M.F.; El-Shazly, M.; Chang, Y.C.; Chen, P.J.; Su, C.H.; Chiu, Y.C.; Illias, A.M.; Chen, C.C.; et al. Lonicerae Japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress. Antioxidants 2022, 11, 1781.
- Wang, Z.; Liu, H.; Fu, R.; Ou, J.; Wang, B. Structural characterization and anti-inflammatory activity of a novel polysaccharide PKP2-1 from Polygonatum kingianum. Front. Nutr. 2023, 10, 1156798.
- Hao, R.; Zhou, X.; Zhao, X.; Lv, X.; Zhu, X.; Gao, N.; Jiang, Y.; Wu, M.; Sun-Waterhouse, D.; Li, D. Flammulina velutipes polysaccharide counteracts cadmium-induced gut injury in mice via modulating gut inflammation, gut microbiota and intestinal barrier. Sci. Total. Environ. 2023, 877, 162910.
- Hu, W.; Yu, A.; Wang, S.; Bai, Q.; Tang, H.; Yang, B.; Wang, M.; Kuang, H. Extraction, Purification, Structural Characteristics, Biological Activities, and Applications of the Polysaccharides from Zingiber officinale Roscoe. (Ginger): A Review. Molecules 2023, 28, 3855.
- Ferreira, S.S.; Correia, A.; Silva, A.M.S.; Wessel, D.F.; Cardoso, S.M.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of pectic polysaccharides from broccoli by-products with in vitro B lymphocyte stimulatory activity. Carbohydr. Polym. 2023, 303, 120432.
- Yuan, L.; Zhong, Z.C.; Liu, Y. Structural characterisation and immunomodulatory activity of a neutral polysaccharide from Sambucus adnata Wall. Int. J. Biol. Macromol. 2020, 154, 1400–1407.
- Zhong, W.; Yang, C.; Zhang, Y.; Yang, D. The prebiotic properties of polysaccharides obtained by differentiated deproteinization methods from Flos Sophorae Immaturus on Lactobacillus fermentum. Front. Microbiol. 2022, 13, 1007267.
- Wang, S.; Peng, Y.; Zhuang, Y.; Wang, N.; Jin, J.; Zhan, Z. Purification, Structural Analysis and Cardio-Protective Activity of Polysaccharides from Radix Astragali. Molecules 2023, 28, 4167.
- Park, J.J.; Lee, W.Y. Anti-glycation effect of Ecklonia cava polysaccharides extracted by combined ultrasound and enzyme-assisted extraction. Int. J. Biol. Macromol. 2021, 180, 684–691.
- Quan, N.; Wang, Y.D.; Li, G.R.; Liu, Z.Q.; Feng, J.; Qiao, C.L.; Zhang, H.F. Ultrasound-Microwave Combined Extraction of Novel Polysaccharide Fractions from Lycium barbarum Leaves and Their In Vitro Hypoglycemic and Antioxidant Activities. Molecules 2023, 28, 3880.
- Xiu, W.; Wang, X.; Yu, S.; Na, Z.; Li, C.; Yang, M.; Ma, Y. Structural Characterization, In Vitro Digestion Property, and Biological Activity of Sweet Corn Cob Polysaccharide Iron (III) Complexes. Molecules 2023, 28, 2961.
- Gao, D.; Chen, H.; Liu, H.; Yang, X.; Guo, P.; Cao, X.; Cai, Y.; Xu, H.; Yang, J. Structure characterization and antioxidant activity analysis of polysaccharides from Lanzhou Lily. Front. Nutr. 2022, 9, 976607.
- Chen, L.; Huang, G.; Hu, J. Preparation, deproteinization, characterization, and antioxidant activity of polysaccharide from cucumber (Cucumis saticus L.). Int. J. Biol. Macromol. 2018, 108, 408–411.
- Liu, J.; Li, T.; Chen, H.; Yu, Q.; Yan, C. Structural characterization and osteogenic activity in vitro of novel polysaccharides from the rhizome of Polygonatum sibiricum. Food. Funct. 2021, 12, 6626–6636.
- Wang, S.; Yang, Y.; Wang, Q.; Wu, Z.; Liu, X.; Chen, S.; Zhou, A. Structural characterization and immunomodulatory activity of a polysaccharide from finger citron extracted by continuous phase-transition extraction. Int. J. Biol. Macromol. 2023, 240, 124491.
- Liu, Y.; Zhang, Y.; Mei, N.; Li, W.; Yang, T.; Xie, J. Three acidic polysaccharides derived from sour jujube seeds protect intestinal epithelial barrier function in LPS induced Caco-2 cell inflammation model. Int. J. Biol. Macromol. 2023, 240, 124435.
- Sun, W.; Kou, X.H.; Wu, C.E.; Fan, G.J.; Li, T.T.; Cheng, X.; Xu, K.; Suo, A.; Tao, Z. Low-temperature plasma modification, structural characterization and anti-diabetic activity of an apricot pectic polysaccharide. Int. J. Biol. Macromol. 2023, 240, 124301.
- Zhang, S.; Ding, C.; Liu, X.; Zhao, Y.; Ding, Q.; Sun, S.; Zhang, J.; Yang, J.; Liu, W.; Li, W. Research Progress on Extraction, Isolation, Structural Analysis and Biological Activity of Polysaccharides from Panax Genus. Molecules 2023, 28, 3733.
- An, F.; Ren, G.; Wu, J.; Cao, K.; Li, M.; Liu, Y.; Liu, Y.; Hu, X.; Song, M.; Wu, R. Extraction, purification, structural characterization, and antioxidant activity of a novel polysaccharide from Lonicera japonica Thunb. Front. Nutr. 2022, 9, 1035760.
- Bi, Z.; Zhao, Y.; Hu, J.; Ding, J.; Yang, P.; Liu, Y.; Lu, Y.; Jin, Y.; Tang, H.; Liu, Y.; et al. A novel polysaccharide from Lonicerae Japonicae Caulis: Characterization and effects on the function of fibroblast-like synoviocytes. Carbohydr. Polym. 2022, 292, 119674.
- Zhang, T.; Liu, H.; Bai, X.; Liu, P.; Yang, Y.; Huang, J.; Zhou, L.; Min, X. Fractionation and antioxidant activities of the water-soluble polysaccharides from Lonicera japonica Thunb. Int. J. Biol. Macromol. 2020, 151, 1058–1066.
- Cai, J.; Liang, Z.; Li, J.; Manzoor, M.F.; Liu, H.; Han, Z.; Zeng, X. Variation in physicochemical properties and bioactivities of Morinda citrifolia L. (Noni) polysaccharides at different stages of maturity. Front. Nutr. 2023, 9, 1094906.
- Sun, H.; Shu, F.; Guan, Y.; Kong, F.; Liu, S.; Liu, Y.; Li, L. Study of anti-fatigue activity of polysaccharide from fruiting bodies of Armillaria gallica. Int. J. Biol. Macromol. 2023, 241, 124611.
- Huang, Y.; Xie, W.; Tang, T.; Chen, H.; Zhou, X. Structural characteristics.; antioxidant and hypoglycemic activities of polysaccharides from Mori Fructus based on different extraction methods. Front. Nutr. 2023, 10, 1125831.
- Chen, G.; Jiang, N.; Zheng, J.; Hu, H.; Yang, H.; Lin, A.; Hu, B.; Liu, H. Structural characterization and anti-inflammatory activity of polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2023, 241, 124386.
- Wang, L.; Lian, J.; Zheng, Q.; Wang, L.; Wang, Y.; Yang, D. Composition analysis and prebiotics properties of polysaccharides extracted from Lepista sordida submerged cultivation mycelium. Front. Microbiol. 2023, 13, 1077322.
- Wu, W.; Huang, T.; Xiang, F. Polyethylene glycol-based ultrasonic-assisted enzymatic extraction, characterization, and antioxidant activity in vitro and in vivo of polysaccharides from Lonicerae japonica leaves. Food. Sci. Nutr. 2019, 7, 3452–3462.
- Wang, P.; Liao, W.; Fang, J.; Liu, Q.; Yao, J.; Hu, M.; Ding, K. A glucan isolated from flowers of Lonicera japonica Thunb. inhibits aggregation and neurotoxicity of Aβ42. Carbohydr. Polym. 2014, 110, 142–147.
- Liao, W.; Hu, X.; Du, Z.; Wang, P.; Ding, K. A homogalacturonan from Lonicera japonica Thunb. disrupts angiogenesis via epidermal growth factor receptor and Delta-like 4 associated signaling. Glycoconj. J. 2022, 39, 725–735.
- Qi, X.; Yu, Y.; Wang, X.; Xu, J.; Wang, X.; Feng, Z.; Zhou, Y.; Xiao, H.; Sun, L. Structural characterization and anti-oxidation activity evaluation of pectin from Lonicera japonica Thunb. Front. Nutr. 2022, 9, 998462.
- Lin, L.; Wang, P.; Du, Z.; Wang, W.; Cong, Q.; Zheng, C.; Jin, C.; Ding, K.; Shao, C. Structural elucidation of a pectin from flowers of Lonicera japonica and its antipancreatic cancer activity. Int. J. Biol. Macromol. 2016, 88, 130–137.
- Zhang, T.; Liu, H.; Ma, P.; Huang, J.; Bai, X.; Liu, P.; Zhu, L.; Min, X. Immunomodulatory effect of polysaccharides isolated from Lonicera japonica Thunb. in cyclophosphamide-treated BALB/c mice. Heliyon 2022, 8, e11876.
- Zhou, X.; Dong, Q.; Kan, X.; Peng, L.; Xu, X.; Fang, Y.; Yang, J. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS ONE 2018, 13, e0204152.
- Qi, X.; Fang, H.; Chen, Z.; Liu, Z.; Yu, X.; Liang, C. Ectopic Expression of a R2R3-MYB Transcription Factor Gene LjaMYB12 from Lonicera japonica Increases Flavonoid Accumulation in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 4494.
- Kumar, D.; Gautam, A.; Rohatgi, S.; Kundu, P.P. Synthesis of vildagliptin loaded acrylamide-g-psyllium/alginate-based core-shell nanoparticles for diabetes treatment. Int. J. Biol. Macromol. 2022, 218, 82–93.
- Wang, D.; Zhao, X.; Liu, Y. Hypoglycemic and hypolipidemic effects of a polysaccharide from flower buds of Lonicera japonica in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2017, 102, 396–404.
- Chopade, P.; Chopade, N.; Zhao, Z.; Mitragotri, S.; Liao, R.; Chandran Suja, V. Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng. Transl. Med. 2022, 8, e10367.
- Brand, A.L.; Lawler, P.E.; Bollinger, J.G.; Li, Y.; Schindler, S.E.; Li, M.; Lopez, S.; Ovod, V.; Nakamura, A.; Shaw, L.M.; et al. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer’s disease: A literature review. Alzheimers. Res. Ther. 2022, 14, 195.
- Imbimbo, B.P.; Ippati, S.; Watling, M.; Imbimbo, C. Role of monomeric amyloid-β in cognitive performance in Alzheimer’s disease: Insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacol. Res. 2023, 187, 106631.
- Sengupta, U.; Nilson, A.N.; Kayed, R. The Role of Amyloid-β Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine 2016, 6, 42–49.
- Tayab, M.A.; Islam, M.N.; Chowdhury, K.A.A.; Tasnim, F.M. Targeting neuroinflammation by polyphenols: A promising therapeutic approach against inflammation-associated depression. Biomed. Pharmacother. 2022, 147, 112668.
- Liu, P.; Bai, X.; Zhang, T.; Zhou, L.; Li, J.; Zhang, L. The protective effect of Lonicera japonica polysaccharide on mice with depression by inhibiting NLRP3 inflammasome. Ann. Transl. Med. 2019, 7, 811.
- Kim, G.; Han, D.W.; Lee, J.H. The Cytoprotective Effects of Baicalein on H2O2-Induced ROS by Maintaining Mitochondrial Homeostasis and Cellular Tight Junction in HaCaT Keratinocytes. Antioxidants 2023, 12, 902.
- Zhang, F.; Zhang, X.; Liang, X.; Wu, K.; Cao, Y.; Ma, T.; Guo, S.; Chen, P.; Yu, S.; Ruan, Q.; et al. Defensing against oxidative stress in Caenorhabditis elegans of a polysaccharide LFP-05S from Lycii fructus. Carbohydr. Polym. 2022, 289, 119433.
- Xie, C.; Ya Likun, M.M.; Luo, Q.L.; Dong, J.C. Role of cellular senescence in inflammatory lung diseases. Cytokine. Growth. Factor. Rev. 2023, 70, 26–40.
- Zhu, J.; Jia, Y.; Wang, C.; Zhou, W.; Shu, Y.; Zhang, K.; Zeng, X.; Guo, R. Lonicera japonica polysaccharides improve longevity and fitness of Caenorhabditis elegans by activating DAF-16. Int. J. Biol. Macromol. 2023, 229, 81–91.
- Zhou, X.; He, G.; Ma, J.; Tang, M.; Tian, G.; Gong, X.; Zhang, H.; Kui, L. Protective Effect of a Novel Polysaccharide from Lonicera japonica on Cardiomyocytes of Mice Injured by Hydrogen Peroxide. Biomed. Res. Int. 2020, 2020, 5279193.
- Qian, L.; Du, M.; Yang, X.; Wang, Q.; Huang, S.; Ma, Y.; Sun, Y. Microanalysis Characterization and Immunomodulatory Effect for Selenium-Enriched Polysaccharide from Morchella esculenta (L.) Pers. Molecules 2023, 28, 2885.
- Feng, J.; Chang, X.; Zhang, Y.; Lu, R.; Meng, X.; Song, D.; Yan, X.; Zhang, J.; Nie, G. Characterization of a polysaccharide HP-02 from Honeysuckle flowers and its immunoregulatory and anti-Aeromonas hydrophila effects in Cyprinus carpio L. Int. J. Biol. Macromol. 2019, 140, 477–483.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021, 71, 209–249.
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020.
- Qin, S.; Li, A.; Yi, M.; Yu, S.; Zhang, M.; Wu, K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J. Hematol. Oncol. 2019, 12, 27.
- Koroukian, S.M.; Booker, B.D.; Vu, L.; Schumacher, F.R.; Rose, J.; Cooper, G.S.; Selfridge, J.E.; Markt, S.C. Receipt of Targeted Therapy and Survival Outcomes in Patients with Metastatic Colorectal Cancer. JAMA Netw. Open 2023, 6, e2250030.
- Chang, T.M.; Chu, P.Y.; Lin, H.Y.; Huang, K.W.; Hung, W.C.; Shan, Y.S.; Chen, L.T.; Tsai, H.J. PTEN regulates invasiveness in pancreatic neuroendocrine tumors through DUSP19-mediated VEGFR3 dephosphorylation. J. Biomed. Sci. 2022, 29, 92.
More
