Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Yiming Liu.
The lithium–sulfur (Li-S) battery is considered to be one of the attractive candidates for breaking the limit of specific energy of lithium-ion batteries and has the potential to conquer the related energy storage market due to its advantages of low-cost, high-energy density, high theoretical specific energy, and environmental friendliness issues.
- lithium–sulfur batteries
- low temperatures
- anode
1. Introduction
In the context of the green and environmental protection society, the demand for rechargeable batteries is ever increasing, and the application range is also gradually becoming wider. Meanwhile, it is a significant challenge to break the theoretical bottleneck for commercial lithium-ion batteries (LIBs), consisting of a low-capacity anode and cathode.
Moreover, with the in-depth research of Goodenough et al. on lithium–sulfur (Li-S) batteries, such as the invention and development of key cathode materials for lithium-ion batteries, the potential development of lithium-ion batteries is being limited [1,2][1][2]. Li-S batteries have been widely considered because of their higher theoretical energy density, stronger environmental protection ability and lower cost, features with great promise when compared to alternative LIBs [3,4][3][4]. Unlike LIBs, working with the ion insertion mechanism [5[5][6],6], Li-S batteries are mainly based on the conversion reaction of active materials [7]. The active materials of the positive and negative electrodes are elemental sulfur and lithium metal, respectively, making the Li-S battery a high energy density, high performance, and low-cost sulfur cathode [8,9,10][8][9][10]. Therefore, Li-S batteries have an outstanding contribution to solving the problem of the subsequent development of the battery industry.
Unfortunately, Li-S batteries still face many challenges, especially at low temperatures. Li-S batteries, at low temperatures, suffer from the slow kinetics of cathode and anode reactions, which in turn leads to low capacity and poor cycle performance [11]. In addition, the shuttle effect of soluble lithium polysulfides (LiPSs, Li2Sx, 2 < x ≤ 8), the insulation of S and insoluble sulfide (Li2S2 and Li2S) [12[12][13],13], the drastic volume change during charge and discharge, and the inherent dendrite growth of the lithium metal anode play a key role in the electrochemical performance [14].
In recent years, Li-S batteries have attracted extensive attention, but this is mainly focused on their performance at room temperature, including the idea of an interlaminar structure [15], designing novel electrode materials with enhanced electrochemical activity and stability [16[16][17],17], and optimizing the electrolyte composition and structure to improve ion transport and reduce the formation of lithium polysulfide [18]. However, there are still few systematic discussions on the operation of Li-S batteries at low temperatures.
2. The Underlying Mechanisms of Li-S Batteries at Low Temperature
The Li-S batteries are composed of a lithium anode, a sulfur cathode, an electrolyte, and a separator, suffering from the dilemma of reduced efficiency or even deactivation when they operate at low temperature. Specifically, the main failure mechanisms of Li-S batteries at low temperature include (i) a high Li ion desolvation energy barrier; (ii) uncontrolled nucleation and deposition of lithium; (iii) LiPSs cluster aggregation; and (iv) cathode passivation caused by Li2S film deposition (Figure 1) [22][19].
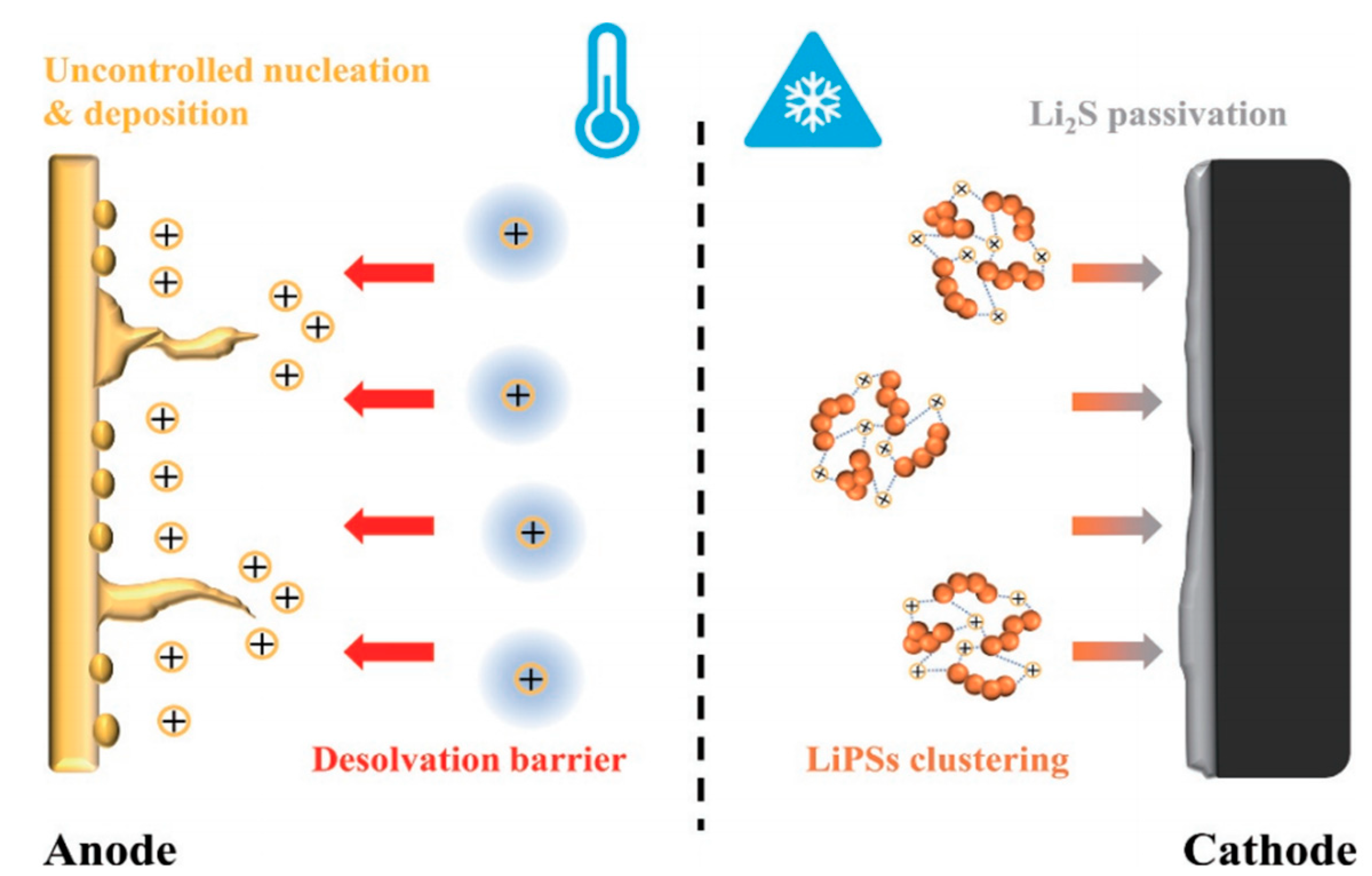
Figure 1. Illustration of Li-S battery failure mechanisms at low temperature.
The high desolvation energy barrier of the Li ions at low temperature was determined to be the key factor leading to the failure of the battery. When the Li ions are bonded with the solvent molecule, the energy barrier for desolvation substantially increases at the electrochemical interface and further weakens local charge transfer capability [23][20]. To this end, the design strategies of solid electrolyte interphase (SEI) should be reconsidered to promote the dissociation of solvent molecules from the primary dissolved sheath of Li ions [24][21], which is favorable for the in-depth investigation of the high lithium ion dissolving energy barrier and would further enhance the performance of the lithium metal anode at low temperature.
The uncontrolled nucleation and deposition of lithium have an extremely negative effect on cycle performance. As the temperature decreases, the large-size deposited Li will evolve into dead lithium with an extensive surface, significantly reducing the Coulomb efficiency [25][22]. Accordingly, it is of great importance to develop effective strategies to mitigate lithium deposition and enhance the activity for Li-S batteries [26][23].
At low temperature, the low-discharge platform of Li-S batteries is firmly affected by the behavior of Li2S4 clusters [27][24]. In other words, the formation of Li2S4 clusters inhibit its conversion to Li2S, eventually leading to slow electrochemical reaction kinetics and capacity loss to the secondary voltage platform [28][25].
Regarding the cathode passivation by the film-like Li2S deposition, the nucleation size of Li2S shrinks with the decreased temperature, which leads to the prepassivation of the electrode surface and disappearance of three-phase interface of the active material, electronic conductor, and ionic conductor (electrolyte), thus terminating the electrochemical reaction. As a result, adjusting the deposition morphology of Li2S is of great significance to improve the capacity and reversibility of the positive electrode at low temperatures [29,30,31,32][26][27][28][29].
In fact, while the processes that limit the performance of low-temperature Li-S batteries are myriad, they all tend to have the same effect: capacity loss. Under a given voltage window, the reversible capacity of Li-S batteries is decreased with the decrease in temperature. For instance, below −20 °C, the reversible capacity of Li-S batteries is less than 25% of the primary capacity. After raising the temperature again to room temperature, the reversible capacity can be recovered as well. On the other hand, due to the irreversible deposition of lithium metal on the anode surface, capacity loss can also occur when the cell is charged at low temperature [33][30]. Both capacity degradation mechanisms for Li-S batteries at freezing temperature are mainly attributed to its increased internal resistance, which is caused by different physicochemical processes [34][31].
Optimizing the cell configurations can improve battery performance and address corresponding issues, which can be approached from various angles, including electrode and electrolyte optimization, and separator/interlayer design. The use of various 3D current collectors, such as porous current collectors, sandwich-type current collectors, and multilayered current collectors, can avoid the cracking. The utilization of binders on the cathode material can also enhance the contact performance between the coating and the electrode, and specific binders can improve specific properties of Li-S batteries. Zhou et al. introduced a binder, ammonium polyphosphate (APP), which not only facilitates the adhesion of electrode coatings but also utilizes its strong affinity with lithium polysulfides to hinder the diffusion and shuttle of polysulfide anions [35][32]. In terms of electrolyte, incorporating new types of electrolytes and using appropriate solvents and salts are commonly employed methods to improve its electrochemical performance. The addition of redox mediators (RMs) in the electrolyte is a highly promising approach for optimizing Li-S battery configurations, as RMs can regulate the oxidation reactions of sulfur [36][33]. Regarding the optimization of separators/interlayers, it can be categorized into three functions: (1) dealing with polysulfide diffusion; (2) improving the electrical conductivity for complete sulfur utilization; and (3) enhancing the kinetics of the conversion reactions of sulfur species. They, respectively, correspond to the key issues of polysulfide dissolution and the Shuttle effect, the insulating nature of sulfur species, and the poor kinetics in the scissoring of S-S bonding for Li-S batteries [37][34].
3. Anode
Since the theoretical specific capacity of sulfur (1673 mAh/g) is much inferior to lithium (3860 mAh/g), the initial energy density is determined by the mass of sulfur in the whole system. Moreover, the instability at the surface and the bulk of the lithium anode during cycling seriously affect the capacity retention and cycle life [38][35]. According to recent progress, Li-S batteries are suffering from the following challenges and difficulties: (1) the Shuttle effect of Li ions [39][36]; (2) the uneven deposition of intermediate-term for SEI [40][37]; and (3) the serious dendrites and self-discharge of the lithium metal anode [4]. Among these factors, the serious dendrites of the lithium metal anode have the greatest impact on safety and stability [41][38]. The formation of lithium dendrites in lithium-ion batteries is attributed to the repeated deposition and stripping of lithium metal on the surface of electrodes during the charging and discharging process. Pore formation and surface defects can occur as a result of this process, leading to a high local potential that promotes the growth of dendrites [42][39]. Other factors such as temperature, current density, composition and concentration of electrolytes, electrode materials and structures, and the charging/discharging states of the battery can also contribute to dendrite formation. Lithium dendrites lead to serious safety issues, as they can cause short circuits, puncture the separator, and initiate uncontrolled reactions [43][40]. In order to overcome these difficulties and obtain stable lithium metal anodes, numerous strategies such as regulating the electrolyte, fabricating an artificial SEI layer, modifying the 3D current collector hosts and/or the lithophilic site, and exploring alternative anode materials have been employed [44][41]. However, research on Li-S batteries operating at low temperatures is still scarce.3.1. Exploration of Substitution and Improvement for Anode Materials
3.1.1. Graphite
Graphite, with its flat intercalation potential, good capacity, and long cycle life, can effectively inhibit volume expansion and dendrite formation in lithium metal anodes. However, the related intrinsic drawbacks, such as poor Li diffusion kinetics in the interlayers, low intercalation potential, and relatively large interfacial resistance [45[42][43],46], usually result in the Li plating on graphite and restricting extensive applications in low-temperature conditions [45,46,47][42][43][44]. Due to the catalytic effect of the metal during the Li ions desolvation process, when mixed with 1 wt% of metal nanoparticles (Cu, Al, Sn), the light graphite oxide was able to deliver 30% of the theoretical capacity at −30 °C and 0.2 °C [48,49][45][46]. In comparison with the original graphite oxide electrode, the graphite oxide with coated and dispersed Sn provided a capacity of 152 and 94 m Ah g−1, respectively, at −30 °C. It was demonstrated that the low-temperature performance of graphite and LTO(Li4Ti5O12) anodes can be effectively improved by using copper nanoparticles on Super-P (Cu/Super-P) as a conductive additive [50,51][47][48]. Coating graphite with Al2O3 may also prevent Li deposition, improving its cryogenic performance [52][49]. The mild oxidation of graphite is expected to solve the problem of reducing the lithium overpotential of the graphite anode. Cao [53][50] and Wu et al. [54][51] innovatively utilized the heat treatment and concentrated nitric acid solution to lightly oxidize graphite, which exhibits better cycling performance at low temperatures. The reduced particle size and number of terminal unsaturated carbon atoms, forming nanoscale voids and channels as well as the formation of chemically bonded SEI, was attributed to this mild oxidation treatment [47][44]. In addition, particle size reduction and structure modification can also improve the low-temperature performance of graphite [55,56][52][53]. The combination of thin graphite sheets with through-holes (porous graphite nanosheets, PGNs) and carbon nanotubes (CNTs) significantly shortens the diffusion path. The dominant mesopores and micropores in PGN-CNT anodes facilitate Li ion transport, resulting in a superior rate and performance at low temperatures (Figure 2a,b). After 500 cycles, the capacity retention was maintained at 90% for 0.75 m LiTFSI 1,3-dioxane (DIOX) electrolyte, and the reversible capacity was above 300 mAhg−1 at 0.1 °C and −20 °C. Ionic liquids were used to perform microwave exfoliation on expanded graphite and synthesized multilayer crystalline graphene (GRAL) (Figure 2c) [57][54]. The high surface area allows efficient electrochemical reactions, as evidenced by the 3–4 times greater capacity of GRAL compared to commercial graphite at −30 °C (Figure 2d). Moreover, oxided mesocarbon microbeads and expanded MCMB were prepared as well. The expanded MCMB with increased interlayer distance delivered 130 and 100 m Ah g−1 at −10 and −40 °C, respectively [58][55].
Figure 2. (a) Scanning electron microscopy (SEM) images of PGN−CNT and (b) its electrochemical performance at high rates and low temperatures with DIOX-based and commercial electrolytes. (c) Transmission electron microscopy (TEM) images of GRAL anode and (d) the electrochemical comparison with SLP30 anode at −30 °C and 0.05/0.1 Ag−1.
3.1.2. Other Related Investigations
Recently, energy-dense Li-S batteries were achieved with the assistance of a gel polymer electrolyte, which was designed by redox chemistry between a Li2S cathode and Si anode. The rationally designed CoN@MCNF/Li2S cathode shows good temperature adaptability at around −20 °C [59][56].3.2. Electrolyte Chemistry Modulation
Jiao et al. [60][57] systematically studied the performance of lithium metal anodes over a range of temperatures. A variety of electrolyte systems have been studied, such as a cosolvent of 1,3-dioxolane (DOL)/1,2-dimethoxyethane (DME), and a cosolvent of ethylene carbonate (EC) and dimethyl carbonate (DMC). Both drupe-like and small spherical deposits of lithium were detected at high temperatures of 60 °C. It was shown that between five degrees and minus fifteen degrees, there will be branching of lithium metal anodes. However, at higher temperatures, the C–C and C–O groups of organics in the SEI are enriched, resulting in a low modulus, flexible SEI structure. In thermodynamic and kinetically favored depositional conditions, low-current density, high Coulombic efficiency as well as a nondendritic structure has been achieved in these electrolytes. Compared with low temperatures, conventional carbonate LiFP6 electrolytes are preferred over poorer SEI layers, with lower Coulombic efficiency and shorter cycle life at high temperature [61][58]. At 0 °C, a homogenous SEI layer was formed that contained uniformly distributed F-containing species, displaying an extended cycle lifetime and increased Coulombic efficiency (Figure 3). The XRF mappings and micro-XANES images illustrate that F and O are inhomogeneously distributed over most of the surface and from the surface to the bulk, which is important to understand. In particular, when the cycle temperature reaches below 60 °C, the internal composition of the SEI is mainly composed of inorganic Li2CO3, and the surface composition is mainly composed of organic oxygen and LiF. These species are produced by the reaction between the electrolyte and the deposited lithium, thus causing rapid failure when building a thick SEI.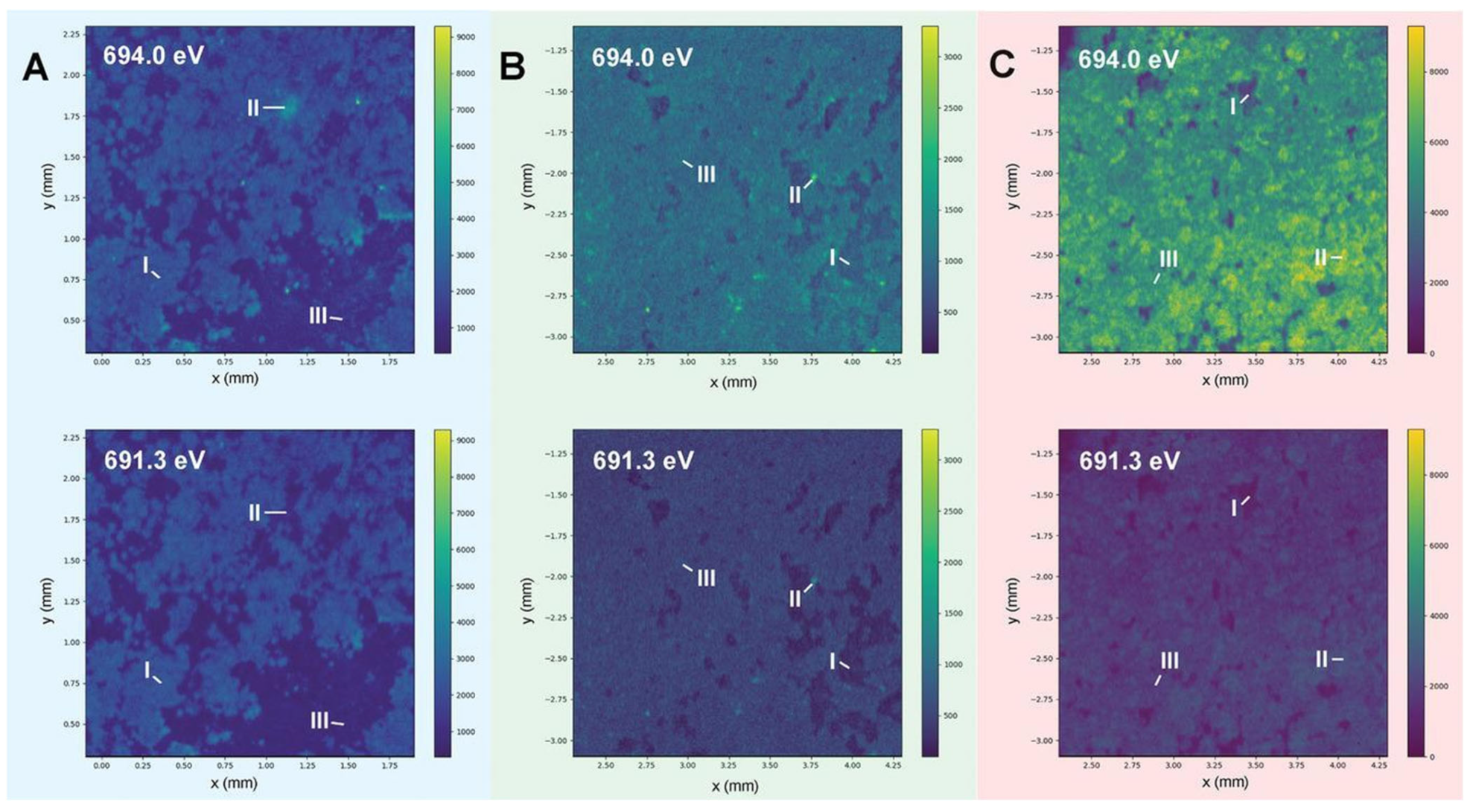
Figure 3. Investigations on temperature effects on the SEI structure using advanced techniques. (A–C) Displays the synchrotron energy-dependent XRF mappings of the cycled lithium metal anode at 0, 25, and 60 °C, respectively.
3.3. Artificial SEI Layer
The problem of the high lithium ion desolubilization energy barrier at low temperatures can be effectively mitigated by artificially adjusting the SEI film. The slow process at the lithium anode occurs when the solvated Li attempts to enter the anode structure, where it must peel off its primary solvated sheath [67][64]. This dynamically challenging process, often referred to as the “charge transfer” component due to its characteristic 50–70 kJ mol−1 activation energy barrier, is the fundamental reason why LIBs cannot be charged at low temperatures. However, the activation energy of the Li ions’ interfacial transfer is different in the presence of different surface films (SEI), and the dynamics of cathode–interface Li ion transfer are also affected by the composition of SEI film [68,69][65][66]. Therefore, the artificially modified SEI film effectively affects the “charge transfer” process, providing a beneficial strategy to solve the high Li ion desolubility barrier problem (Figure 4).
Figure 4. Schematic illustration of the proposed (a) mosaic structure, (b) multilayered structure of the SEI. (c) Schematic open-circuit energy diagram of battery electrolyte. ΦA and ΦC are the anode and cathode work functions. Eg is the window of the electrolyte for thermodynamic stability. A µA > LUMO and/or a µC < HOMO requires a kinetic stability for the formation of an SEI layer.
3.4. The 3D Current Collector Hosts and/or Lithophilic Site Modification
The 3D current collector hosts and lithophile site modification are effective to achieve high-performance lithium anodes and fast conversion kinetics [45][42]; their feasibility and reliability in extreme hot and cold conditions, however, have been studied relatively infrequently. Peng and co-workers [72][67] used chemical vapor deposition to produce graphite-coated Ni-Fe foam (graphite @Ni-Fe). It exhibited good cycle performance, and the capacity retention rate was 98.10% after 100 cycles at −50 °C and 0.4 A cm−2, with a Coulomb efficiency over 98.3%. At present, zinc batteries are performing at low temperature, but lithium batteries are not.References
- Schipper, F.; Aurbach, D. A brief review: Past, present and future of lithium ion batteries. Russ. J. Electrochem. 2016, 52, 1095–1121.
- Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. Tribute to John B. Goodenough: From Magnetism to Rechargeable Batteries. Adv. Energy Mater. 2021, 11, 2170006.
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in Lithium–Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mater. 2021, 33, 2003666.
- Wei, Z.; Ren, Y.; Sokolowski, J.; Zhu, X.; Wu, G. Mechanistic understanding of the role separators playing in advanced lithium-sulfur batteries. InfoMat 2020, 2, 483–508.
- Zeng, Z.; Liu, X.; Jiang, X.; Liu, Z.; Peng, Z.; Feng, X.; Chen, W.; Xia, D.; Ai, X.; Yang, H.; et al. Enabling an intrinsically safe and high-energy-density 4.5 V-class Li-ion battery with nonflammable electrolyte. InfoMat 2020, 2, 984–992.
- Rojaee, R.; Plunkett, S.; Rasul, M.G.; Cheng, M.; Jabbari, V.; Shahbazian-Yassar, R. Interfacial engineering of lithium-polymer batteries with in situ UV cross-linking. InfoMat 2021, 3, 1016–1027.
- Wang, T.; He, J.; Cheng, X.-B.; Zhu, J.; Lu, B.; Wu, Y. Strategies toward High-Loading Lithium–Sulfur Batteries. ACS Energy Lett. 2023, 8, 116–150.
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li-Sulfur Battery Systems: An Integral Perspective. Adv. Energy Mater. 2015, 5, 1500212.
- Knoop, J.E.; Ahn, S. Recent advances in nanomaterials for high-performance Li–S batteries. J. Energy Chem. 2020, 47, 86–106.
- Hong, X.; Wang, R.; Liu, Y.; Fu, J.; Liang, J.; Dou, S. Recent advances in chemical adsorption and catalytic conversion materials for Li–S batteries. J. Energy Chem. 2020, 42, 144–168.
- Huang, S.; Liu, L.; Wang, Y.; Shang, Y.; Zhang, L.; Wang, J.; Zheng, Y.; Schmidt, O.G.; Yang, H.Y. Elucidating the reaction kinetics of lithium–sulfur batteries by operando XRD based on an open-hollow 2 cathode. J. Mater. Chem. A 2019, 7, 6651–6658.
- Zhuang, Z.; Kang, Q.; Wang, D.; Li, Y. Single-atom catalysis enables long-life, high-energy lithium-sulfur batteries. Nano Res. 2020, 13, 1856–1866.
- Xiao, Z.; Yang, Z.; Li, Z.; Li, P.; Wang, R. Synchronous Gains of Areal and Volumetric Capacities in Lithium–Sulfur Batteries Promised by Flower-like Porous Ti3C2Tx Matrix. ACS Nano 2019, 13, 3404–3412.
- Wang, R.; Cui, W.; Chu, F.; Wu, F. Lithium metal anodes: Present and future. J. Energy Chem. 2020, 48, 145–159.
- Jiao, L.; Zhang, C.; Geng, C.; Wu, S.; Li, H.; Lv, W.; Tao, Y.; Chen, Z.; Zhou, G.; Li, J.; et al. Capture and Catalytic Conversion of Polysulfides by In Situ Built TiO2-MXene Heterostructures for Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1900219.
- Yao, Y.-X.; Zhang, X.-Q.; Li, B.-Q.; Yan, C.; Chen, P.-Y.; Huang, J.-Q.; Zhang, Q. A compact inorganic layer for robust anode protection in lithium-sulfur batteries. InfoMat 2020, 2, 379–388.
- Sun, K.; Peng, Z. Intermetallic interphases in lithium metal and lithium ion batteries. InfoMat 2021, 3, 1083–1109.
- Chen, D.; Liu, Y.; Xia, C.; Han, Y.; Sun, Q.; Wang, X.; Chen, W.; Jian, X.; Lv, W.; Ma, J.; et al. Polybenzimidazole functionalized electrolyte with Li-wetting and self-fluorination functionalities for practical Li metal batteries. InfoMat 2022, 4, e12247.
- Jiao, Y.; Wang, F.; Ma, Y.; Luo, S.; Li, Y.; Hu, A.; He, M.; Li, F.; Chen, D.; Chen, W.; et al. Challenges and advances on low-temperature rechargeable lithium-sulfur batteries. Nano Res. 2022.
- Hatzell, K.B. Make ion–solvent interactions weaker. Nat. Energy 2021, 6, 223–224.
- Xu, K.; von Cresce, A.; Lee, U. Differentiating Contributions to “Ion Transfer” Barrier from Interphasial Resistance and Li+ Desolvation at Electrolyte/Graphite Interface. Langmuir 2010, 26, 11538–11543.
- Thenuwara, A.C.; Shetty, P.P.; McDowell, M.T. Distinct Nanoscale Interphases and Morphology of Lithium Metal Electrodes Operating at Low Temperatures. Nano Lett. 2019, 19, 8664–8672.
- Bai, P.; Li, J.; Brushett, F.R.; Bazant, M.Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 2016, 9, 3221–3229.
- Gupta, A.; Bhargav, A.; Jones, J.-P.; Bugga, R.V.; Manthiram, A. Influence of Lithium Polysulfide Clustering on the Kinetics of Electrochemical Conversion in Lithium–Sulfur Batteries. Chem. Mater. 2020, 32, 2070–2077.
- Lang, S.-Y.; Xiao, R.-J.; Gu, L.; Guo, Y.-G.; Wen, R.; Wan, L.-J. Interfacial Mechanism in Lithium–Sulfur Batteries: How Salts Mediate the Structure Evolution and Dynamics. J. Am. Chem. Soc. 2018, 140, 8147–8155.
- Yu, S.-H.; Huang, X.; Schwarz, K.; Huang, R.; Arias, T.A.; Brock, J.D.; Abruña, H.D. Direct visualization of sulfur cathodes: New insights into Li–S batteries via operando X-ray based methods. Energy Environ. Sci. 2018, 11, 202–210.
- Chu, H.; Noh, H.; Kim, Y.-J.; Yuk, S.; Lee, J.-H.; Lee, J.; Kwack, H.; Kim, Y.; Yang, D.-K.; Kim, H.-T. Achieving three-dimensional lithium sulfide growth in lithium-sulfur batteries using high-donor-number anions. Nat. Commun. 2019, 10, 188.
- Zhou, G.; Tian, H.; Jin, Y.; Tao, X.; Liu, B.; Zhang, R.; Seh, Z.W.; Zhuo, D.; Liu, Y.; Sun, J.; et al. Catalytic oxidation of Li2S on the surface of metal sulfides for Li-S batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 840–845.
- Gerber, L.C.H.; Frischmann, P.D.; Fan, F.Y.; Doris, S.E.; Qu, X.; Scheuermann, A.M.; Persson, K.; Chiang, Y.-M.; Helms, B.A. Three-Dimensional Growth of Li2S in Lithium–Sulfur Batteries Promoted by a Redox Mediator. Nano Lett. 2016, 16, 549–554.
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery—A low-temperature aging study. J. Power Sources 2015, 275, 799–807.
- Li, Q.; Lu, D.; Zheng, J.; Jiao, S.; Luo, L.; Wang, C.-M.; Xu, K.; Zhang, J.-G.; Xu, W. Li+-Desolvation Dictating Lithium-Ion Battery’s Low-Temperature Performances. ACS Appl. Mater. Interfaces 2017, 9, 42761–42768.
- Zhou, G.; Liu, K.; Fan, Y.; Yuan, M.; Liu, B.; Liu, W.; Shi, F.; Liu, Y.; Chen, W.; Lopez, J.; et al. An Aqueous Inorganic Polymer Binder for High Performance Lithium-Sulfur Batteries with Flame-Retardant Properties. ACS Cent. Sci. 2018, 4, 260–267.
- Kong, L.; Peng, H.-J.; Huang, J.-Q.; Zhang, Q. Review of nanostructured current collectors in lithium–sulfur batteries. Nano Res. 2017, 10, 4027–4054.
- Jeong, Y.C.; Kim, J.H.; Nam, S.; Park, C.R.; Yang, S.J. Rational Design of Nanostructured Functional Interlayer/Separator for Advanced Li–S Batteries. Adv. Funct. Mater. 2018, 28, 1707411.
- Kong, L.L.; Wang, L.; Ni, Z.C.; Liu, S.; Li, G.R.; Gao, X.P. Lithium–Magnesium Alloy as a Stable Anode for Lithium–Sulfur Battery. Adv. Funct. Mater. 2019, 29, 8756.
- Zhang, B.W.; Sun, B.; Fu, P.; Liu, F.; Zhu, C.; Xu, B.M.; Pan, Y.; Chen, C. A Review of the Application of Modified Separators in Inhibiting the “shuttle effect” of Lithium-Sulfur Batteries. Membranes 2022, 12, 790.
- Wang, Z.; Shen, X.; Li, S.; Wu, Y.; Yang, T.; Liu, J.; Qian, T.; Yan, C. Low-temperature Li-S batteries enabled by all amorphous conversion process of organosulfur cathode. J. Energy Chem. 2022, 64, 496–502.
- Xiang, J.W.; Yang, L.Y.; Yuan, L.X.; Yuan, K.; Zhang, Y.; Huang, Y.Y.; Lin, J.; Pan, F.; Huang, Y.H. Alkali-Metal Anodes: From Lab to Market. JOULE 2019, 3, 2334–2363.
- Wang, J.N.; Yi, S.S.; Liu, J.W.; Sun, S.Y.; Liu, Y.P.; Yang, D.W.; Xi, K.; Gao, G.X.; Abdelkader, A.; Yan, W.; et al. Suppressing the Shuttle Effect and Dendrite Growth in Lithium-Sulfur Batteries. ACS Nano 2020, 14, 9819–9831.
- Jin, C.B.; Sheng, O.W.; Luo, J.M.; Yuan, H.D.; Fang, C.; Zhang, W.K.; Huang, H.; Gan, Y.P.; Xia, Y.; Liang, C.; et al. 3D lithium metal embedded within lithiophilic porous matrix for stable lithium metal batteries. Nano Energy 2017, 37, 177–186.
- Hu, Y.; Hu, A.; Wang, J.; Niu, X.; Zhou, M.; Chen, W.; Lei, T.; Huang, J.; Li, Y.; Xue, L.; et al. Strong intermolecular polarization to boost polysulfide conversion kinetics for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 9771–9779.
- Cai, W.; Yao, Y.X.; Zhu, G.L.; Yan, C.; Jiang, L.L.; He, C.; Huang, J.Q.; Zhang, Q. A review on energy chemistry of fast-charging anodes. Chem. Soc. Rev. 2020, 49, 3806–3833.
- Collins, G.A.; Geaney, H.; Ryan, K.M. Alternative anodes for low temperature lithium-ion batteries. J. Mater. Chem. A 2021, 9, 14172–14213.
- Zhang, N.; Deng, T.; Zhang, S.; Wang, C.; Chen, L.; Wang, C.; Fan, X. Critical Review on Low-Temperature Li-Ion/Metal Batteries. Adv. Mater. 2022, 34, e2107899.
- Nobili, F.; Dsoke, S.; Mecozzi, T.; Marassi, R. Metal-oxidized graphite composite electrodes for lithium-ion batteries. Electrochim. Acta 2005, 51, 536–544.
- Nobili, F.; Mancini, M.; Dsoke, S.; Tossici, R.; Marassi, R. Low-temperature behavior of graphite–tin composite anodes for Li-ion batteries. J. Power Sources 2010, 195, 7090–7097.
- Marinaro, M.; Nobili, F.; Birrozzi, A.; Eswara Moorthy, S.K.; Kaiser, U.; Tossici, R.; Marassi, R. Improved low-temperature electrochemical performance of Li4Ti5O12 composite anodes for Li-ion batteries. Electrochim. Acta 2013, 109, 207–213.
- Marinaro, M.; Mancini, M.; Nobili, F.; Tossici, R.; Damen, L.; Marassi, R. A newly designed Cu/Super-P composite for the improvement of low-temperature performances of graphite anodes for lithium-ion batteries. J. Power Sources 2013, 222, 66–71.
- Friesen, A.; Hildebrand, S.; Horsthemke, F.; Börner, M.; Klöpsch, R.; Niehoff, P.; Schappacher, F.M.; Winter, M. Al2O3 coating on anode surface in lithium ion batteries: Impact on low temperature cycling and safety behavior. J. Power Sources 2017, 363, 70–77.
- Cao, X.; Kim, J.H.; Oh, S.M. The effects of oxidation on the surface properties of MCMB-6-28. Electrochim. Acta 2002, 47, 4085–4089.
- Wu, Y.P.; Jiang, C.; Wan, C.; Holze, R. Modified natural graphite as anode material for lithium ion batteries. J. Power Sources 2002, 111, 329–334.
- Ji, Y.; Zhang, Y.; Wang, C.-Y. Li-Ion Cell Operation at Low Temperatures. J. Electrochem. Soc. 2013, 160, A636–A649.
- Xu, J.; Wang, X.; Yuan, N.; Hu, B.; Ding, J.; Ge, S. Graphite-based lithium ion battery with ultrafast charging and discharging and excellent low temperature performance. J. Power Sources 2019, 430, 74–79.
- Raccichini, R.; Varzi, A.; Chakravadhanula, V.S.K.; Kübel, C.; Balducci, A.; Passerini, S. Enhanced low-temperature lithium storage performance of multilayer graphene made through an improved ionic liquid-assisted synthesis. J. Power Sources 2015, 281, 318–325.
- Zhao, G.; Wei, Z.; Zhang, N.; Sun, K. Enhanced low temperature performances of expanded commercial mesocarbon microbeads (MCMB) as lithium ion battery anodes. Mater. Lett. 2012, 89, 243–246.
- Meng, X.; Liu, Y.; Wang, Z.; Zhang, Y.; Wang, X.; Qiu, J. A quasi-solid-state rechargeable cell with high energy and superior safety enabled by stable redox chemistry of Li2S in gel electrolyte. Energy Environ. Sci. 2021, 14, 2278–2290.
- Han, Y.; Jie, Y.; Huang, F.; Chen, Y.; Lei, Z.; Zhang, G.; Ren, X.; Qin, L.; Cao, R.; Jiao, S. Enabling Stable Lithium Metal Anode through Electrochemical Kinetics Manipulation. Adv. Funct. Mater. 2019, 29, 4629.
- Adair, K.R.; Banis, M.N.; Zhao, Y.; Bond, T.; Li, R.; Sun, X. Temperature-Dependent Chemical and Physical Microstructure of Li Metal Anodes Revealed through Synchrotron-Based Imaging Techniques. Adv Mater 2020, 32, e2002550.
- Atkinson, R.W.; Carter, R.; Love, C.T. Operational strategy to stabilize lithium metal anodes by applied thermal gradient. Energy Storage Mater. 2019, 22, 18–28.
- Dong, X.; Lin, Y.; Li, P.; Ma, Y.; Huang, J.; Bin, D.; Wang, Y.; Qi, Y.; Xia, Y. High-Energy Rechargeable Metallic Lithium Battery at −70 °C Enabled by a Cosolvent Electrolyte. Angew. Chem. Int. Ed. 2019, 58, 5623–5627.
- Sharifi, O.; Golmohammad, M.; Soozandeh, M.; Mehranjani, A.S. Improved Ga-doped Li7La3Zr2O12 garnet-type solid electrolytes for solid-state Li-ion batteries. J. Solid State Eelectrochemistry 2023, 1–12.
- Alizadeh, S.M.; Moghim, I.; Golmohammad, M. Synthesis and characterization of highly conductive Ga/Y co-doped LLZO by facile combustion sol-gel method. Solid State Ion. 2023, 397, 116260.
- Liu, Q.; Jiang, L.; Zheng, P.; Sun, J.; Liu, C.; Chai, J.; Li, X.; Zheng, Y.; Liu, Z. Recent Advances in Stability Issues of Inorganic Solid Electrolytes and Composite Solid Electrolytes for All-Solid-State Batteries. Chem. Rec. 2022, 22, e202200116.
- Yan, Q.; Whang, G.; Wei, Z.; Ko, S.-T.; Sautet, P.; Tolbert, S.H.; Dunn, B.S.; Luo, J. A Perspective on interfacial engineering of lithium metal anodes and beyond. Appl. Phys. Lett. 2020, 117, 8417.
- Katorova, N.S.; Luchkin, S.Y.; Rupasov, D.P.; Abakumov, A.M.; Stevenson, K.J. Origins of irreversible capacity loss in hard carbon negative electrodes for potassium-ion batteries. J. Chem. Phys. 2020, 152, 194704.
- Xu, K.; Lam, Y.F.; Zhang, S.S.; Jow, T.R.; Curtis, T.B. Solvation sheath of Li+ in nonaqueous electrolytes and its implication of graphite/electrolyte interface chemistry. J. Phys. Chem. C 2007, 111, 7411–7421.
- Chu, P.; Zhao, H.L.; Wang, J.; Xie, H.L.; Han, C.Q.; Yang, Z. Bifunctional 3D foam negative current collector toward stable liquid metal battery. J. Alloys Compd. 2022, 903, 3952.
More
