Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Nahum Mendez-Sanchez.
Cholestasis is a condition characterized by decrease in bile flow due to progressive pathological states that lead to chronic cholestatic liver diseases which affect the biliary tree at the intrahepatic and extrahepatic level. Cholestasis can pose risks to overall health, as the reduced flow of bile can hinder the absorption of fat-soluble vitamins and nutrients, leading to deficiencies. Additionally, it can induce other complications other such as cirrhosis, liver failure, malignancies and bone disease. Early diagnosis and adequate management are crucial in treating cholestasis in order to alleviate symptoms, prevent complications and address the underlying cause.
- cholestasis
- bile acids
- ursodeoxycholic acid
- fibrosis
1. Introduction
Cholestasis is a condition characterized by a decrease in bile flow due to progressive pathological states that lead to chronic cholestatic liver diseases (CLD) which affect the biliary tree at the intrahepatic level and extrahepatic level [1]. Those conditions share commons mechanism and etiologies, either genetical or immunological. The two most prevalent of CLD are primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) [2,3][2][3].
Cholestasis induces symptoms such as pruritus and fatigue but also important complications such as cirrhosis, liver failure, malignancies, bone disease and nutritional deficiencies that merit close follow-up and specific interventions to improve quality of life [4]. As CLD progress to liver cirrhosis, there will be an increase in mortality but also an important impact in quality of life and economic burden due to comorbidities related with liver failure. CLD represent high costs to patients because of a reduction in outpatient activities but also from hospitalizations, mostly due to cirrhosis complications and liver transplantation (LT) [5]. In a study carried out from 1988 to 2018, CLD, and particularly PBC and PSC, accounted for 14.2% of LT. About 10% to 40% of those patients will have a recurrence of primary disease after LT [6].
2. Pathophysiology of Cholangiopathies
The general sequence of events leading to cholestasis and its associated damage can be summarized as: (1) ductular reaction (DR), (2) biliary stasis (intrinsic and/or extrinsic obstruction), (3) modification of bile components to a cytotoxic profile and (4) a proinflammatory and profibrotic state. It is essential to know biology of cholangiocytes and the general aspects of pathophysiology to understand the therapeutic targets of the different pharmacological options (Figure 1).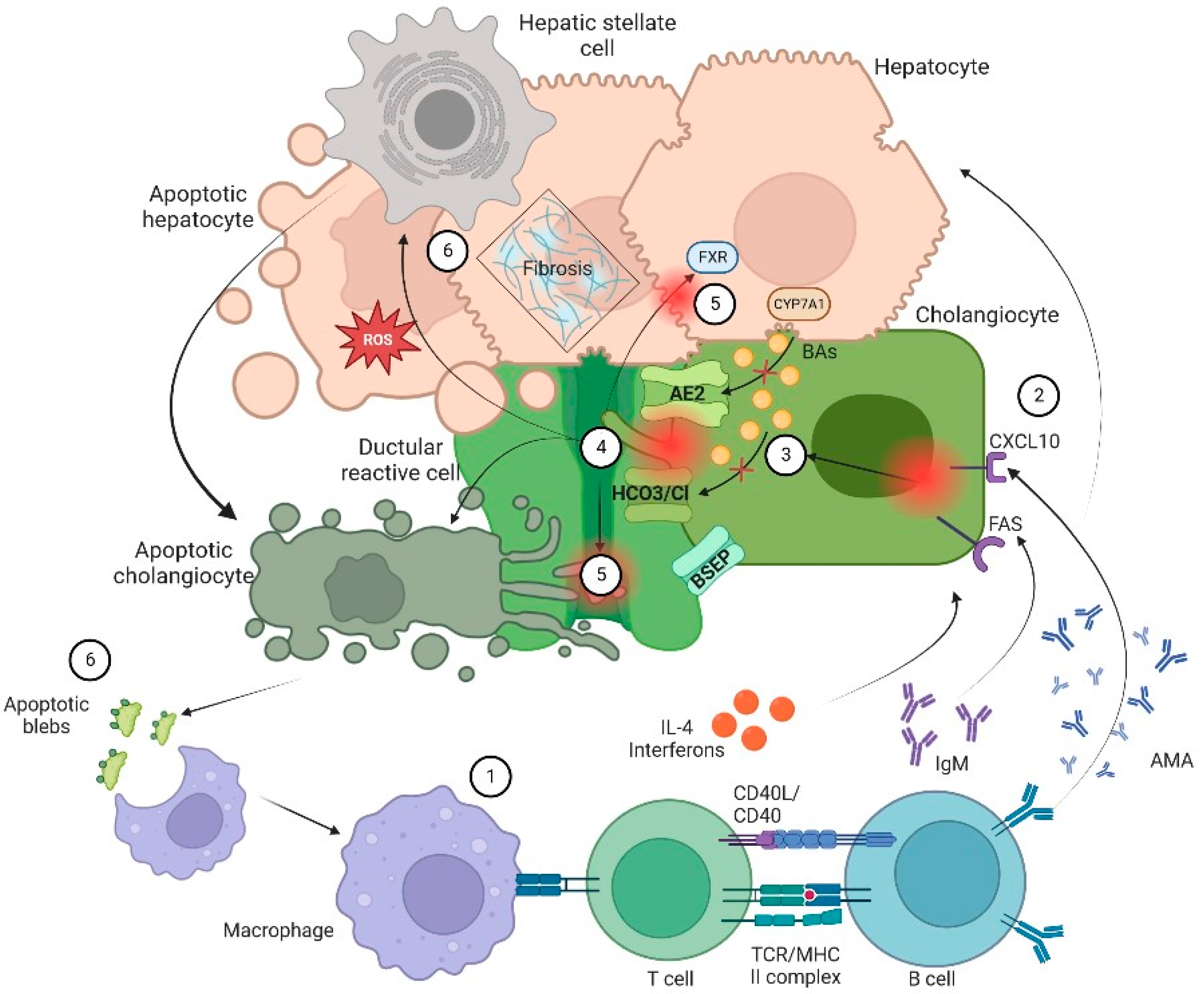
Figure 1. Pathophysiology of cholangiopathies. In general, patients with CLD are in a proinflammatory state due to an exposition to a microbial and immunogenic environment with mitochondrial autoantigens in the form of apoptotic blebs that induce an immune response. (1) Dysregulation of the innate and adaptive immune response results in directed damage to cholangiocytes. (2) Small cholangiocytes act as antigen presenting cells and produce a variety of cytokines such as IL-6 and IL-8 and increase the expression of CXCL10. The latter will allow an increase in the production of IFNγ and other chemoattractants that result in T-cell infiltration and production of anti-mitochondrial antibody by B cells that ultimately increase production of bile. On the other hand, (3) there is a failure of biliary transporters (AE2, BSEP and the HCO3 umbrella) due to direct damage of cytotoxic T cells with mitochondrial injury on biliary cells. Hence, cholangiocytes lose their protection against toxic hydrophobic bile acids (BAs) and reduce BAs transportation in the context of bile overproduction. (4) Inflammation and injury lead to ductular reaction (DR), infiltration of leukocytes and lymphocytes that activate liver progenitor cells, thus increasing matrix protein levels through different proteins such as TGFβ1/2. Moreover, bile salts will be acidified becoming hydrophobic; eventually these toxic bile salts cross the membrane leading to apoptosis. (5) In addition, the constant bile duct insult led to retention of hydrophobic BAs promoting local injury and altering the enterohepatic circulation of bile. Finally, there is a dysregulation in farnesoid X receptor (FXR) activation that may lead to a profibrotic state. (6) Apoptosis and cell injury promotes formation of reactive oxygen species (ROS) both in liver cells and cholangiocytes. Furthermore, apoptosis releases apoptotic blebs that perpetuate a proinflammatory state. All those processes induce cells to evolve toward an exhausted, profibrogenic phenotype which can contribute to the development of hepatic stellate cell-mediated liver fibrosis.
2.1. Cholangiocytes Biology
Cholangiocytes are the metabolically active epithelium that lines the bile ducts, accounting for 3% to 5% of the total of liver cells. Furthermore, cholangiocytes are heterogeneous both in functions and morphology. For instance, small cholangiocytes possess an especial plasticity under stress conditions. In fact, small cholangiocytes are more resistant to injury than large cholangiocytes and proliferate instead of dying, unlike the large ones. On the other hand, large cholangiocytes are involved in bile secretion and are characterized by the expression of anion exchangers, bile acids (BAs) transporters and secretin receptors. Cholangiocytes express water channels such as aquaporins, transporters such as the sodium/glucose cotransporter 1 (SGLT1) and important exchangers, mainly the Cl−/HCO3− exchanger. Therefore, the main functions of cholangiocytes consist of bile production, regulation of bile volume and composition and their transport. They are also involved in modulation of liver injury and the repairing mechanism [6,7][6][7]. Regarding bile production, about 40% of the daily bile output is made by cholangiocytes. That process starts when secretin binds to its receptor on the basolateral membrane of cholangiocytes and activates the cyclic adenosine monophosphate (cAMP) signaling pathway which in turn activates the apical chloride channels leading to a release of chloride ions that drives bicarbonate secretion by activating the AE2 chloride/bicarbonate exchanger helping to the alkalinity of bile. In the case of bile output and BAs transport, it is important to notice that cholangiocytes are exposed to high concentrations of bile on their apical side. BAs are transported into cholangiocytes through the apical sodium-dependent bile acid transporter (ASBT) on their apical membrane; once inside, BAs can stimulate a secretin-induced cAMP secretory response as well as stimulate bicarbonate secretion, hence regulating bile flow [7]. In addition to the latter functions, cholangiocytes have important immune activity, as they express and secrete interleukins 6 (IL-6) and 8 (IL-8), and monocyte chemotactic protein-1 (MCP1) through the TLR4-NF-κB and TLR4-MAPK signaling pathway which is initiated by proinflammatory cytokines such as TNF-α or by bacterial products. Moreover, cholangiocytes interact with CD4+ and CD8+ T cells via IL-8 and MCP1, but also by expressing adhesion molecules. It is possible that cholangiocytes may act as antigen presenting cells since they also express major histocompatibility complex class I and class II. Thus, cholangiocytes not only regulate bile metabolism and transport, but act as inflammatory mediators in CLD and play an important role in the progression of liver fibrosis [6,7,8][6][7][8].2.2. Ductular Reaction
As wresearchers have previously explained, cholangiocytes participate in the formation and modification of bile through a series of transmembrane channels, transporters and exchangers that are expressed in the apical or basolateral domain [7]. There are multiple triggers or promoters in the activation of cholangiocytes or DR in which the number of ductules increases, accompanied by infiltration of leukocytes and lymphocytes, activation of liver progenitor cells and an increase in matrix protein levels [8]. Initially, DR is a response to an aggressor stimulus; it tries to limit and eliminate the harmful factor. If DR is perpetuated, proinflammatory pathways (Notch and Hedgehog) induce cholangiocytes maturation, fibronectin deposition and cytokine transcription (interleukin-6, interleukin-8, tumor necrosis factor and various growth factors) that generate histological and structural changes that condition cholestasis. Furthermore, cholangiocytes recognize the presence of pathogens through pattern recognition receptors (PRR), and the activation of these PRRs triggers a signaling cascade which results in the expression of various cytokines, immunoglobulins, adhesion molecules and a strong cellular response (CD4+ T cells, CD8+ T cells, B cells, macrophages, and natural killer cells) that can release pro- and anti-inflammatory molecules and an angiogenic, fibrogenic and proliferative factor. This is the basis for consideration of immunomodulatory therapies for CLD [9].2.3. Biliary Stasis
Biliary stasis is due to impaired bile flow and impaired secretion by hepatocytes. An altered transport is another predisposing factor for cholestatic status, RD, increase BAs concentration and mitochondrial dysfunction [6]. The dysfunction of apical and basolateral cotransporters of the cholangiocytes generates cholestasis impairing secretion by the canalicular membrane. Primary BAs are synthesized in the liver where they undergo conjugation to form bile salts and are then secreted in the bile canaliculi to reach the intestine where primary bile salts undergo dehydroxylation and deconjugation by the intestinal microbiota and form secondary BAs. BAs are then passively reabsorbed in the upper intestine and terminal ileum via ASBT. Once reabsorbed, BAs are transport via the organic solute transporter (OST) α/β to the portal circulation, thus returning to the hepatocyte by other two transporters, either the sodium-taurocholate cotransporter polypeptide (NTCP) or the organic anion transporter polypeptide (OATP). In addition, the enterohepatic circulation of BAs is regulated by different feedback mechanisms that protect hepatocytes from BAs cytotoxicity, which is the most important the negative feedback of the nuclear farnesoid X receptor (FXR). In the enterocytes, BAs bind to FXR to decrease its synthesis via CYP7A1. This pathway can also be suppressed by the activity of fibroblast enteral growth factor 19 (FGF-19) that binds to its hepatocyte receptor and by fibroblast growth factor 4/β-klotho (FGFR4/β-klotho) complex to inhibit transcription of the CYP7A1 gene. FGF19 is an endocrine gastrointestinal hormone that controls the metabolism of BAs through its effects on CYP7A1, which is the first and rate-limiting enzyme in the classic pathway of BAs’ synthesis [10]. In cholestasis, high levels of BAs induce FGF-19 expression. The increased concentration of FGF-19 in the gut stimulates activation of the FGFR4/β-klotho receptor in the liver which is associated with malignancy risk [11]. On the other hand, FXR activation leads to downregulation of the intestinal BAs transporter ASBT, the hepatic uptake transporters NTCP and OATP, and upregulation of bile salt export pump (BSEP) of hepatic efflux transporters (Figure 1) (Figure 2) [6,7,8,9][6][7][8][9].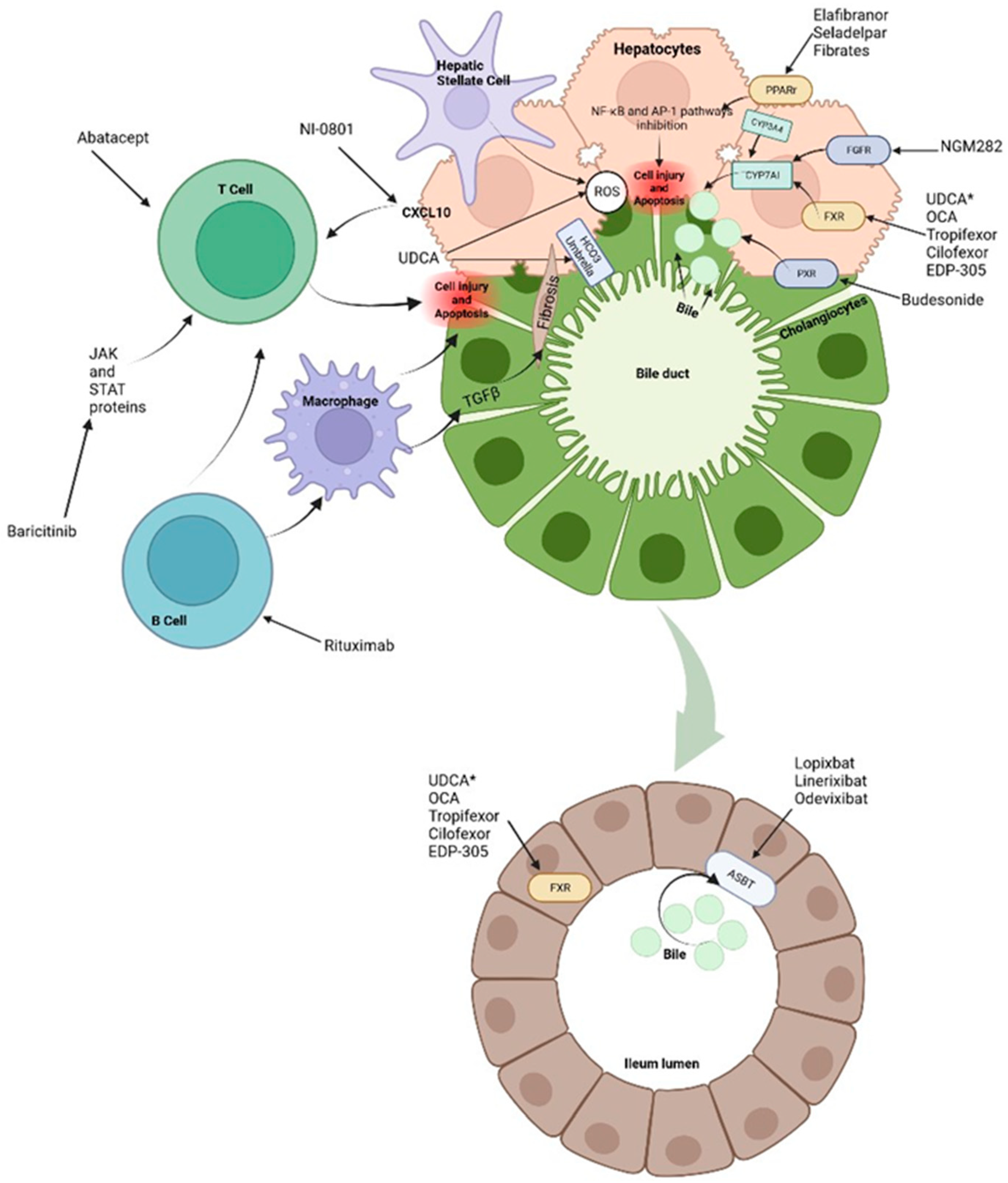
Figure 2. Treatments and molecular targets. In the liver, FXR agonists, PPAr agonist and FGF agonist improve cholestasis by decreasing BAs production and output via CYP7A1 inhibition. PPAr agonist and FRX also modulate the proinflammatory state by suppression of inflammatory pathways such as NF-kB. This results in ROS and interleukin reduction as well as decrease in hepatic stellate cells and immune cells activation. Immunomodulatory therapies, such as Rituximab or Baricitinib are aimed at blocking antigen presentation and the direct damage to liver cells. It is important to note that apoptosis releases apoptotic blebs which perpetuates a proinflammatory state and the formation ROS both in liver cells and cholangiocytes. Among other mechanisms, * UDCA serves as a partial agonist of FXR, improves BAs flow and enhances ions channels. Its effects on the biliary HCO3− umbrella, near the apical surface, prevents the permeation of hydrophobic BAs; hence, contributing to the reduction in cell injury. Intestinal FXR agonists enhance the alleviation of cholestasis in the digestive system by reducing the overall size of the BAs pool that is reabsorbed. The events triggered by FXR in the gut liver axis probably represent a therapeutic option through the decrease in the excess of BAs in the liver. Finally, about 90–95% of BAs are reabsorbed into the terminal ileum via ASBT. Hence, the inhibition of ASBT reduces the overload of BAs in the liver. The decrease in ASBT expression increases the excretion of fecal BAs and the total concentration of BAs in liver.
2.4. Citotoxic Profile of Biliary Acids
The leakage and accumulation of BAs in hepatocytes at notably high concentrations can trigger the activation of cholangiocytes, resulting in chronic inflammation, proliferation, apoptosis, and, as the damage progresses, fibrosis [1,2,3,4,5,6,7,8,9][1][2][3][4][5][6][7][8][9]. BAs are composed of four steroid rings that form a hydrocarbon network which has hydrophobic and hydrophilic regions and a balance which is variable and explains the differences in their biological properties including choleretic potency, solubilization properties and activation of BAs receptors. Hydrophobic BAs are potent detergents whereas hydrophilic BAs are not; they lack membrane-disrupting properties, thus being non-hepatotoxic, even in high concentrations. This is relevant for the therapeutic use of hydrophilic BAs, such as ursodeoxycholic acid (UDCA), tauroursodeoxycholic acid (TUDCA) and new semisynthetic BAs derivatives such as 24-norursodeoxycholic acid (Nor-UDCA) in the treatment of liver diseases [12]. Hydrophilic BAs may enhance alkalinization of bile by increasing bicarbonate secretion, therefore protecting cholangiocytes from bile cytotoxic effects. In addition, hydrophilic BAs bind in the intestines and bile ducts to nuclear and surface receptors such as FXR, TGR5 and Pregnane X receptor (PXR) to modulate inflammatory pathways [1,9][1][9].2.5. Profibrotic State
Ultimately, proliferation of cholangiocytes can lead to disruption of cell cycle leading to biliary fibrosis. By secreting cytokines and chemokines via β-catenin and higher levels of intracellular cAMP, cholangiocytes attract macrophages that in turn active myofibroblasts and the transforming growth factor beta (TGFβ) [3,13][3][13]. A proinflammatory local state characterized by senescence and apoptosis may lead to ductopenia and increase other proliferative factors leading to local angiogenesis and fibrosis. At same time, there is recruitment adaptative and innate immune cells that may active mesenchymal cells and endothelial cells (Figure 2) [13].References
- Sanjel, B.; Shim, W.S. Recent advances in understanding the molecular mechanisms of cholestatic pruritus: A review. Biochim. Biophys. Acta Mol. Basis 2020, 1866, 165958.
- Gazda, J.; Drazilova, S.; Janicko, M.; Jarcuska, P. The Epidemiology of Primary Biliary Cholangitis in European Countries: A Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 9151525.
- Tabibian, J.H.; Ali, A.H.; Lindor, K.D. Primary Sclerosing Cholangitis, Part 1: Epidemiology, Etiopathogenesis, Clinical Features, and Treatment. Gastroenterol. Hepatol. 2018, 14, 293–304.
- Malik, A.; Kardashian, A.A.; Zakharia, K.; Bowlus, C.L.; Tabibian, J.H. Preventative care in cholestatic liver disease: Pearls for the specialist and subspecialist. Liver Res. 2019, 3, 118–127.
- Gerussi, A.; Restelli, U.; Croce, D.; Bonfanti, M.; Invernizzi, P.; Carbone, M. Cost of illness of Primary Biliary Cholangitis—A population-based study. Dig. Liver Dis. 2021, 53, 1167–1170.
- Yokoda, R.T.; Rodriguez, E.A. Review: Pathogenesis of cholestatic liver diseases. World J. Hepatol. 2020, 12, 423–435.
- Méndez-Sánchez, N. Bile Acids in Health and Disease Foreword. Ann. Hepatol. 2017, 16 (Suppl. S1), s3.
- Desmet, V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011, 458, 251–259.
- Goldstein, J.; Levy, C. Novel and emerging therapies for cholestatic liver diseases. Liver Int. 2018, 38, 1520–1535.
- Hirschfield, G.M.; Chazouillères, O.; Drenth, J.P.; Thorburn, D.; Harrison, S.A.; Landis, C.S.; Mayo, M.J.; Muir, A.J.; Trotter, J.F.; Leeming, D.J.; et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: A multicenter, randomized, double-blind, placebo-controlled phase 2 trial. J. Hepatol. 2019, 70, 483–493.
- Méndez-Sánchez, N. Management of primary biliary cholangitis: The importance to identify patients’ non-responders to standard treatment. Minerva Med. 2018, 109, 407–409.
- Cabrera, D.; Arab, J.P.; Arrese, M. UDCA, NorUDCA, and TUDCA in Liver Diseases: A Review of Their Mechanisms of Action and Clinical Applications. Handb. Exp. Pharmacol. 2019, 256, 237–264.
- Fabris, L.; Fiorotto, R.; Spirli, C.; Cadamuro, M.; Mariotti, V.; Perugorria, M.J.; Banales, J.M.; Strazzabosco, M. Pathobiology of inherited biliary diseases: A roadmap to understand acquired liver diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 497–511.
More
