Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lowell D. Kispert and Version 3 by Dean Liu.
Carotenoids are a large and diverse group of compounds that have been shown to have a wide range of potential health benefits. While some carotenoids have been extensively studied, numerous others have not received as much attention. In numerous studies, using EPR (electron paramagnetic resonance) techniques in correlation with DFT (density functional theory) calculations, rwesearchers have characterized about 20 conventional carotenoids - their electron transfer from the carotenoid molecule to form the radical cation, and further proton loss from the radical cation to form neutral radicals (radicals with no charge). Several conventional carotenoids are briefly discussed here.
- xanthophylls
- electron transfer
- radical cation
- carotenoid neutral radicals
1. Introduction
Carotenoids are a class of more than 1200 naturally occurring pigments synthesized by plants, algae and photosynthetic bacteria [1]. Carotenoids are important for human health because they act as antioxidants, helping to protect cells from damage caused by reactive oxygen species (ROS). They have been associated with a reduced risk of chronic diseases such as type 2 diabetes [2], cancer [3][4][3,4], heart disease or age-related macular degeneration [5]. Another important role is their provitamin A activity or the capability of some dietary carotenoids to form vitamin A by the action of dioxygenase enzymes [6]. For example, the dioxygenase enzyme β-Carotene 15-15′-oxygenase (BCO1) catalyzes the oxidative cleavage of dietary β-Carotene to retinal (vitamin A aldehyde) [7], which can be further reduced to retinol (vitamin A) or oxidized to retinoic acid (the biologically active form of vitamin A). Carotenoids such as β-carotene and α-carotene β-cryptoxanthin are considered provitamin A carotenoids, but any carotenoid with at least one unchanged β-ionone ring in its structure can have provitamin A activity. In addition to their health benefits, carotenoids in plants are also involved in photosynthesis. They are no longer considered just accessory pigments [8]; they have essential roles in photosynthesis [9], helping to capture light energy and transfer it to chlorophyll molecules and also protecting plants from damage caused by excess light and other environmental stresses [10]. Similarly, in the human body, carotenoids photoprotect against damage by intense light and harmful free radicals, and also maintain the structural and functional integrity of biological membranes. The mechanisms through which carotenoids may exert their health effects are complex, and further studies, including clinical studies, are needed to provide a comprehensive understanding. Carotenoids are indispensable for life. The world of carotenoids is endless. Their number and resourcefulness are immense, and only a tiny fraction of the 1200 carotenoids has been studied to date. Their complexity lies not only in their number, but in their different yet alike structures (given by the different functional groups and similar polyene chains) and their interactions with a multitude of different environments.
2. Studies of Electron and Proton Loss of Conventional Carotenoids by DFT and EPR
Carotenoids are prone to oxidation. RWesearchers have studied the oxidation of carotenoids adsorbed on solid artificial matrices, such as silica alumina or imbedded in the pores of molecular sieves MCM-41, where their radical cation is formed by electron transfer from the carotenoid molecule to the solid matrix [11][11]. Some of the most well-known carotenoids that reswearchers studied using EPR methods in combination with DFT include lycopene, β-carotene, zeaxanthin, canthaxanthin, lutein, astaxanthin and cis-bixin (Figure 1). With normal EPR at the X-band frequency (9 GHz), the carotenoid radical cation exhibits a single unresolved peak with giso = 2.0027, characteristic of organic π-radicals. In the year 1999, the EPR signal previously not resolved at the X-band frequency was resolved at a higher frequency (330 GHz) [11][12]. At higher frequencies, from 327 to 670 GHz, the unresolved line resolves into two peaks as a result of the g-anisotropy of g⊥ = 2.0023 and g|| = 2.0032, characteristic of a cylindrically symmetrical π-radical cation. Determining the g-tensors from high-field spectra is important for learning about molecular structure from its principal values. The difference gxx − gyy decreases with increasing chain length. When gxx − gyy approaches zero, the g-tensor becomes cylindrically symmetrical with gxx = gyy = g⊥ and gzz = g||. This applies for the all-trans carotenoid radical cations and allows differentiation between carotenoid radical cations with cylindrical symmetry and other C-H organic radicals of different symmetry. The lack of temperature dependence of the EPR line widths over the range of 5–80 K at 327 GHz also suggests a rapid rotation of methyl groups even at 5 K, which averages out the proton couplings from three oriented β-protons. This results in isotropic β-proton couplings from rotating methyl groups [11][12].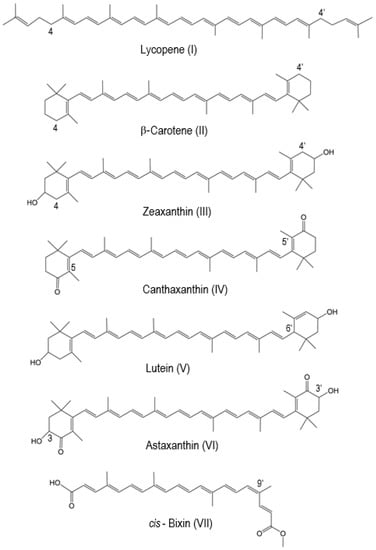
Figure 1. The structures of selected natural carotenoids.
Table 1. The most favorable proton loss positions for selected carotenoids in our studies.
| Carotenoid | The Most Favorable Proton Loss from the Radical Cation | Reference | |
|---|---|---|---|
| Lycopene | C4(4′) | [14] | |
| β-carotene | C4(4′) | [15] | |
| Zeaxanthin | C4(4′) | [15][16] | [15,16] |
| Canthaxanthin | C5(5′) | [15] | |
| Lutein | C6′ | [15] | |
| Astaxanthin | C3(C3′) | [17] | |
| cis | -Bixin | C9′ | [20] |
