This review summarizes the last findings in Anterior Chamber Angle evaluation, focusing on new instruments and their application to the clinical practice. Special attention will be given to the comparison between these new techniques and traditional slit-lamp gonioscopy.
- diagnosis
- trabecular meshwork
- anterior chamber angle
- iridocorneal angle
- angle closure glaucoma
1. Introduction
Evaluation of the anterior chamber angle (ACA) is an essential part of the ophthalmological examination, instrumental to achieve pertinent relevant information on glaucoma patients as well as on non-glaucomatous subjects[1] [1]. In patients with glaucoma or glaucoma suspicion, a careful assessment of the ACA should always be performed, allowing direct visualization of the main structures cause of the aqueous humor drainage, directly affecting intraocular pressure[1][2] [1,2]. Several findings may be associated with an impaired aqueous humor outflow, among them abnormal iris insertion, abnormal pigmentation of the trabecular meshwork (TM), presence of synechiae, blood in the Schlemm’s canal (SC), angle recession, abnormal blood vessels in the angle, evidence of anterior segment dysgenesis, and other abnormalities [3].
Although less prevalent worldwide than primary open-angle glaucoma, primary angle-closure glaucoma prevalence has been estimated to be 22 million people in 2013, with the highest numbers in people of Asian ancestry[4] [4]. In China, it was estimated that 9 million people have a significant angle closure and more than 28 million people have an anatomic trait predisposing to primary angle closure glaucoma (an “occludable” drainage angle)[5] [5]. Despite these data, the definition of “occludable angle” is in fact unclear both in the literature and in the authoritative clinical guidelines on glaucoma. According to the most widely used classification, an occludable angle is defined as an angle in which the TM is not gonioscopically identifiable in more than 90° of angle circumference[6][7] [6,7]. However, in 2004 Foster et al. reported that this definition should be reconsidered, and suggested that angles between 10° and 20° should be defined as angles with a “probable” and a “possible” risk of closure, respectively[8] [8]. It should be noted that the management of occludable angles is also poorly defined. In a recent randomized controlled trial on patients with primary angle closure suspicion[9] [9], prophylactic peripheral iriditomy had little effect on preventing the progression towards primary angle closure. These results may be justified, at least partially, if the low rate of progression from primary angle closure suspicion to angle-closure glaucoma found in this study is properly taken into account.
It has been demonstrated that without an appropriate gonioscopic evaluation, the vast majority of chronic angle closure varieties may be mistaken for open-angle glaucoma[10][11] [10,11]. In this respect, Varma et al. found that approximately 10% of patients diagnosed with primary open-angle glaucoma were actually affected by angle closure glaucoma[12] [12]. Slit-lamp gonioscopy is still the clinical reference standard for the assessment of the irido-corneal angle, playing a fundamental role in the distinction between open and closed angle glaucoma, and consequently in the determination of the future disease management [10][11][10,11]. However, gonioscopy is performed in approximately half of the ophthalmological visits, and assessment of the ACA during the follow-up is poor, even among glaucoma specialists[13] [13]. It has been demonstrated that the repeatability of gonioscopy is higher when the examination is performed by highly experienced vs. novice personnel, as the regular practice and the retraining are likely to improve and maintain the performance [14]. This has been found in a collaborative care glaucoma clinic, where a“fair to moderate”agreement in gonioscopy was achieved between experienced optometrists and glaucoma specialists[15] [15].
Several imaging technologies have been developed in recent years, to make the evaluation of the ACA more quantitative and practical: ultrasound biomicroscopy (UBM), gonio-photographic systems (GPS), limbal anterior chamber depth measurement (LACDM), known also as Van Herick test, scanning peripheral anterior chamber depth analyzer (SPAC), Scheimpflug photography (SP), and anterior segment optical coherence tomography (AS-OCT), which may provide a more objective evaluation of the ACA structures[16] [16]. Deep learning algorithms have been recently introduced as well, to automate the analysis of angle images[17] [17].
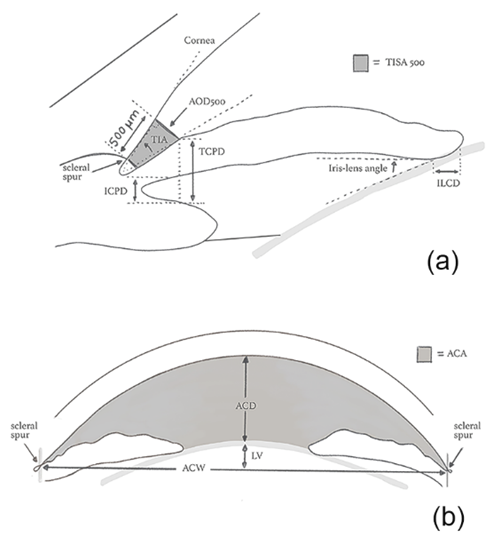
A variety of quantitative parameters describing the anterior segment anatomic features have been proposed, namely: angle opening distance (AOD), angle recess area (ARA), trabecular-iris angle (TIA), trabecular-iris space area (TISA), trabecular-ciliary process distance (TCPD), anterior chamber width (ACW), peripheral anterior chamber depth (ACD), anterior chamber volume (ACV), and Van Herick’s grading (VHG) (Figure 1).
Figure 1. Quantitative parameters of the irido-corneal angle (a) and of the anterior chamber (b). ACA: Anterior chamber angle; ACD: Anterior chamber depth; ACW: Anterior chamber width; AOD: Angle opening distance; ICPD: Iris-ciliary process distance; ILCD: Iris-lens contact distance; LV: Lens vault; TIA: Trabecular-iris angle; TISA: Trabecular iris space area; TCPD: Trabecular-ciliary process distance.
2. Future Directions
Nowadays, a wide range of instruments allows the ophthalmologists exploring the ACA configuration, helping in the detection of narrow angles and in the diagnosis of angle closure (Table 2). Among traditional contact techniques, gonioscopy has been consistently considered for a long time the gold standard of ACA evaluation, although it is a time-consuming examination, requiring a long learning curve and good ocular surface conditions. Similarly, the UBM examination, even more suitable to identify anterior angle structures, requires contact with the eye and a well-trained operator to be properly carried out. Conversely, non-contact techniques such as AS-OCT are non-invasive methods allowing for a quick measurement of a wide number of angle parameters.
Table 2. Area under the curve (AUC) with 95% Confidence Interval (CI) for the detection of angle closure and narrow angle. Data reported by techniques and demographics.
|
Technique |
AUC |
95% CI |
Main Ethnicity (%) |
Mean Age (SD) (y) |
|
Angle closure detection |
|
|
|
|
|
EyeCam [54] |
0.98 |
0.93–1.00 |
Chinese (70.4%) |
60.7 (12.6) |
|
Manual grading EyeCam [51] |
0.88 |
0.81–0.96 |
Chinese (72.9%) |
60.5 (12.9) |
|
Automated grading EyeCam [51] |
0.74 |
0.63–0.85 |
Chinese (72.9%) |
60.5 (12.9) |
|
Visante AS-OCT [54] |
0.85 |
0.76–0.92 |
Chinese (70.4%) |
60.7 (12.6) |
|
Visante AS-OCT [98] |
0.76 |
0.74–0.78 |
Chinese (86.8%) |
60.8 (6.8) |
|
CASIA SS-1000 SS-OCT [107] |
0.84 |
0.81–0.88 |
Chinese (87.3%) |
61.8 (6.7) |
|
Deep Learning algorithm |
0.93 |
0.92–0.94 |
Chinese (100%) |
61.1 (8.1) |
|
Narrow angle detection |
|
|
|
|
|
Ultrasound Biomicroscopy (ARA 750) [37] |
0.97 |
0.92–1.00 |
White (58.3%) |
42.9 (n/a) |
|
Scheimpflug Photography [84] |
0.93 |
0.90–0.96 |
Indian (100%) |
56.2 (6.5) |
|
Scanning peripheral ACD analyzer [76] |
0.79 |
0.70–0.87 |
Chinese (94.5%) |
65.5 (8.2) |
|
Modified Van Herick [76] |
0.87 |
0.80–0.94 |
Chinese (94.5%) |
65.5 (8.2) |
|
Technique |
AUC |
95% CI |
Main Ethnicity (%) |
Mean Age (SD) (y) |
|
Angle closure detection |
|
|
|
|
|
EyeCam |
0.98 |
0.93–1.00 |
Chinese (70.4%) |
60.7 (12.6) |
|
Manual grading EyeCam |
0.88 |
0.81–0.96 |
Chinese (72.9%) |
60.5 (12.9) |
|
Automated grading EyeCam |
0.74 |
0.63–0.85 |
Chinese (72.9%) |
60.5 (12.9) |
|
Visante AS-OCT |
0.85 |
0.76–0.92 |
Chinese (70.4%) |
60.7 (12.6) |
|
Visante AS-OCT |
0.76 |
0.74–0.78 |
Chinese (86.8%) |
60.8 (6.8) |
|
CASIA SS-1000 SS-OCT |
0.84 |
0.81–0.88 |
Chinese (87.3%) |
61.8 (6.7) |
|
Deep Learning algorithm |
0.93 |
0.92–0.94 |
Chinese (100%) |
61.1 (8.1) |
|
Narrow angle detection |
|
|
|
|
|
Ultrasound Biomicroscopy (ARA 750) |
0.97 |
0.92–1.00 |
White (58.3%) |
42.9 (n/a) |
|
Scheimpflug Photography |
0.93 |
0.90–0.96 |
Indian (100%) |
56.2 (6.5) |
|
Scanning peripheral ACD analyzer |
0.79 |
0.70–0.87 |
Chinese (94.5%) |
65.5 (8.2) |
|
Modified Van Herick |
0.87 |
0.80–0.94 |
Chinese (94.5%) |
65.5 (8.2) |
ACD: Anterior chamber depth; ARA: Angle recess area; AS-OCT: Anterior segment OCT; AUC: Area under the curve; SD: Standard deviation; SS-OCT: Swept source OCT.
All the aforementioned techniques have advantages and disadvantages, summarized in this review, which the clinician should be made aware of, to meaningfully exploit the available resources. Even if a great amount of information and quantitative data on the irido-corneal angle may be achieved, interpretation is not always easy, especially taking into account the lack of normative databases and longitudinal studies [18][133]. Moreover, the vast majority of studies investigating the diagnostic ability of these instruments has been performed in populations of Chinese ethnicity (Table 2), leaving the question open on how they may perform in mixed-race populations. Finally, none of these methods may be considered a reliable substitute of slit-lamp gonioscopy, that remains the clinical reference standard in the diagnosis and management of narrow angles, according to the clinical guidelines[18] [133].
In more recent years, ACA evaluation has improved with the introduction of deep learning algorithms, potentially valuable in the screening of populations at high risk of primary angle closure, and with poor access to eye care. As a medical specialty that is highly dependent on ancillary imaging tests, the application of artificial intelligence is rapidly increasing in ophthalmology, and the future of deep-learning applications in glaucoma is certainly bright. However, additional studies are required to evaluate the usefulness of the deep learning algorithms in different populations and their applicability to different devices. The importance of telemedicine and the use of virtual ophthalmology has recently also increased, especially in the setting of the so-called COVID-19 era[19] [134]. Gonio-photographs and AS-OCT imaging have been already used for remote evaluation and screening of angle closure, with encouraging results[20] [135]. Although these techniques are likely to require further improvements, they may be considered as auxiliary solutions, in a time of social distancing.
Reference
(we'll rearrange the references after you submitted it)
- Pillunat, L.E.; Erb, C.; Junemann, A.G.M.; Kimmich, F. Micro-Invasive Glaucoma Surgery (Migs): A Review of Surgical Procedures Using Stents. Ophthalmol. 2017, 11, 1583–1600.
- Schacknow, P.N.; Samples, J.R. The Glaucoma Book: A Practical, Evidence-Based Approach to Patient Care; Springer-Verlag: New York, NY, USA, 2010.
- Teixeira, F.; Sousa, D.C.; Leal, I.; Barata, A.; Neves, C.M.; Pinto, L.A. Automated Gonioscopy Photography for Iridocorneal Angle Grading. J. Ophthalmol. 2020, 30, 112–118.
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090.
- Foster, P.J.; Johnson, G.J. Glaucoma in China: How Big Is the Problem? J. Ophthalmol. 2001, 85, 1277–1282.
- Arkell, S.M.; Lightman, D.A.; Sommer, A.; Taylor, H.R.; Korshin, O.M.; Tielsch, J.M. The Prevalence of Glaucoma among Eskimos of Northwest Alaska. Arch Ophthalmol. 1987, 105, 482–485.
- Foster, P.J.; Buhrmann, R.; Quigley, H.A.; Johnson, G.J. The Definition and Classification of Glaucoma in Prevalence Surveys. J. Ophthalmol. 2002, 86, 238–242.
- Foster, P.J.; Aung, T.; Nolan, W.P.; Machin, D.; Baasanhu, J.; Khaw, P.T.; Alsbirk, P.H.; Lee, P.S.; Seah, S.K.; Johnson, G.J. Defining Occludable Angles in Population Surveys: Drainage Angle Width, Peripheral Anterior Synechiae, and Glaucomatous Optic Neuropathy in East Asian People. J. Ophthalmol. 2004, 88, 486–490.
- He, M.; Jiang, Y.; Huang, S.; Chang, D.S.; Munoz, B.; Aung, T.; Foster, P.J.; Friedman, D.S. Laser Peripheral Iridotomy for the Prevention of Angle Closure: A Single-Centre, Randomised Controlled Trial. Lancet 2019, 393, 1609–1618.
- Day, A.C.; Baio, G.; Gazzard, G.; Bunce, C.; Azuara-Blanco, A.; Munoz, B.; Friedman, D.S.; Foster, P.J. The Prevalence of Primary Angle Closure Glaucoma in European Derived Populations: A Systematic Review. J. Ophthalmol. 2012, 96, 1162–1167.
- Bonomi, L.; Marchini, G.; Marraffa, M.; Bernardi, P.; de Franco, I.; Perfetti, S.; Varotto, A. Epidemiology of Angle-Closure Glaucoma: Prevalence, Clinical Types, and Association with Peripheral Anterior Chamber Depth in the Egna-Neumarket Glaucoma Study. Ophthalmology 2000, 107, 998–1003.
- Varma, D.K.; Simpson, S.M.; Rai, A.S.; Ahmed, I.I.K. Undetected Angle Closure in Patients with a Diagnosis of Open-Angle Glaucoma. J. Ophthalmol. 2017, 52, 373–378.
- Quigley, H.A.; Friedman, D.S.; Hahn, S.R. Evaluation of Practice Patterns for the Care of Open-Angle Glaucoma Compared with Claims Data: The Glaucoma Adherence and Persistency Study. Ophthalmology 2007, 114, 1599–1606.
- Campbell, P.; Redmond, T.; Agarwal, R.; Marshall, L.R.; Evans, B.J. Repeatability and Comparison of Clinical Techniques for Anterior Chamber Angle Assessment. Ophthalmic Physiol. Opt. 2015, 35, 170–178.
- Phu, J.; Wang, H.; Khuu, S.K.; Zangerl, B.; Hennessy, M.P.; Masselos, K.; Kalloniatis, M. Anterior Chamber Angle Evaluation Using Gonioscopy: Consistency and Agreement between Optometrists and Ophthalmologists. Vis. Sci. 2019, 96, 751–760.
- de Leon, J.M.S.; Tun, T.A.; Perera, S.A.; Aung, T. Angle Closure Imaging: A Review. Ophthalmol. Rep. 2013, 1, 80–88.
- Porporato, N.; Baskaran, M.; Husain, R.; Aung, T. Recent Advances in Anterior Chamber Angle Imaging. Eye 2020, 34, 51–59.
- Xu, B.Y.; Pardeshi, A.A.; Burkemper, B.; Richter, G.M.; Lin, S.C.; McKean-Cowdin, R.; Varma, R. Differences in Anterior Chamber Angle Assessments between Gonioscopy, Eyecam, and Anterior Segment Oct: The Chinese American Eye Study. Vis. Sci. Technol. 2019, 8, 5.
- Murakami, Y.; Wang, D.; Burkemper, B.; Lin, S.C.; Varma, R. A Population-Based Assessment of the Agreement between Grading of Goniophotographic Images and Gonioscopy in the Chinese-American Eye Study (Ches). Ophthalmol. Vis. Sci. 2016, 57, 4512–4516.
- Ritch, R. Pigment Dispersion Syndrome. J. Ophthalmol. 1998, 126, 442–445.