You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Fang-Chi Li.
Apical periodontitis is the inflammation and destruction of periradicular tissues, mediated by microbial factors originating from the infected pulp space. This bacteria-mediated inflammatory disease is known to interfere with root development in immature permanent teeth.
- apical periodontitis
- regenerative endodontics
1. Introduction
Normal periapical healthy tissues consist of cementum, the periodontal ligament (PDL), and an alveolar bone. Apical periodontitis is the inflammation of the periapical tissues and is mainly mediated by the microbes and their byproducts in the root canal lumen [1,2][1][2]. The interaction between the microbial virulence factors or the pathogen-associated molecular pattern (PAMP) and the host immune response disrupts tissue homeostasis, resulting in the inflammation and destruction of periapical tissues [2]. In apical periodontitis, differences in cellular crosstalk and signaling from cell-to-cell and cell-to-extracellular matrix (ECM) interactions influence the expression of inflammatory mediators/cytokines, regulating the periapical host response to microbes. The degradation of the ECM, involving the periapical bone, cementum, and dentin, also contributes to disease progression [2,3][2][3]. Apical periodontitis in immature permanent teeth impairs further root development. This incompletely developed immature permanent tooth presents important clinical challenges. Most notably, thin and short roots compromise a tooth’s mechanical integrity, increasing its predisposition to root fracture [4,5][4][5]. Typically, the healing of apical periodontitis is considered crucial for the continuation of root development [6].
2. Apical Periodontitis
Apical periodontitis is the inflammation and destruction of periradicular tissues mediated by microbial virulence factors of endodontic origin. Dental pulp offers the first line of defense against invading microbial threats in a susceptible tooth. Thus, in the initial step of the disease process, the dental pulp becomes inflamed, infected, and necrotic due to autogenous oral microflora. The invasion of pulp space by the microbes and the egress of microbes/byproducts into the periapical region can induce a range of periapical inflammatory responses. The periapical host response activates several classes of cells and an array of intercellular messengers, antibodies, and effector molecules [2] (Figure 1). Intracanal bacteria and their byproducts, including proteins, carbohydrates, and lipids, that form pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) induce immune responses by host cells via the activation of pathogen-recognizing receptors (PRRs) in the host immune cells (e.g., Toll-like receptors) [12][7]. Among different modulins that induce the formation of cytokine networks and host tissue pathology, lipopolysaccharides (LPS) are a key component. They are the major constituent of the cell wall of Gram-negative bacteria and have been shown to act as endotoxins that elicit a variety of immune responses in odontoblasts, fibroblasts, stem cells associated with dentin–pulp/periodontal tissues, endothelial cells, and macrophages [2,13][2][8]. LPSs not only signal the endothelial cells to express adhesion molecules but also activate macrophages to produce several molecular mediators, such as tumor necrosis factor-α (TNF-α) and interleukins (IL) [14][9]. Via Toll-like receptor 4 (TLR-4), LPSs also activate specific pathways in the host cells, resulting in chemokine/cytokine production [12,14][7][9].
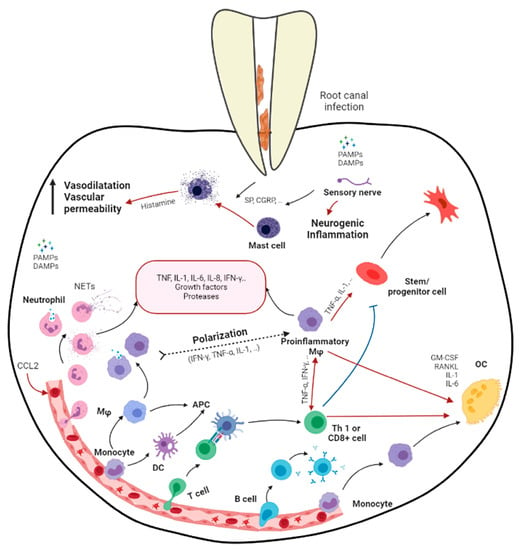
Figure 1. Schematic diagram demonstrating the immune responses in apical periodontitis resulting from the bacterial infection in root canal system (produced by Dr. Hebatullah Hussein). Black arrows indicate a differentiation path or secretion of immune mediators. Black dashed arrows indicate a hypothetical differentiation path. Red arrows indicate induction. Blue arrows indicate inhibition. APC, antigen presenting cell; CGRP, calcitonin gene-related peptide; DAMPs, damage-associated molecular patterns; GM-CSF, granulocyte/monocyte colony-stimulating factor; Mφ, macrophage; OC, osteoclast; PAMPs, pathogen-associated molecular patterns; RANKL, receptor activator of nuclear factor κB ligand; SP, substance P; Treg, regulatory T cell. Created with BioRender.com, accessed on 5 November 2021.
Host-derived mediators, which are induced by the infective process, are critical in stimulating periapical inflammation and tissue destruction. These mediators play important roles in combating infection but may do so at the price of promoting tissue damage. Tissue destruction in the periapex involves the resorption of bone and its replacement by granulation and/or cystic transformation, which is extensively infiltrated by leukocytes. Currently, it has been established that the pathogenic effects of pulpal infections on the periapical tissue are predominantly indirect and operating via the stimulation of host-derived soluble mediators such as chemokines/cytokines rather than by the direct necrotizing effects of bacteria on tissue [15][10].
Generally, the earliest periapical response to inflammation involves the migration of polymorphonuclear neutrophils (PMNs) and monocytes [16,17][11][12]. The massive infiltration of neutrophils is characteristic of the acute phases of apical periodontitis. Chemokines such as IL-8, monocyte chemoattractant peptide-1 (MCP-1), and macrophage-derived chemokine (MDC) are present in periapical tissues and are likely to be involved in stimulating periapical monocytes and leukocyte infiltration [18][13]. The pro-inflammatory cytokines IL-1 and TNF-α are expressed early in response to infection and subsequently induce the production of downstream mediators such as IL-6 and IL-8 [19][14]. IL-lα/β, TNF-α/β, IL-6, and IL-11 possess varying levels of bone-resorptive activity. IL-1β shows 500-fold more potent bone-resorptive activity than TNFs [20][15]. In addition, IL-1 and TNF-α contribute to tissue destruction by inducing PGE2 and matrix metalloproteinases [21,22][16][17]. On the other hand, IL-2, 4, 5, 6, 10, and 13 are secreted by macrophages and T helper 2 (TH2) during inflammation, suppressing IL-1 production [15,23][10][18]. IL-4 and IFN-γ may also inhibit IL-1-stimulated bone resorption [24,25][19][20].
Members of the transforming growth factors type beta (TGF-β) superfamily are critical regulators of cell growth, differentiation, repair, and inflammation [26][21]. During the early phases of inflammation, TGF-β1 acts a chemoattractant for monocytes and lymphocytes, recruiting them to the site of injury, and in the later phases, exerts a potent suppressive effect on the proliferation and differentiation of both T- and B-lymphocytes [27][22]. Furthermore, TGF-β1 inhibits the production and antagonizes the biological activities of IL-1, IL-2, IL-6, TNF-α, and lFN-γ while inhibiting bone resorption [28][23]. TGF-β1 is a powerful negative regulator of inflammation, stimulating collagen synthesis, neovascularization, and fibroblast proliferation [29][24]. Regardless of the formidable defense mechanisms mentioned above, the host immune response is unable to eliminate the microbes that are well established in the morphological complexities of the infected root canal, which is beyond the reaches of the host immune mechanism [2]. Therefore, apical periodontitis, although it may present clinically as symptomatic or asymptomatic, may not be a self-resolving inflammatory process.
3. Effects of Apical Periodontitis in Immature Roots
The complete development of the root takes three years after tooth eruption is complete. An immature root is shorter, features a thinner dentin wall, and lacks apical constriction. The apical aspect of the immature root contains the “apical papilla”, which is the congregation of progenitor stem cells, is of ecto-mesenchymal origin, and has the potential to form the dentin–pulp complex [30,31][25][26]. In immature teeth with apical periodontitis, the intracanal microbial virulence factors interfere with the process of root development resulting in hindered root maturation. These teeth with immature roots are prone to fracture and subsequently tooth loss [32,33][27][28].
An earlier study showed that the apical papilla of immature permanent teeth with deep caries had extremely reduced cellularity, while the Hertwig’s epithelial root sheath (HERS) was discontinuous or absent in cases with pulpitis and pulp necrosis [34][29]. It is known that young patients have a stronger host immune mechanism than older patients. This is attributed to the efficient blood circulation at the open root apex, allowing the transportation of the cellular and molecular components of innate and adaptive immune responses to the canal space [35][30]. Clinical investigations have revealed that the apical papilla was able to survive the process of pulp necrosis [36,37][31][32]. This was attributed to the apical location of the apical papilla, which benefits from collateral circulation, and/or endothelial trans-differentiation, which induces angiogenesis during inflammation [31,37][26][32]. It appears that stem cells from the apical papilla (SCAP) and surrounding stem cells are equipped to receive nutrients and oxygen via diffusion from the surrounding apical tissues for survival and for maintaining their differentiation potential in adverse conditions, such as apical periodontitis and abscesses. Therefore, the current regime of treatment has shifted from conventional root canal treatment and apexification to regenerative endodontic procedures aimed at promoting root development in immature permanent teeth with pulpal necrosis and apical periodontitis [38][33].
References
- Kakehashi, S.; Stanley, H.R.; Fitzgerald, R.J. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral. Surg. Oral. Med. Oral. Pathol. 1965, 20, 340–349.
- Nair, P. Pathogenesis of Apical Periodontitis and the Causes of Endodontic Failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381.
- Márton, I.J.; Kiss, C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol. Immunol. 2000, 15, 139–150.
- Trope, M. Treatment of the Immature Tooth with a Non–Vital Pulp and Apical Periodontitis. Dent. Clin. N. Am. 2010, 54, 313–324.
- Danwittayakorn, S.; Banomyong, D.; Ongchavalit, L.; Ngoenwiwatkul, Y.; Porkaew, P. Comparison of the Effects of Intraradicular Materials on the Incidence of Fatal Root Fracture in Immature Teeth Treated with Mineral Trioxide Aggregate Apexification: A Retrospective Study. J. Endod. 2019, 45, 977–984.e1.
- Diogenes, A.; Hargreaves, K.M. Microbial Modulation of Stem Cells and Future Directions in Regenerative Endodontics. J. Endod. 2017, 43, S95–S101.
- Yumoto, H.; Hirao, K.; Hosokawa, Y.; Kuramoto, H.; Takegawa, D.; Nakanishi, T.; Matsuo, T. The roles of odontoblasts in dental pulp innate immunity. Jpn. Dent. Sci. Rev. 2018, 54, 105–117.
- Henderson, B.; Poole, S.; Wilson, M. Bacterial modulins: A novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996, 60, 316–341.
- Marinho, A.C.; Martinho, F.C.; Leite, F.R.; Nascimento, G.G.; Gomes, B.P. Proinflammatory Activity of Primarily Infected Endodontic Content against Macrophages after Different Phases of the Root Canal Therapy. J. Endod. 2015, 41, 817–823.
- Stashenko, P.; Teles, R.; D’Souza, R. Periapical Inflammatory Responses and Their Modulation. Crit. Rev. Oral Biol. Med. 1998, 9, 498–521.
- Akamine, A.; Hashiguchi, I.; Toriya, Y.; Maeda, K. Immunohistochemical examination on the localization of macrophages and plasma cells in induced rat periapical lesions. Endod. Dent. Traumatol. 1994, 10, 121–128.
- Kawashima, N.; Okiji, T.; Kosaka, T.; Suda, H. Kinetics of macrophages and lymphoid cells during the development of experimentally induced periapical lesions in rat molars: A quantitative immunohistochemical study. J. Endod. 1996, 22, 311–316.
- Rahimi, P.; Wang, C.Y.; Stashenko, P.; Lee, S.K.; Lorenzo, J.A.; Graves, D.T. Monocyte chemoattractant protein-1 expression and monocyte recruitment in osseous inflammation in the mouse. Endocrinology 1995, 136, 2752–2759.
- Tani-Ishii, N.; Wang, C.-Y.; Stashenko, P. Immunolocalization of bone-resorptive cytokines in rat pulp and periapical lesions following surgical pulp exposure. Oral Microbiol. Immunol. 1995, 10, 213–219.
- Stashenko, P.; Dewhirst, F.E.; Peros, W.J.; Kent, R.L.; Ago, J.M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J. Immunol. 1987, 138, 1464–1468.
- Saito, S.; Ngan, P.; Saito, M.; Kim, K.; Lanese, R.; Shanfeld, J.; Davidovitch, Z. Effects of cytokines on prostaglandin E and cAMP levels in human periodontal ligament fibroblasts in vitro. Arch. Oral Biol. 1990, 35, 387–395.
- Meikle, M.C.; Atkinson, S.J.; Ward, R.V.; Murphy, G.; Reynolds, J.J. Gingival fibroblasts degrade type I collagen films when stimulated with tumor necrosis factor and interleukin 1: Evidence that breakdown is mediated by metalloproteinases. J. Periodontal Res. 1898, 24, 207–213.
- Mosmann, T.R. Regulation of Immune Responses by T Cells with Different Cytokine Secretion Phenotypes: Role of a New Cytokine, Cytokine Synthesis Inhibitory Factor (IL10). Int. Arch. Allergy Appl. Immunol. 1991, 94, 110–115.
- Takahashi, N.; Mundy, G.R.; Roodman, G.D. Recombinant human interferon-gamma inhibits formation of human osteoclast-like cells. J. Immunol. 1986, 137, 3544–3549.
- Watanabe, K.; Tanaka, Y.; Morimoto, I.; Yahata, K.; Zeki, K.; Fujihira, T.; Yamashita, U.; Eto, S. Interleukin-4 as a potent inhibitor of bone resorption. Biochem. Biophys. Res. Commun. 1990, 172, 1035–1041.
- Massagué, J.; Cheifetz, S.; Laiho, M.; Ralph, D.A.; Weis, F.M.; Zentella, A. Transforming growth factor-beta. Cancer Surv. 1992, 12, 81–103.
- Wahl, S.M. Transforming growth factor beta: The good, the bad, and the ugly. J. Exp. Med. 1994, 180, 1587–1590.
- Espevik, T.; Waage, A.; Faxvaag, A.; Shalaby, M.R. Regulation of interleukin-2 and interleukin-6 production from T-cells: Involvement of interleukin-1 beta and transforming growth factor-beta. Cell Immunol. 1990, 126, 47–56.
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2.
- D’Souza, R.; Qin, C.D. Development of the Pulpodentin Complex. In Seltzer and Bender’s Dental Pulp; Hargreaves, K.M., Goodis, H.E., Eds.; Quintessence Publishing Co. Inc.: Chicago, IL, USA, 2002; pp. 13–40.
- Huang, G.T.-J.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The Hidden Treasure in Apical Papilla: The Potential Role in Pulp/Dentin Regeneration and BioRoot Engineering. J. Endod. 2008, 34, 645–651.
- Cvek, M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod. Dent. Traumatol. 1992, 8, 45–55.
- Kahler, S.L.; Shetty, S.; Andreasen, F.M.; Kahler, B. The Effect of Long-term Dressing with Calcium Hydroxide on the Fracture Susceptibility of Teeth. J. Endod. 2018, 44, 464–469.
- Ricucci, D.; Siqueira, J.F.; Loghin, S.; Lin, L.M. Pulp and apical tissue response to deep caries in immature teeth: A histologic and histobacteriologic study. J. Dent. 2017, 56, 19–32.
- Horan, M.A.; Ashcroft, G.S. Ageing, Defence Mechanisms and the Immune System. Age Ageing 1997, 26, 15–19.
- Yoo, Y.-J.; Oh, J.-H.; Lee, W.; Woo, K.M. Regenerative Characteristics of Apical Papilla–derived Cells from Immature Teeth with Pulpal and Periapical Pathosis. J. Endod. 2016, 42, 1626–1632.
- Chrepa, V.; Pitcher, B.; Henry, M.A.; Diogenes, A. Survival of the Apical Papilla and Its Resident Stem Cells in a Case of Advanced Pulpal Necrosis and Apical Periodontitis. J. Endod. 2017, 43, 561–567.
- Hargreaves, K.M.; Giesler, T.; Henry, M.; Wang, Y. Regeneration potential of the young permanent tooth: What does the future hold? J. Endod. 2008, 34, S51–S56.
More
