The microRNAs (miRNAs), lncRNAs (long ncRNAs), and circRNAs (circular RNAs) with significant regulatory and structural roles make up approximately 99% of the human genome, which does not contain proteins. Non-coding RNAs (ncRNA) have been discovered to be essential novel regulators of cardiovascular risk factors and cellular processes, making them significant prospects for advanced diagnostics and prognosis evaluation. Cases of cardiovascular diseases (CVDs) are rising due to limitations in the existing therapeutic approach; most of the treatment options are based on the coding transcripts that encode proteins. Various investigations have shown the role of nc-RNA in the early diagnosis and treatment of CVDs. Furthermore, the development of novel diagnoses and treatments based on miRNAs, lncRNAs, and circRNAs could be more helpful in the clinical management of patients with CVDs.
- cardiovascular disease
- microRNAs
- diagnosis
- long noncoding RNA
- ncRNAs
- therapy
1. Introduction
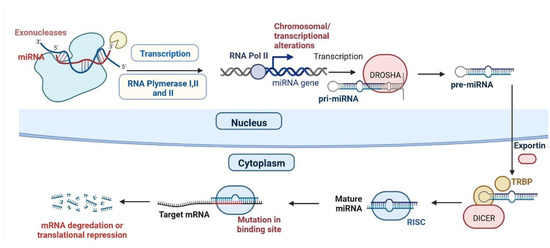
2. miRNAs and CVDs
| S.N. | Type of Disease | miRNA | Regulation | Importance | Reference |
|---|---|---|---|---|---|
| 1 | Cardiac hypertrophy (CH) | miR-208a | Up-regulation | Cardiac remodelling | [13] |
| 2 | CH | miR-19a/b | Up-regulation | Cardiac remodelling in response to angiotensin II infusion | [14] |
| 3 | CH | miR-155 | Up-regulation | Cardiac remodelling | [15] |
| 4 | CH | miR-199a | Up-regulation | Maintenance of cell size in cardiomyocytes | [16] |
| 5 | CH | miR-1, | Down-regulation | Induces cardiac hypertrophy. | [17] |
| 6 | CH | miR-101 | Down-regulation | Inhibit cardiac hypertrophy signalling | [18] |
| 7 | CH | miR-185 | Down-regulation | Inhibit CH hypertrophy signalling | [19] |
| 8 | CH | miR-34a | Down-regulation | Regulation of Ang II-induced cardi myocyte hypertrophy | [20] |
| Cardiomyocyte | |||||
| Downstream Targets: Pdk4, Sgk1 | |||||
| [ | |||||
| 56 | |||||
| ] | |||||
| 74 | |||||
| MI | |||||
| miR-21 | |||||
| Down-regulated | |||||
| Fibroblast, Downstream Targets Pten; Sprouty-1, collagens | |||||
| [ | |||||
| 57 | |||||
| ] | |||||
| 75 | |||||
| MI | |||||
| miR-24 | |||||
| Up-regulated | |||||
| Anti-apoptosis in Cardiomyocyte, fibroblast, endothelial cell; | |||||
| Downstream Targets Bim; Furin; Gata2, Pak4 | |||||
| [ | |||||
| 58 | |||||
| ] | |||||
| 76 | |||||
| MI | |||||
| miR-29 | |||||
| Down-regulated | |||||
| Cardiomyocyte, fibroblast | |||||
| Downstream Targets: Mcl-1; Collagens | |||||
| [ | |||||
| 59 | |||||
| ] | |||||
| 77 | |||||
| MI | |||||
| miR-92a | |||||
| Up-regulated | |||||
| Endothelial cell | |||||
| Downstream Targets: Itga5 | [ | 60 | ] | ||
| 78 | MI | miR-101 | Down-regulated | Cardiac remodelling Downstream Targets: Collagens |
[61] |
| 79 | MI | miR-126 | Down-regulated | Protects against myocardial ischemia-reperfusion injury | [62] |
2.1. miRNAs and HF
2.2. Arrhythmias
2.3. miRNAs and ACS and MI
2.4. miRNAs and Atherosclerosis
2.5. miRNAs and RHD
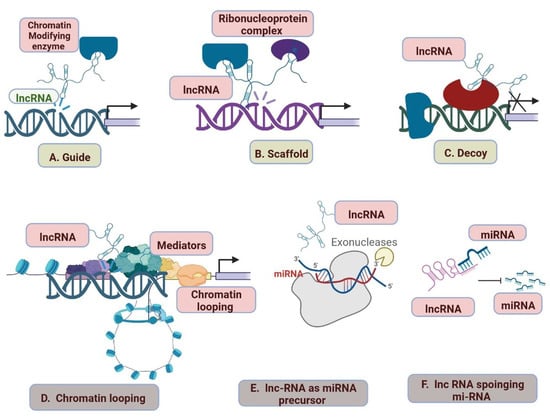
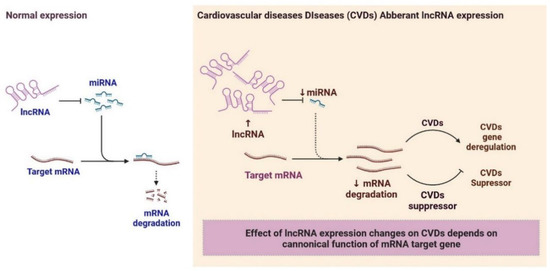
2.6. LncRNAs and Cardiovascular Diseases
| Type of Disease | lncRNA | Regulations | Importance | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Myocardial infraction (MI) | aHIF | Regulations of the angiogenesis process and a biomarker | Inhibits the autophagy of cardiac cells during MI | [98] | ||||||
| 2 | MI | ANRIL | Regulates myocardial cell apoptosis in AMI | Protection of cardiomyocytes | [99] | ||||||
| 3 | MI | APF | APF lncRNA regulates autophagy | Acting as a sponge for miRNA- 188-3p. |
[98] | ||||||
| 4 | MI | CARL | Regulates mitochondrial fission and apoptosis | Acting as a sponge for miRNA-539. |
[98] | ||||||
| 5 | MI | CDR1AS | Inhibiting the autophagy of cardiac cells during MI | Biomarker. | [98] | ||||||
| 6 | MI | FTX | Regulates cardiomyocytes | Act as a sponge for miRNA- 29b-1-5. |
[100] | ||||||
| 7 | MI | GAS5 | Regulates the protection of cardiomyocytes against hypoxic injury | Act as a sponge for miRNA-142; improves apoptosis by negatively regulating sema3a. |
[98] | ||||||
| 8 | MI | H19 | Regulates autophagy | Induction of cardiac remodeling, autophagy, and biomarker. | [98] | ||||||
| 9 | CH | miR-145 | Down-regulation | Inhibits isoproterenol-induced cardiomyocyte hypertrophy | |||||||
| 9 | MI | HOTAIR | [ | Regulates cardioprotective | 21] | ||||||
| Act as a sponge for miRNA-1 and as a | biomarker. | [ | 101 | ] | 10 | CH | miR-150 | Down-regulation | Reduces the immunosuppression function of Myeloid-derived suppressor cells (MDSCs) | [22] | |
| 10 | MI | KCNQ1OT1 | Down-regulation of lncRNA KCNQ1OT1 protects against myocardial ischemia/reperfusion injury | Biomarker for left ventricular dysfunction. | [102] | 11 | CH | miR-378 | Down-regulation | Act as negative regulator for CH | [23] |
| 11 | MI | LIPCAR | Down-regulated | Biomarker for cardiac remodelling. | [103] | 12 | Heart failure (HF) | miR-125b | Up-regulation | Conduction of Cardiac fibrosis (CF) | [24] |
| 12 | MI | Lnc-Ang362 | Upregulation | Promotion of CF | [104] | 13 | HF | miR-22, | Up-regulation | Regulator for cardiac remodelling | |
| MI | MALAT1 | Down-regulation | Regulation of cardiomyocytes apoptosis and autophagy through miRNA- 558; and biomarker. |
[105] | 14 | HF | miR-92b | Up-regulation | Related to the left atrium diameter, left ventricular end-diastolic dimension | [25] | |
| 15 | |||||||||||
| 13 | MI | MDRL | Regulates mitochondrial fission | Reduction of mitochondrial fission and apoptosis acting as a sponge for miRNA-361. |
[106] | HF | miR-320a | Up-regulation | CF through activation of the IL6/STAT3 axis. | [26] | |
| 14 | MI | MEG3 | Regulates cardiomyocytes | Regulation of cardiomyocytes apoptosis. | [107] | 16 | HF | miR-423-5p | Up-regulation | Upregulated in human failing myocardium | [27] |
| 15 | MI | MHRT | Regulates cardiomyocytes | Regulation of cardiomyocytes apoptosis and biomarker. | [108] | 17 | HF | miR-200b | Up-regulation | Regulation of multiple cellular pathways in HF | |
| 16 | MI | MIAT | [ | Regulates CF | 28] | ||||||
| Regulation of cardiac hypertrophy and fibrosis acting as a sponge for | miRNA-150 and -93. | [ | 98 | ] | 18 | HF | miR-622 | Up-regulation | Improves blood vessel growth | [29] | |
| 17 | MI | Mirt1/2 | Regulates cardiomyocytes | Regulation of cardiac remodelling. | [98] | 19 | HF | miR-1228 | Up-regulation | Marker for systolic HF | [30] |
| 18 | MI | n379519 | Regulates CF | Promotion of cardiac fibrosis through miRNA-30. | [109] | 20 | HF | miR-208b | Up-regulation | pathogenesis of DCM | [31] |
| 21 | HF | miR-499 | Up-regulation | Cardiac development | [32] | ||||||
| 22 | HF | miR-223 | Up-regulation | Altered in post-MI HF in humans | [33] | ||||||
| 23 | HF | miR-1254 | Up-regulation | Altered in post-MI HF in humans | [34] | ||||||
| 24 | HF | miR-1306 | Up-regulation | Elected to explore novel circulating markers for HF | [35] | ||||||
| 25 | HF | miR-18a | Down-regulation | CF through the Notch2 pathway. | [36] | ||||||
| 26 | HF | miR-26b | Down-regulation | Controlling critical signalling pathways, such as BMP/ SMAD1 signalling | [37] | ||||||
| 27 | HF | miR-27a | Down-regulation | Inhibiting miR-27a-3p mitigated CH phenotype induced by Ang II (Angiotensin -II) | [38] | ||||||
| 28 | HF | miR-30e | Down-regulation | The overexpression of miR-30c reduces the level of connective tissue growth | [39] | ||||||
| 29 | HF | miR-106a | Down-regulation | Notch 3 pathway in ischemic heart injury. | [40] | ||||||
| 30 | HF | miR-199a | Down-regulation | Improves contractile function | [41] | ||||||
| 31 | HF | miR-652 | Down-regulation | Marker for predicting acute coronary syndrome | [42] | ||||||
| 32 | HF | miR-1 | Down-regulation | Systolic HF | [43] | ||||||
| 33 | HF | miR-126 | Down-regulation | Activation of the vascular endothelial growth factor (VEGM) signalling pathway in the endothelium. | [44] | ||||||
| 34 | HF | miR-423 | Down-regulation | It is a circulating biomarker for heart failure. | [45] | ||||||
| 35 | |||||||||||
| 19 | MI | NONRATT021972 | Regulates cardiomyocytes | Promotion of cardiac function. | [110] | ||||||
| 20 | MI | NRF | Regulates cardiomyocyte necrosis. | Regulation of cardiomyocyte necrosis. | [111] | ||||||
| 21 | MI | NRON | Up-regulated | Wisper in cardiac fibroblast; Biomarker | [98] | ||||||
| 22 | MI | PCFL | Up-regulated | Promotion of cardiac fibrosis through miRNA-378. | [98] | ||||||
| 23 | MI | TTTY15 | Up-regulated | Induction of cardiomyocyte injury by hypoxia targeting miRNA-455. | [98] | ||||||
| 24 | MI | UCA1 | Regulates cardiomyocytes | Regulated ischemia and hypoxia of cardiomyocytes; Biomarker. | [111] | ||||||
| 25 | MI | UIHTC | Regulates cardiomyocytes against MI | Promotion of mitochondrial function. | [98] | ||||||
| 26 | MI | Wisper | Regulates cardiac fibroblast | MI-induced fibrosis and cardiac dysfunction | [112] | ||||||
| 27 | MI | ZFAS1 | Regulates cardiomyocyte | Induction of cardiomyocyte apoptosis, cardiac contractility reduction, and biomarker. |
[113] | ||||||
| 28 | Coronary heart disease | aHIF | Up-regulated | Biomarker. | [114] | ||||||
| 29 | Coronary heart disease | ANRIL | Down-regulates | Diagnostic and prognostic indicator for CHD | [114] | ||||||
| 30 | Coronary heart disease | APOA1-AS | Up-regulations increase the risk of CHD | Biomarker. | [115] | Cardiac electrical and structural remodelling (CE and SR) | miR-1 | ||||
| 31 | Coronary heart disease | AWPPH | Regulates apoptosis. | Promotion of ECs apoptosis. | [116] | ||||||
| 32 | Coronary heart disease | BACE1-AS | dysregulation | dysregulation of the BACE1/BACE1-AS/Aβ axis is associated with HF. | [Down-regulated | Increased altered conduction Increased CF |
[46] | ||||
| 117 | ] | ||||||||||
| 33 | Coronary heart disease | BANCR | Differentially expressed | Promotion of VSMCs proliferation and migration. | [118] | ||||||
| 34 | Coronary heart disease | CHROME | Up-regulated | Regulation of cellular cholesterol homeostasis | [119] | 36 | CE and SR | miR-26 | Down-regulated | Increase inwardly rectifying channel | [47] |
| 35 | Coronary heart disease | CoroMarker | Differentially expressed | novel biomarker for the diagnosis | [120] | 37 | CE and SR | miR-29 | Down-regulated | Increased CF | [48] |
| 36 | Coronary heart disease | EGOT | Differentially expressed | Biomarker. | [121] | 38 | CE and SR | miR-30 | Down-regulated | Increased CF | [49 |
| 37 | ] | ||||||||||
| Coronary heart disease | H19 | Differentially expressed | Biomarker. | [ | 122] | 39 | CE and SR | miR-133 | Down-regulated | Increased CF | |
| 38 | Coronary heart disease | [ | HOTTIP | 50 | ] | ||||||
| Up-regulates | Promotes ECs proliferation and migration | [ | 123 | ] | 40 | CE and SR | miR-328 | Up-regulated | Shortened atrial action potential duration by targeting | [51] | |
| 39 | Coronary heart disease | HRCR | Regulates hypertrophic Ca2+ signaling pathway | Regulation of cardiomyocytes apoptosis and proliferation. | [124] | 41 | CE and SR | miR-499 | Up-regulated | Altered conduction | |
| 40 | by targeting | [ | 52 | ] | |||||||
| Coronary heart disease | LIPCAR | Differentially expressed | Biomarker. | [ | 103] | 42 | CE and SR | miR-21 | Up-regulated | Inhibition of fibroblast proliferation | |
| 41 | Coronary heart disease | [ | lincRNA-p21 | 53 | ] | ||||||
| Regulates cardiac remodelling and heart failure | Regulation of cardiomyocytes apoptosis and proliferation. | [ | 125 | ] | 43 | Acute coronary syndrome (ACS) and myocardial infarction (MI) | miR-1 | Up-regulated | |||
| 42 | marker of cardiomyocyte injury | Coronary heart disease | [ | 54 | ] | ||||||
| LINC00968 | Up-regulated | Promotion of ECs proliferation and migration acting as a sponge for | miRNA-9 | [126] | 44 | ACS and MI | miR-133a | Up-regulated | Development of VF (Ventricular fibrillation) | [55] | |
| 43 | Coronary heart disease | MALAT1 | Differentially expressed | Biomarker. | [127] | 45 | ACS and MI | miR-208a | Up-regulated | Regulates the cardiac stress response. | [56 |
| 44 | ] | ||||||||||
| Coronary heart disease | MIAT | Differentially expressed | Biomarker | [ | 128] | 46 | ACS and MI | miR-499-5p | Up-regulated | Associated with cardiac injury and also with cardio protection | [57] |
| 45 | Coronary heart disease | NEXN-AS1 | Differentially expressed | Mitigation of atherosclerosis. | [129] | 47 | ACS and MI | miR-126, | Down-regulated | downregulated in the region adjacent to MI areas | [58] |
| 46 | Coronary heart disease | SMILR | Differentially expressed | Biomarker. | [130] | 48 | ACS and MI | miR-221/222 | Down-regulated | Severity of the coronary artery lesions | [59] |
| 47 | Arterial Hypertension | AK098656 | Up-regulated | Regulation of arteries of resistance and a biomarker | [131] | 49 | ACS and MI | miR-29 | Dysregulation | Involved in CF multiple collagens, fibrillin’s, and elastin | [60] |
| 48 | Arterial Hypertension | ANRIL | Regulates endothelial cell activities | Increase of susceptibility to higher systolic blood pressure conferred by polymorphisms. |
[132] | 50 | ACS and MI | miR-145 | Up-regulated | Significantly upregulated in mice in response to chronic hypoxia and that genetic ablation | |
| 49 | Arterial Hypertension | [ | GAS5 | 61 | ] | ||||||
| Regulates ECs and VSMCs function | Regulation of ECs and VSMCs function acting as endogenous RNA | competing of miRNA-21; and a biomarker. GAS5 Targets miR-194-3p. miR-194-3 | [ | 133] | 51 | ACS and MI | miR-21 | Up-regulated | Up-regulated in the hypoxia | [62] | |
| 50 | Arterial Hypertension | Giver | Regulates VSMCs dysfunction. | Promotion of VSMCs dysfunction. | [134] | 52 | ACS and MI | miR-206 | Up-regulated | Normal and hypertensive mouse PASMCs. | [63] |
| 51 | Arterial Hypertension | Lnc-Ang362 | Regulates VSMCs | Regulation of VSMCs proliferation through miRNA-221 and -222. | 53 | ACS and MI | miR-328 | Down-regulated | Regulates Hypoxic Pulmonary Hypertension | [64] | |
| [ | 135 | ] | 54 | Pulmonary arterial hypertension (PAH) | miR-204 | Down-regulated | Hypoxia related to pulmonary arterial hypertension | [65] | |||
| 55 | Atherosclerosis (AS) | miR-33 | Dysregulation | Promising strategy to reverse autophagy dysfunction in atherosclerosis. | [66] | ||||||
| 56 | |||||||||||
| 52 | Arterial Hypertension | NR_027032 | Differentially expressed | Biomarker. | [136] | ||||||
| 53 | Arterial Hypertension | NR_034083 | Differentially expressed | Biomarker. | [137] | ||||||
| 54 | Arterial Hypertension | NR_104181 | Differentially expressed | Biomarker. | [136](AS) | miR-122 | Up | Significantly up-regulated in patients with atherosclerotic lesion | [67] | ||
| 55 | Heart failure | ANRIL | Differentially expressed | Biomarker. | [138] | 57 | (AS) | miR-126 | Down-regulated | [68] | |
| 56 | Heart failure | BACE1-AS | Regulates apoptosis. | Promotion of ECs apoptosis. | 58 | (AS) | miR-1 | Down-regulated | Downregulation of miR-10a enhances IκB/NF-κB activation | [69] | |
| [ | 139 | ] | 59 | (AS) | miR-221/222 | Down-regulated | Down-regulatesSuppression of PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) in the progression of atherosclerosis | Induction of Pathological cardiac remodelling.[70] | |||
| [ | 141 | ] | 60 | Congenital heart diseases (CHDs) | miR-1275, miR-27b, miR-421 | Up-regulated | usually developing hearts | [13] | |||
| 61 | |||||||||||
| 59 | Heart failure | CHRF | Up-regulated | Endogenous sponge to miRNA-489 activity. | [142] | CHD | |||||
| 60 | miR-122, miR-1201 | Down-regulated | developing hearts | [ | 14] | ||||||
| Heart failure | HEAT2 | Up-regulated | Biomarker. | [ | 143] | 62 | CHD | miR-222, miR-337-5p, miR-363, miR-424, miR-424, miR-660, miR-708, miR-421, miR-19a, miR-130b, miR-146b-5p, miR-154, miR-155, miR-181c, miR-181d and miR-192, | Up-regulated | tetralogy of Fallot | [15] |
| 61 | Heart failure | HOTAIR | 63 | CHDs | miR-181a, miR-720, miR-29c and miR-940 | Down-regulated | tetralogy of Fallot | [16] | |||
| 64 | CHD | miR-181c | Up-regulated | ventricular septal defect | [17] | ||||||
| CHD | miR-1-1 | Down-regulated | ventricular septal defect | [18] | |||||||
| 65 | |||||||||||
| 57 | Heart failure | Chaer | Dysregulation | Induction of Pathological cardiac remodelling.Up-regulated | LncRNA HOTAIR may function as a miR-19-sponge to modulate PTEN levels Biomarker. | [144] | |||||
| 62 | Heart failure | LIPCAR | Up-regulated | Biomarker. | [145] | ||||||
| 63 | Heart failure | lincRNA-ROR | Regulates CH | Regulation of cardiac hypertrophy acting as a sponge for miRNA-133. | [146] | ||||||
| 64 | Heart failure | CHD | miR-106a, miR-144, miR-451, miR-486-3p, miR-486-5p, hsa-let-7e, miR-16, miR-18a, miR-25, miR-93, and miR-505 | Up-regulated | transposition of the great arteries | [19] | |||||
| 66 | CHD | miR-873 | Up-regulated | Cyanotic CHD | [20] | ||||||
| [ | 140 | ] | |||||||||
| 58 | Heart failure | Chast | LOC285194 | Up-regulated | overexpression suppressed MKN45 and HGC-27 cell proliferation and promoted cell apoptosis; Biomarker. | [144] | |||||
| 65 | Heart failure | MEG3 | Regulates CF | Regulation of cardiac fibrosis and diastolic dysfunction | [147] | ||||||
| 66 | Heart failure | MHRT | Regulates of chromatin re-modellers | Regulation of chromatin remodels and biomarker. | [148] | 67 | CHD | miR-182 | Down-regulated | Cyanotic CHD | [21] |
| 67 | Heart failure | MIAT | Regulates CH | Regulation of cardiac hypertrophy acting as a sponge for miRNA-150. | [149] | 68 | CHD | ||||
| 68 | miR-498 | Heart failure | NRONUp-regulated | Ventricular septal defect | |||||||
| Upregulated | Biomarker. | [ | 150 | ] | 69 | CHD | miR-379-5p, miR-409-3p, miR-433, hsa-let-7e-5p, miR-155-5p, miR-222-3p, and miR-487b | Down-regulated | Ventricular septal defect | [22] | |
| 69 | Heart failure | RNY5 | Dysregulation | Biomarker. | [151] | 70 | CHD | hsa-let-7b, hsa-let-7a, and miR-486 | Up-regulated | Atrioventricular septal defect and atrial septal defect | [23] |
| 71 | |||||||||||
| 70 | Heart failure | SOX2-OT | Dysregulation | Biomarker. | [152] | CHD | miR-19b, miR-22, miR-29c, miR-375 | Up-regulated | Atrioventricular septal defect and atrial septal defect | [22][23 | |
| 71 | ] | ||||||||||
| Heart failure | SRA1 | Dysregulation | Biomarker. | [ | 153] | 72 | Myocrdial infraction (I) | miR-1 | Cardiomyocyte Downstream Targets: Ncx-1; KCNJ2, GJA1; IGF-1 |
[55] | |
| 73 | MI | miR-15 | Up-regulated |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021.
- Sreeniwas Kumar, A.; Sinha, N. Cardiovascular Disease in India: A 360 Degree Overview. Med. J. Armed Forces India 2020, 76, 1–3.
- Cuadrado-Godia, E.; Ois, A.; Roquer, J. Heart Failure in Acute Ischemic Stroke. CCR 2010, 6, 202–213.
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488.
- Schwalm, J.D.; McKee, M.; Huffman, M.D.; Yusuf, S. Resource Effective Strategies to Prevent and Treat Cardiovascular Disease. Circulation 2016, 133, 742–755.
- Sallam, T.; Sandhu, J.; Tontonoz, P. Long Noncoding RNA Discovery in Cardiovascular Disease: Decoding Form to Function. Circ. Res. 2018, 122, 155–166.
- Zhang, C.; Han, B.; Xu, T.; Li, D. The Biological Function and Potential Mechanism of Long Non-coding RNAs in Cardiovascular Disease. J. Cell. Mol. Med. 2020, 24, 12900–12909.
- Correia, C.C.M.; Rodrigues, L.F.; de Avila Pelozin, B.R.; Oliveira, E.M.; Fernandes, T. Long Non-Coding RNAs in Cardiovascular Diseases: Potential Function as Biomarkers and Therapeutic Targets of Exercise Training. ncRNA 2021, 7, 65.
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.-M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-Coding RNAs in Cardiovascular Diseases: Diagnostic and Therapeutic Perspectives. Eur. Heart J. 2018, 39, 2704–2716.
- Lu, P.; Ding, F.; Xiang, Y.K.; Hao, L.; Zhao, M. Noncoding RNAs in Cardiac Hypertrophy and Heart Failure. Cells 2022, 11, 777.
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854.
- Marinescu, M.-C.; Lazar, A.-L.; Marta, M.M.; Cozma, A.; Catana, C.-S. Non-Coding RNAs: Prevention, Diagnosis, and Treatment in Myocardial Ischemia–Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 2728.
- Huang, X.-H.; Li, J.-L.; Li, X.-Y.; Wang, S.-X.; Jiao, Z.-H.; Li, S.-Q.; Liu, J.; Ding, J. MiR-208a in Cardiac Hypertrophy and Remodeling. Front. Cardiovasc. Med. 2021, 8, 773314.
- Liu, K.; Hao, Q.; Wei, J.; Li, G.-H.; Wu, Y.; Zhao, Y.-F. MicroRNA-19a/b-3p Protect the Heart from Hypertension-Induced Pathological Cardiac Hypertrophy through PDE5A. J. Hypertens. 2018, 36, 1847–1857.
- Seok, H.Y.; Chen, J.; Kataoka, M.; Huang, Z.-P.; Ding, J.; Yan, J.; Hu, X.; Wang, D.-Z. Loss of MicroRNA-155 Protects the Heart From Pathological Cardiac Hypertrophy. Circ. Res. 2014, 114, 1585–1595.
- Yan, M.; Yang, S.; Meng, F.; Zhao, Z.; Tian, Z.; Yang, P. MicroRNA 199a-5p Induces Apoptosis by Targeting JunB. Sci. Rep. 2018, 8, 6699.
- Wehbe, N.; Nasser, S.; Pintus, G.; Badran, A.; Eid, A.; Baydoun, E. MicroRNAs in Cardiac Hypertrophy. Int. J. Mol. Sci. 2019, 20, 4714.
- Wei, L.; Yuan, M.; Zhou, R.; Bai, Q.; Zhang, W.; Zhang, M.; Huang, Y.; Shi, L. MicroRNA-101 Inhibits Rat Cardiac Hypertrophy by Targeting Rab1a. J. Cardiovasc. Pharmacol. 2015, 65, 357–363.
- Kim, J.O.; Song, D.W.; Kwon, E.J.; Hong, S.-E.; Song, H.K.; Min, C.K.; Kim, D.H. MiR-185 Plays an Anti-Hypertrophic Role in the Heart via Multiple Targets in the Calcium-Signaling Pathways. PLoS ONE 2015, 10, e0122509.
- Huang, J.; Sun, W.; Huang, H.; Ye, J.; Pan, W.; Zhong, Y.; Cheng, C.; You, X.; Liu, B.; Xiong, L.; et al. MiR-34a Modulates Angiotensin II-Induced Myocardial Hypertrophy by Direct Inhibition of ATG9A Expression and Autophagic Activity. PLoS ONE 2014, 9, e94382.
- Li, R.; Yan, G.; Zhang, Q.; Jiang, Y.; Sun, H.; Hu, Y.; Sun, J.; Xu, B. MiR-145 Inhibits Isoproterenol-induced Cardiomyocyte Hypertrophy by Targeting the Expression and Localization of GATA6. FEBS Lett. 2013, 587, 1754–1761.
- Liu, W.; Liu, Y.; Zhang, Y.; Zhu, X.; Zhang, R.; Guan, L.; Tang, Q.; Jiang, H.; Huang, C.; Huang, H. MicroRNA-150 Protects Against Pressure Overload-Induced Cardiac Hypertrophy: M ICRO RNA-150 M ODULATES C ARDIAC H YPERTROPHY. J. Cell. Biochem. 2015, 116, 2166–2176.
- Ganesan, J.; Ramanujam, D.; Sassi, Y.; Ahles, A.; Jentzsch, C.; Werfel, S.; Leierseder, S.; Loyer, X.; Giacca, M.; Zentilin, L.; et al. MiR-378 Controls Cardiac Hypertrophy by Combined Repression of Mitogen-Activated Protein Kinase Pathway Factors. Circulation 2013, 127, 2097–2106.
- Zhang, B.; Mao, S.; Liu, X.; Li, S.; Zhou, H.; Gu, Y.; Liu, W.; Fu, L.; Liao, C.; Wang, P. MiR-125b Inhibits Cardiomyocyte Apoptosis by Targeting BAK1 in Heart Failure. Mol. Med. 2021, 27, 72.
- Huang, Z.-P.; Wang, D.-Z. MiR-22 in Cardiac Remodeling and Disease. Trends Cardiovasc. Med. 2014, 24, 267–272.
- Li, F.; Li, S.-S.; Chen, H.; Zhao, J.-Z.; Hao, J.; Liu, J.-M.; Zu, X.-G.; Cui, W. MiR-320 Accelerates Chronic Heart Failure with Cardiac Fibrosis through Activation of the IL6/STAT3 Axis. Aging 2021, 13, 22516–22527.
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p As a Circulating Biomarker for Heart Failure. Circ. Res. 2010, 106, 1035–1039.
- Zhang, F.; Cheng, N.; Du, J.; Zhang, H.; Zhang, C. MicroRNA-200b-3p Promotes Endothelial Cell Apoptosis by Targeting HDAC4 in Atherosclerosis. BMC Cardiovasc. Disord 2021, 21, 172.
- Shen, N.-N.; Wang, J.-L.; Fu, Y. The MicroRNA Expression Profiling in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 856358.
- Peterlin, A.; Počivavšek, K.; Petrovič, D.; Peterlin, B. The Role of MicroRNAs in Heart Failure: A Systematic Review. Front. Cardiovasc. Med. 2020, 7, 161.
- Zhao, X.; Wang, Y.; Sun, X. The Functions of MicroRNA-208 in the Heart. Diabetes Res. Clin. Pract. 2020, 160, 108004.
- Khanaghaei, M.; Tourkianvalashani, F.; Hekmatimoghaddam, S.; Ghasemi, N.; Rahaie, M.; Khorramshahi, V.; Sheikhpour, A.; Heydari, Z.; Pourrajab, F. Circulating MiR-126 and MiR-499 Reflect Progression of Cardiovascular Disease; Correlations with Uric Acid and Ejection Fraction. Heart Int. 2016, 11, heartint.500022.
- Zhang, M.-W.; Shen, Y.-J.; Shi, J.; Yu, J.-G. MiR-223-3p in Cardiovascular Diseases: A Biomarker and Potential Therapeutic Target. Front. Cardiovasc. Med. 2021, 7, 610561.
- De Gonzalo-Calvo, D.; Cediel, G.; Bär, C.; Núñez, J.; Revuelta-Lopez, E.; Gavara, J.; Ríos-Navarro, C.; Llorente-Cortes, V.; Bodí, V.; Thum, T.; et al. Circulating MiR-1254 Predicts Ventricular Remodeling in Patients with ST-Segment-Elevation Myocardial Infarction: A Cardiovascular Magnetic Resonance Study. Sci. Rep. 2018, 8, 15115.
- Chen, X.; Li, C.; Li, J.; Sheng, L.; Liu, X. Upregulation of MiR-1306-5p Decreases Cerebral Ischemia/Reperfusion Injury in Vitro by Targeting BIK. Biosci. Biotechnol. Biochem. 2019, 83, 2230–2237.
- Yuan, L.; Tang, C.; Li, D.; Yang, Z. MicroRNA-18a Expression in Female Coronary Heart Disease and Regulatory Mechanism on Endothelial Cell by Targeting Estrogen Receptor. J. Cardiovasc. Pharmacol. 2018, 72, 277–284.
- Icli, B.; Dorbala, P.; Feinberg, M.W. An Emerging Role for the MiR-26 Family in Cardiovascular Disease. Trends Cardiovasc. Med. 2014, 24, 241–248.
- Tian, C.; Hu, G.; Gao, L.; Hackfort, B.T.; Zucker, I.H. Extracellular Vesicular MicroRNA-27a* Contributes to Cardiac Hypertrophy in Chronic Heart Failure. J. Mol. Cell. Cardiol. 2020, 143, 120–131.
- Yang, J.; Yang, X.-S.; Fan, S.-W.; Zhao, X.-Y.; Li, C.; Zhao, Z.-Y.; Pei, H.-J.; Qiu, L.; Zhuang, X.; Yang, C.-H. Prognostic Value of MicroRNAs in Heart Failure: A Meta-Analysis. Medicine 2021, 100, e27744.
- Guan, X.; Wang, L.; Liu, Z.; Guo, X.; Jiang, Y.; Lu, Y.; Peng, Y.; Liu, T.; Yang, B.; Shan, H.; et al. MiR-106a Promotes Cardiac Hypertrophy by Targeting Mitofusin 2. J. Mol. Cell. Cardiol. 2016, 99, 207–217.
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA Therapy Stimulates Uncontrolled Cardiac Repair after Myocardial Infarction in Pigs. Nature 2019, 569, 418–422.
- Chi, X.; Jiang, Y.; Chen, Y.; Lv, L.; Chen, J.; Yang, F.; Zhang, X.; Pan, F.; Cai, Q. Upregulation of MicroRNA MiR-652-3p Is a Prognostic Risk Factor for Hepatocellular Carcinoma and Regulates Cell Proliferation, Migration, and Invasion. Bioengineered 2021, 12, 7519–7528.
- Kura, B.; Kalocayova, B.; Devaux, Y.; Bartekova, M. Potential Clinical Implications of MiR-1 and MiR-21 in Heart Disease and Cardioprotection. Int. J. Mol. Sci. 2020, 21, 700.
- Wang, X.; Lian, Y.; Wen, X.; Guo, J.; Wang, Z.; Jiang, S.; Hu, Y. Expression of MiR-126 and Its Potential Function in Coronary Artery Disease. Afr. Health Sci. 2017, 17, 474.
- Rizzacasa, B.; Morini, E.; Mango, R.; Vancheri, C.; Budassi, S.; Massaro, G.; Maletta, S.; Macrini, M.; D’Annibale, S.; Romeo, F.; et al. MiR-423 Is Differentially Expressed in Patients with Stable and Unstable Coronary Artery Disease: A Pilot Study. PLoS ONE 2019, 14, e0216363.
- Fathi, M.; Gharakhanlou, R.; Rezaei, R. The Changes Of Heart MiR-1 And MiR-133 Expressions Following Physiological Hypertrophy Due To Endurance Training. Cell J. 2020, 22, 133–140.
- Luo, X.; Pan, Z.; Shan, H.; Xiao, J.; Sun, X.; Wang, N.; Lin, H.; Xiao, L.; Maguy, A.; Qi, X.-Y.; et al. MicroRNA-26 Governs Profibrillatory Inward-Rectifier Potassium Current Changes in Atrial Fibrillation. J. Clin. Investig. 2013, 123, 1939–1951.
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.-D.; Esfandyari, D.; Papadopoulou, A.S.; De Strooper, B.; et al. Cardiac Myocyte MiR-29 Promotes Pathological Remodeling of the Heart by Activating Wnt Signaling. Nat. Commun. 2017, 8, 1614.
- Li, J.; Salvador, A.M.; Li, G.; Valkov, N.; Ziegler, O.; Yeri, A.; Yang Xiao, C.; Meechoovet, B.; Alsop, E.; Rodosthenous, R.S.; et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ. Res. 2021, 128, e1–e23.
- Li, N.; Zhou, H.; Tang, Q. MiR-133: A Suppressor of Cardiac Remodeling? Front. Pharmacol. 2018, 9, 903.
- Huang, H.; Chen, H.; Liang, X.; Chen, X.; Chen, X.; Chen, C. Upregulated MiR-328-3p and Its High Risk in Atrial Fibrillation: A Systematic Review and Meta-Analysis with Meta-Regression. Medicine 2022, 101, e28980.
- Ling, T.-Y.; Wang, X.-L.; Chai, Q.; Lau, T.-W.; Koestler, C.M.; Park, S.J.; Daly, R.C.; Greason, K.L.; Jen, J.; Wu, L.-Q.; et al. Regulation of the SK3 Channel by MicroRNA-499—Potential Role in Atrial Fibrillation. Heart Rhythm. 2013, 10, 1001–1009.
- Cardin, S.; Guasch, E.; Luo, X.; Naud, P.; Le Quang, K.; Shi, Y.; Tardif, J.-C.; Comtois, P.; Nattel, S. Role for MicroRNA-21 in Atrial Profibrillatory Fibrotic Remodeling Associated With Experimental Postinfarction Heart Failure. Circ. Arrhythmia Electrophysiol. 2012, 5, 1027–1035.
- Girmatsion, Z.; Biliczki, P.; Bonauer, A.; Wimmer-Greinecker, G.; Scherer, M.; Moritz, A.; Bukowska, A.; Goette, A.; Nattel, S.; Hohnloser, S.H.; et al. Changes in MicroRNA-1 Expression and IK1 up-Regulation in Human Atrial Fibrillation. Heart Rhythm. 2009, 6, 1802–1809.
- Wexler, Y.; Nussinovitch, U. The Diagnostic Value of Mir-133a in ST Elevation and Non-ST Elevation Myocardial Infarction: A Meta-Analysis. Cells 2020, 9, 793.
- Wang, J.; Xu, L.; Tian, L.; Sun, Q. Circulating MicroRNA-208 Family as Early Diagnostic Biomarkers for Acute Myocardial Infarction: A Meta-Analysis. Medicine 2021, 100, e27779.
- Hoekstra, M. MicroRNA-499-5p: A Therapeutic Target in the Context of Cardiovascular Disease. Ann. Transl. Med. 2016, 4, 539.
- Ling, H.; Guo, Z.; Shi, Y.; Zhang, L.; Song, C. Serum Exosomal MicroRNA-21, MicroRNA-126, and PTEN Are Novel Biomarkers for Diagnosis of Acute Coronary Syndrome. Front. Physiol. 2020, 11, 654.
- Yu, X.; Xu, J.; Song, M.; Zhang, L.; Li, Y.; Han, L.; Tang, M.; Zhang, W.; Zhong, M.; Wang, Z. Associations of Circulating MicroRNA-221 and 222 With the Severity of Coronary Artery Lesions in Acute Coronary Syndrome Patients. Angiology 2022, 73, 579–587.
- Rusu-Nastase, E.G.; Lupan, A.-M.; Marinescu, C.I.; Neculachi, C.A.; Preda, M.B.; Burlacu, A. MiR-29a Increase in Aging May Function as a Compensatory Mechanism Against Cardiac Fibrosis Through SERPINH1 Downregulation. Front. Cardiovasc. Med. 2022, 8, 810241.
- Caruso, P.; Dempsie, Y.; Stevens, H.C.; McDonald, R.A.; Long, L.; Lu, R.; White, K.; Mair, K.M.; McClure, J.D.; Southwood, M.; et al. A Role for MiR-145 in Pulmonary Arterial Hypertension: Evidence From Mouse Models and Patient Samples. Circ. Res. 2012, 111, 290–300.
- Parikh, V.N.; Jin, R.C.; Rabello, S.; Gulbahce, N.; White, K.; Hale, A.; Cottrill, K.A.; Shaik, R.S.; Waxman, A.B.; Zhang, Y.-Y.; et al. MicroRNA-21 Integrates Pathogenic Signaling to Control Pulmonary Hypertension: Results of a Network Bioinformatics Approach. Circulation 2012, 125, 1520–1532.
- Jalali, S.; Ramanathan, G.K.; Parthasarathy, P.T.; Aljubran, S.; Galam, L.; Yunus, A.; Garcia, S.; Cox, R.R.; Lockey, R.F.; Kolliputi, N. Mir-206 Regulates Pulmonary Artery Smooth Muscle Cell Proliferation and Differentiation. PLoS ONE 2012, 7, e46808.
- Guo, L.; Qiu, Z.; Wei, L.; Yu, X.; Gao, X.; Jiang, S.; Tian, H.; Jiang, C.; Zhu, D. The MicroRNA-328 Regulates Hypoxic Pulmonary Hypertension by Targeting at Insulin Growth Factor 1 Receptor and L-Type Calcium Channel-A1C. Hypertension 2012, 59, 1006–1013.
- Courboulin, A.; Paulin, R.; Giguère, N.J.; Saksouk, N.; Perreault, T.; Meloche, J.; Paquet, E.R.; Biardel, S.; Provencher, S.; Côté, J.; et al. Role for MiR-204 in Human Pulmonary Arterial Hypertension. J. Exp. Med. 2011, 208, 535–548.
- Ouimet, M.; Ediriweera, H.; Afonso, M.S.; Ramkhelawon, B.; Singaravelu, R.; Liao, X.; Bandler, R.C.; Rahman, K.; Fisher, E.A.; Rayner, K.J.; et al. MicroRNA-33 Regulates Macrophage Autophagy in Atherosclerosis. ATVB 2017, 37, 1058–1067.
- Wu, X.; Du, X.; Yang, Y.; Liu, X.; Liu, X.; Zhang, N.; Li, Y.; Jiang, X.; Jiang, Y.; Yang, Z. Inhibition of MiR-122 Reduced Atherosclerotic Lesion Formation by Regulating NPAS3-Mediated Endothelial to Mesenchymal Transition. Life Sci. 2021, 265, 118816.
- Boon, R.A.; Dimmeler, S. MicroRNA-126 in Atherosclerosis. ATVB 2014, 34, 449–454.
- Šatrauskienė, A.; Navickas, R.; Laucevičius, A.; Krilavičius, T.; Užupytė, R.; Zdanytė, M.; Ryliškytė, L.; Jucevičienė, A.; Holvoet, P. Mir-1, MiR-122, MiR-132, and MiR-133 Are Related to Subclinical Aortic Atherosclerosis Associated with Metabolic Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 1483.
- Song, J.; Ouyang, Y.; Che, J.; Li, X.; Zhao, Y.; Yang, K.; Zhao, X.; Chen, Y.; Fan, C.; Yuan, W. Potential Value of MiR-221/222 as Diagnostic, Prognostic, and Therapeutic Biomarkers for Diseases. Front. Immunol. 2017, 8, 56.
- Saheera, S.; Krishnamurthy, P. Cardiovascular Changes Associated with Hypertensive Heart Disease and Aging. Cell Transpl. 2020, 29, 096368972092083.
- Vavassori, C.; Cipriani, E.; Colombo, G.I. Circulating MicroRNAs as Novel Biomarkers in Risk Assessment and Prognosis of Coronary Artery Disease. Eur. Cardiol. 2022, 17, e06.
- Knezevic, I.; Patel, A.; Sundaresan, N.R.; Gupta, M.P.; Solaro, R.J.; Nagalingam, R.S.; Gupta, M. A Novel Cardiomyocyte-Enriched MicroRNA, MiR-378, Targets Insulin-like Growth Factor 1 Receptor. J. Biol. Chem. 2012, 287, 12913–12926.
- Gozuacik, D.; Akkoc, Y.; Ozturk, D.G.; Kocak, M. Autophagy-Regulating MicroRNAs and Cancer. Front. Oncol. 2017, 7, 65.
- Ikeda, S.; He, A.; Kong, S.W.; Lu, J.; Bejar, R.; Bodyak, N.; Lee, K.-H.; Ma, Q.; Kang, P.M.; Golub, T.R.; et al. MicroRNA-1 Negatively Regulates Expression of the Hypertrophy-Associated Calmodulin and Mef2a Genes. Mol. Cell Biol. 2009, 29, 2193–2204.
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B.A. Heart Failure With Preserved Ejection Fraction In Perspective. Circ. Res. 2019, 124, 1598–1617.
- Wong, L.; Wang, J.; Liew, O.; Richards, A.; Chen, Y.-T. MicroRNA and Heart Failure. Int. J. Mol. Sci. 2016, 17, 502.
- Schulte, C. Diagnostic and Prognostic Value of Circulating MicroRNAs in Heart Failure with Preserved and Reduced Ejection Fraction. WJC 2015, 7, 843.
- Iwasaki, Y.; Nishida, K.; Kato, T.; Nattel, S. Atrial Fibrillation Pathophysiology: Implications for Management. Circulation 2011, 124, 2264–2274.
- Ultimo, S.; Zauli, G.; Martelli, A.M.; Vitale, M.; McCubrey, J.A.; Capitani, S.; Neri, L.M. Cardiovascular Disease-Related MiRNAs Expression: Potential Role as Biomarkers and Effects of Training Exercise. Oncotarget 2018, 9, 17238–17254.
- Osbourne, A.; Calway, T.; Broman, M.; McSharry, S.; Earley, J.; Kim, G.H. Downregulation of Connexin43 by MicroRNA-130a in Cardiomyocytes Results in Cardiac Arrhythmias. J. Mol. Cell. Cardiol. 2014, 74, 53–63.
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651.
- Sayed, A.S.M.; Xia, K.; Yang, T.-L.; Peng, J. Circulating MicroRNAs: A Potential Role in Diagnosis and Prognosis of Acute Myocardial Infarction. Dis. Markers 2013, 35, 561–566.
- Zhou, S.; Jin, J.; Wang, J.; Zhang, Z.; Freedman, J.H.; Zheng, Y.; Cai, L. MiRNAS in Cardiovascular Diseases: Potential Biomarkers, Therapeutic Targets and Challenges. Acta Pharm. Sin. 2018, 39, 1073–1084.
- Halushka, P.V.; Goodwin, A.J.; Halushka, M.K. Opportunities for MicroRNAs in the Crowded Field of Cardiovascular Biomarkers. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 211–238.
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547.
- Ali Sheikh, M.S.; Alduraywish, A.; Almaeen, A.; Alruwali, M.; Alruwaili, R.; Alomair, B.M.; Salma, U.; Hedeab, G.M.; Bugti, N.; A.M.Abdulhabeeb, I. Therapeutic Value of MiRNAs in Coronary Artery Disease. Oxidative Med. Cell. Longev. 2021, 2021, 8853748.
- Andreou, I.; Sun, X.; Stone, P.H.; Edelman, E.R.; Feinberg, M.W. MiRNAs in Atherosclerotic Plaque Initiation, Progression, and Rupture. Trends Mol. Med. 2015, 21, 307–318.
- Uray, K.; Major, E.; Lontay, B. MicroRNA Regulatory Pathways in the Control of the Actin–Myosin Cytoskeleton. Cells 2020, 9, 1649.
- Nappi, F.; Iervolino, A.; Avtaar Singh, S.S.; Chello, M. MicroRNAs in Valvular Heart Diseases: Biological Regulators, Prognostic Markers and Therapeutical Targets. Int. J. Mol. Sci. 2021, 22, 12132.
- Bielska, A.; Niemira, M.; Kretowski, A. Recent Highlights of Research on MiRNAs as Early Potential Biomarkers for Cardiovascular Complications of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3153.
- Fang, Y.; Xu, Y.; Wang, R.; Hu, L.; Guo, D.; Xue, F.; Guo, W.; Zhang, D.; Hu, J.; Li, Y.; et al. Recent Advances on the Roles of LncRNAs in Cardiovascular Disease. J. Cell. Mol. Med. 2020, 24, 12246–12257.
- Bär, C.; Chatterjee, S.; Thum, T. Long Noncoding RNAs in Cardiovascular Pathology, Diagnosis, and Therapy. Circulation 2016, 134, 1484–1499.
- Uchida, S.; Dimmeler, S. Long Noncoding RNAs in Cardiovascular Diseases. Circ. Res. 2015, 116, 737–750.
- Ounzain, S.; Pedrazzini, T. Super-Enhancer Lncs to Cardiovascular Development and Disease. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1953–1960.
- Su, W.; Huo, Q.; Wu, H.; Wang, L.; Ding, X.; Liang, L.; Zhou, L.; Zhao, Y.; Dan, J.; Zhang, H. The Function of LncRNA-H19 in Cardiac Hypertrophy. Cell Biosci. 2021, 11, 153.
- Wolska, M.; Jarosz-Popek, J.; Junger, E.; Wicik, Z.; Porshoor, T.; Sharif, L.; Czajka, P.; Postula, M.; Mirowska-Guzel, D.; Czlonkowska, A.; et al. Long Non-Coding RNAs as Promising Therapeutic Approach in Ischemic Stroke: A Comprehensive Review. Mol. Neurobiol. 2021, 58, 1664–1682.
- Xie, L.; Zhang, Q.; Mao, J.; Zhang, J.; Li, L. The Roles of LncRNA in Myocardial Infarction: Molecular Mechanisms, Diagnosis Biomarkers, and Therapeutic Perspectives. Front. Cell Dev. Biol. 2021, 9, 680713.
- Yang, J.; Huang, X.; Hu, F.; Fu, X.; Jiang, Z.; Chen, K. LncRNA ANRIL Knockdown Relieves Myocardial Cell Apoptosis in Acute Myocardial Infarction by Regulating IL-33/ST2. Cell Cycle 2019, 18, 3393–3403.
- Long, B.; Li, N.; Xu, X.-X.; Li, X.-X.; Xu, X.-J.; Guo, D.; Zhang, D.; Wu, Z.-H.; Zhang, S.-Y. Long Noncoding RNA FTX Regulates Cardiomyocyte Apoptosis by Targeting MiR-29b-1-5p and Bcl2l2. Biochem. Biophys. Res. Commun. 2018, 495, 312–318.
- Cantile, M.; Di Bonito, M.; Tracey De Bellis, M.; Botti, G. Functional Interaction among LncRNA HOTAIR and MicroRNAs in Cancer and Other Human Diseases. Cancers 2021, 13, 570.
- Li, X.; Dai, Y.; Yan, S.; Shi, Y.; Han, B.; Li, J.; Cha, L.; Mu, J. Down-Regulation of LncRNA KCNQ1OT1 Protects against Myocardial Ischemia/Reperfusion Injury Following Acute Myocardial Infarction. Biochem. Biophys. Res. Commun. 2017, 491, 1026–1033.
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating Long Noncoding RNA, LIPCAR, Predicts Survival in Patients With Heart Failure. Circ. Res. 2014, 114, 1569–1575.
- Chen, G.; Huang, S.; Song, F.; Zhou, Y.; He, X. Lnc-Ang362 Is a pro-Fibrotic Long Non-Coding RNA Promoting Cardiac Fibrosis after Myocardial Infarction by Suppressing Smad7. Arch. Biochem. Biophys. 2020, 685, 108354.
- Bu, S.; Singh, K.K. Epigenetic Regulation of Autophagy in Cardiovascular Pathobiology. Int. J. Mol. Sci. 2021, 22, 6544.
- Nukala, S.B.; Jousma, J.; Cho, Y.; Lee, W.H.; Ong, S.-G. Long Non-Coding RNAs and MicroRNAs as Crucial Regulators in Cardio-Oncology. Cell Biosci. 2022, 12, 24.
- Wu, H.; Zhao, Z.-A.; Liu, J.; Hao, K.; Yu, Y.; Han, X.; Li, J.; Wang, Y.; Lei, W.; Dong, N.; et al. Long Noncoding RNA Meg3 Regulates Cardiomyocyte Apoptosis in Myocardial Infarction. Gene Ther. 2018, 25, 511–523.
- Zhang, J.; Gao, C.; Meng, M.; Tang, H. Long Noncoding RNA MHRT Protects Cardiomyocytes against H2O2-Induced Apoptosis. Biomol. Ther. 2016, 24, 19–24.
- Wang, X.; Yong, C.; Yu, K.; Yu, R.; Zhang, R.; Yu, L.; Li, S.; Cai, S. Long Noncoding RNA (LncRNA) N379519 Promotes Cardiac Fibrosis in Post-Infarct Myocardium by Targeting MiR-30. Med. Sci. Monit. 2018, 24, 3958–3965.
- Magadum, A.; Singh, N.; Kurian, A.A.; Munir, I.; Mehmood, T.; Brown, K.; Sharkar, M.T.K.; Chepurko, E.; Sassi, Y.; Oh, J.G.; et al. Pkm2 Regulates Cardiomyocyte Cell Cycle and Promotes Cardiac Regeneration. Circulation 2020, 141, 1249–1265.
- Wang, K.; Liu, F.; Liu, C.-Y.; An, T.; Zhang, J.; Zhou, L.-Y.; Wang, M.; Dong, Y.-H.; Li, N.; Gao, J.-N.; et al. The Long Noncoding RNA NRF Regulates Programmed Necrosis and Myocardial Injury during Ischemia and Reperfusion by Targeting MiR-873. Cell Death Differ. 2016, 23, 1394–1405.
- Micheletti, R.; Plaisance, I.; Abraham, B.J.; Sarre, A.; Ting, C.-C.; Alexanian, M.; Maric, D.; Maison, D.; Nemir, M.; Young, R.A.; et al. The Long Noncoding RNA Wisper Controls Cardiac Fibrosis and Remodeling. Sci. Transl. Med. 2017, 9, eaai9118.
- Jiao, L.; Li, M.; Shao, Y.; Zhang, Y.; Gong, M.; Yang, X.; Wang, Y.; Tan, Z.; Sun, L.; Xuan, L.; et al. LncRNA-ZFAS1 Induces Mitochondria-Mediated Apoptosis by Causing Cytosolic Ca2+ Overload in Myocardial Infarction Mice Model. Cell Death Dis. 2019, 10, 942.
- Dueñas, A.; Expósito, A.; Aranega, A.; Franco, D. The Role of Non-Coding RNA in Congenital Heart Diseases. JCDD 2019, 6, 15.
- Lu, M.; Lu, Q.; Zhang, Y.; Tian, G. ApoB/ApoA1 Is an Effective Predictor of Coronary Heart Disease Risk in Overweight and Obesity. J. Biomed. Res. 2011, 25, 266–273.
- Li, X.; Song, F.; Sun, H. Long Non-coding RNA AWPPH Interacts with ROCK2 and Regulates the Proliferation and Apoptosis of Cancer Cells in Pediatric T-cell Acute Lymphoblastic Leukemia. Oncol. Lett. 2020, 20, 239.
- Li, Y.; Fang, J.; Zhou, Z.; Zhou, Q.; Sun, S.; Jin, Z.; Xi, Z.; Wei, J. Downregulation of LncRNA BACE1-AS Improves Dopamine-Dependent Oxidative Stress in Rats with Parkinson’s Disease by Upregulating MicroRNA-34b-5p and Downregulating BACE1. Cell Cycle 2020, 19, 1158–1171.
- Mao, J.; Zhou, Y.; Lu, L.; Zhang, P.; Ren, R.; Wang, Y.; Wang, J. Identifying a Serum Exosomal-Associated LncRNA/CircRNA-MiRNA-MRNA Network in Coronary Heart Disease. Cardiol. Res. Pract. 2021, 2021, 6682183.
- Hennessy, E.J.; van Solingen, C.; Scacalossi, K.R.; Ouimet, M.; Afonso, M.S.; Prins, J.; Koelwyn, G.J.; Sharma, M.; Ramkhelawon, B.; Carpenter, S.; et al. The Long Noncoding RNA CHROME Regulates Cholesterol Homeostasis in Primates. Nat. Metab. 2019, 1, 98–110.
- Guo, F.; Sha, Y.; Hu, B.; Li, G. Correlation of Long Non-Coding RNA LncRNA-FA2H-2 With Inflammatory Markers in the Peripheral Blood of Patients With Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 682959.
- Toni, L.; Hailu, F.; Sucharov, C.C. Dysregulated Micro-RNAs and Long Noncoding RNAs in Cardiac Development and Pediatric Heart Failure. Am. J. Physiol.-Heart Circ. Physiol. 2020, 318, H1308–H1315.
- Huang, Y.; Wang, L.; Mao, Y.; Nan, G. Long Noncoding RNA-H19 Contributes to Atherosclerosis and Induces Ischemic Stroke via the Upregulation of Acid Phosphatase 5. Front. Neurol. 2019, 10, 32.
- Sun, Y.; Huang, S.; Wan, C.; Ruan, Q.; Xie, X.; Wei, D.; Li, G.; Lin, S.; Li, H.; Wu, S. Knockdown of LncRNA ENST00000609755.1 Confers Protection Against Early OxLDL-Induced Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 650212.
- Wang, F.; Cai, X.; Jiao, P.; Liu, Y.; Yuan, B.; Zhang, P.; Liu, H.; Ma, L. Relationship between Long Non-Coding RNA and Prognosis of Patients with Coronary Heart Disease after Percutaneous Coronary Intervention: A Protocol for Systematic Review and Meta-Analysis. Medicine 2020, 99, e23525.
- Wu, G.; Cai, J.; Han, Y.; Chen, J.; Huang, Z.-P.; Chen, C.; Cai, Y.; Huang, H.; Yang, Y.; Liu, Y.; et al. LincRNA-P21 Regulates Neointima Formation, Vascular Smooth Muscle Cell Proliferation, Apoptosis, and Atherosclerosis by Enhancing P53 Activity. Circulation 2014, 130, 1452–1465.
- Wang, Q.-C.; Wang, Z.-Y.; Xu, Q.; Chen, X.-L.; Shi, R.-Z. LncRNA Expression Profiles and Associated CeRNA Network Analyses in Epicardial Adipose Tissue of Patients with Coronary Artery Disease. Sci. Rep. 2021, 11, 1567.
- Cao, C.; Zhen, W.; Yu, H.; Zhang, L.; Liu, Y. LncRNA MALAT1/MiR-143 Axis Is a Potential Biomarker for in-Stent Restenosis and Is Involved in the Multiplication of Vascular Smooth Muscle Cells. Open Life Sci. 2021, 16, 1303–1312.
- Saygili, H.; Bozgeyik, I.; Yumrutas, O.; Akturk, E.; Bagis, H. Differential Expression of Long Noncoding RNAs in Patients with Coronary Artery Disease. Mol. Syndr. 2021, 12, 372–378.
- Hu, Y.-W.; Guo, F.-X.; Xu, Y.-J.; Li, P.; Lu, Z.-F.; McVey, D.G.; Zheng, L.; Wang, Q.; Ye, J.H.; Kang, C.-M.; et al. Long Noncoding RNA NEXN-AS1 Mitigates Atherosclerosis by Regulating the Actin-Binding Protein NEXN. J. Clin. Investig. 2019, 129, 1115–1128.
- Liao, J.; Wang, J.; Liu, Y.; Li, J.; Duan, L. Transcriptome Sequencing of LncRNA, MiRNA, MRNA and Interaction Network Constructing in Coronary Heart Disease. BMC Med. Genom. 2019, 12, 124.
- Jin, L.; Lin, X.; Yang, L.; Fan, X.; Wang, W.; Li, S.; Li, J.; Liu, X.; Bao, M.; Cui, X.; et al. AK098656, a Novel Vascular Smooth Muscle Cell–Dominant Long Noncoding RNA, Promotes Hypertension. Hypertension 2018, 71, 262–272.
- Gholami, L.; Ghafouri-Fard, S.; Mirzajani, S.; Arsang-Jang, S.; Taheri, M.; Dehbani, Z.; Dehghani, S.; Houshmand, B.; Amid, R.; Sayad, A.; et al. The LncRNA ANRIL Is Down-Regulated in Peripheral Blood of Patients with Periodontitis. Non-Coding RNA Res. 2020, 5, 60–66.
- Luo, Y.; Guo, J.; Xu, P.; Gui, R. Long Non-Coding RNA GAS5 Maintains Insulin Secretion by Regulating Multiple MiRNAs in INS-1 832/13 Cells. Front. Mol. Biosci. 2020, 7, 559267.
- Das, S.; Zhang, E.; Senapati, P.; Amaram, V.; Reddy, M.A.; Stapleton, K.; Leung, A.; Lanting, L.; Wang, M.; Chen, Z.; et al. A Novel Angiotensin II–Induced Long Noncoding RNA Giver Regulates Oxidative Stress, Inflammation, and Proliferation in Vascular Smooth Muscle Cells. Circ. Res. 2018, 123, 1298–1312.
- Yu, B.; Wang, S. Angio-LncRs: LncRNAs That Regulate Angiogenesis and Vascular Disease. Theranostics 2018, 8, 3654–3675.
- Jusic, A.; Devaux, Y. On behalf of the EU-CardioRNA COST Action (CA17129) Noncoding RNAs in Hypertension. Hypertension 2019, 74, 477–492.
- Han, Y.; Ali, M.K.; Dua, K.; Spiekerkoetter, E.; Mao, Y. Role of Long Non-Coding RNAs in Pulmonary Arterial Hypertension. Cells 2021, 10, 1892.
- El Azzouzi, H.; Doevendans, P.A.; Sluijter, J.P.G. Long Non-Coding RNAs in Heart Failure: An Obvious Lnc. Ann. Transl. Med. 2016, 4, 182.
- Greco, S.; Zaccagnini, G.; Fuschi, P.; Voellenkle, C.; Carrara, M.; Sadeghi, I.; Bearzi, C.; Maimone, B.; Castelvecchio, S.; Stellos, K.; et al. Increased BACE1-AS Long Noncoding RNA and β-Amyloid Levels in Heart Failure. Cardiovasc. Res. 2017, 113, 453–463.
- Ottaviani, L.; Martins, P.A.D.C. Non-coding RNAs in cardiac hypertrophy. J. Physiol. 2017, 595, 4037–4050.
- Gomes, C.P.d.C.; Schroen, B.; Kuster, G.M.; Robinson, E.L.; Ford, K.; Squire, I.B.; Heymans, S.; Martelli, F.; Emanueli, C.; Devaux, Y.; et al. Regulatory RNAs in Heart Failure. Circulation 2020, 141, 313–328.
- Fan, J.; Li, H.; Xie, R.; Zhang, X.; Nie, X.; Shi, X.; Zhan, J.; Yin, Z.; Zhao, Y.; Dai, B.; et al. LncRNA ZNF593-AS Alleviates Contractile Dysfunction in Dilated Cardiomyopathy. Circ. Res. 2021, 128, 1708–1723.
- Wang, S.; Lv, T.; Chen, Q.; Yang, Y.; Xu, L.; Zhang, X.; Wang, E.; Hu, X.; Liu, Y. Transcriptome Sequencing and LncRNA-MiRNA-MRNA Network Construction in Cardiac Fibrosis and Heart Failure. Bioengineered 2022, 13, 7118–7133.
- Greco, S.; Zaccagnini, G.; Perfetti, A.; Fuschi, P.; Valaperta, R.; Voellenkle, C.; Castelvecchio, S.; Gaetano, C.; Finato, N.; Beltrami, A.P.; et al. Long Noncoding RNA Dysregulation in Ischemic Heart Failure. J. Transl. Med. 2016, 14, 183.
- Santer, L.; López, B.; Ravassa, S.; Baer, C.; Riedel, I.; Chatterjee, S.; Moreno, M.U.; González, A.; Querejeta, R.; Pinet, F.; et al. Circulating Long Noncoding RNA LIPCAR Predicts Heart Failure Outcomes in Patients Without Chronic Kidney Disease. Hypertension 2019, 73, 820–828.
- Sato, M.; Kadomatsu, T.; Miyata, K.; Warren, J.S.; Tian, Z.; Zhu, S.; Horiguchi, H.; Makaju, A.; Bakhtina, A.; Morinaga, J.; et al. The LncRNA Caren Antagonizes Heart Failure by Inactivating DNA Damage Response and Activating Mitochondrial Biogenesis. Nat. Commun. 2021, 12, 2529.
- Pinheiro, A.; Naya, F.J. The Key Lnc (RNA)s in Cardiac and Skeletal Muscle Development, Regeneration, and Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 84.
- Han, P.; Chang, C.-P. Long Non-Coding RNA and Chromatin Remodeling. RNA Biol. 2015, 12, 1094–1098.
- Yang, L.; Deng, J.; Ma, W.; Qiao, A.; Xu, S.; Yu, Y.; Boriboun, C.; Kang, X.; Han, D.; Ernst, P.; et al. Ablation of LncRNA Miat Attenuates Pathological Hypertrophy and Heart Failure. Theranostics 2021, 11, 7995–8007.
- Zheng, Y.; Zhang, Y.; Zhang, X.; Dang, Y.; Cheng, Y.; Hua, W.; Teng, M.; Wang, S.; Lu, X. Novel LncRNA-MiRNA-MRNA Competing Endogenous RNA Triple Networks Associated Programmed Cell Death in Heart Failure. Front. Cardiovasc. Med. 2021, 8, 747449.
- Garcia-Padilla, C.; Lozano-Velasco, E.; Garcia-Lopez, V.; Aranega, A.; Franco, D.; Garcia-Martinez, V.; Lopez-Sanchez, C. Comparative Analysis of Non-Coding RNA Transcriptomics in Heart Failure. Biomedicines 2022, 10, 3076.
- Ou, Y.; Liao, C.; Li, H.; Yu, G. LncRNA SOX2OT/Smad3 Feedback Loop Promotes Myocardial Fibrosis in Heart Failure. IUBMB Life 2020, 72, 2469–2480.
- Di Salvo, T.G.; Guo, Y.; Su, Y.R.; Clark, T.; Brittain, E.; Absi, T.; Maltais, S.; Hemnes, A. Right Ventricular Long Noncoding RNA Expression in Human Heart Failure. Pulm. Circ. 2015, 5, 135–161.
