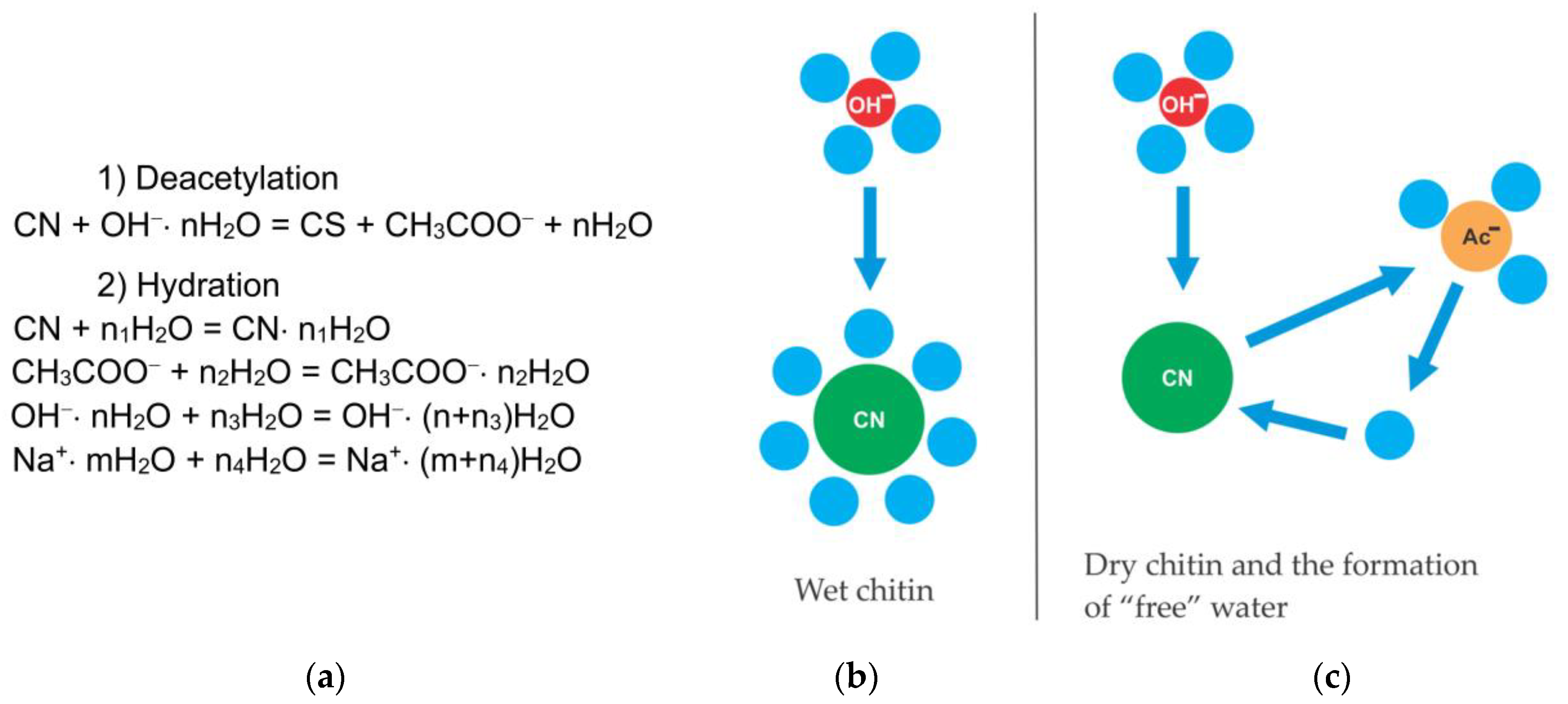
Chitosan can be obtained from chitin chemically or by using enzymatic preparations. From a chemical point of view, both acids and alkalis can be used to deacetylate chitin. However, alkaline deacetylation is used more often since glycosidic bonds are very sensitive to an acidic environment, in which they are destroyed. A mechanism for the chitin deacetylation reaction is proposed, taking into account its kinetic features in which the decisive role is assigned to the effects of hydration. It has been shown that the rate of chitin deacetylation increases with a decrease in the degree of hydration of hydroxide ions in a concentrated alkali solution. When the alkali concentration is less than the limit of complete hydration, the reaction practically does not occur. Hypotheses have been put forward to explain the decrease in the rate of the reaction in the second flat portion of the kinetic curve. The first hypothesis is the formation of “free” water, leading to the hydration of chitin molecules and a decrease in the reaction rate. The second hypothesis postulates the formation of a stable amide anion of chitosan, which prevents the nucleophilic attack of the chitin macromolecule by hydroxide ions.
- chitin
- chitosan
- deacetylation
- kinetics
- reaction mechanism
- hydration
1. Introduction
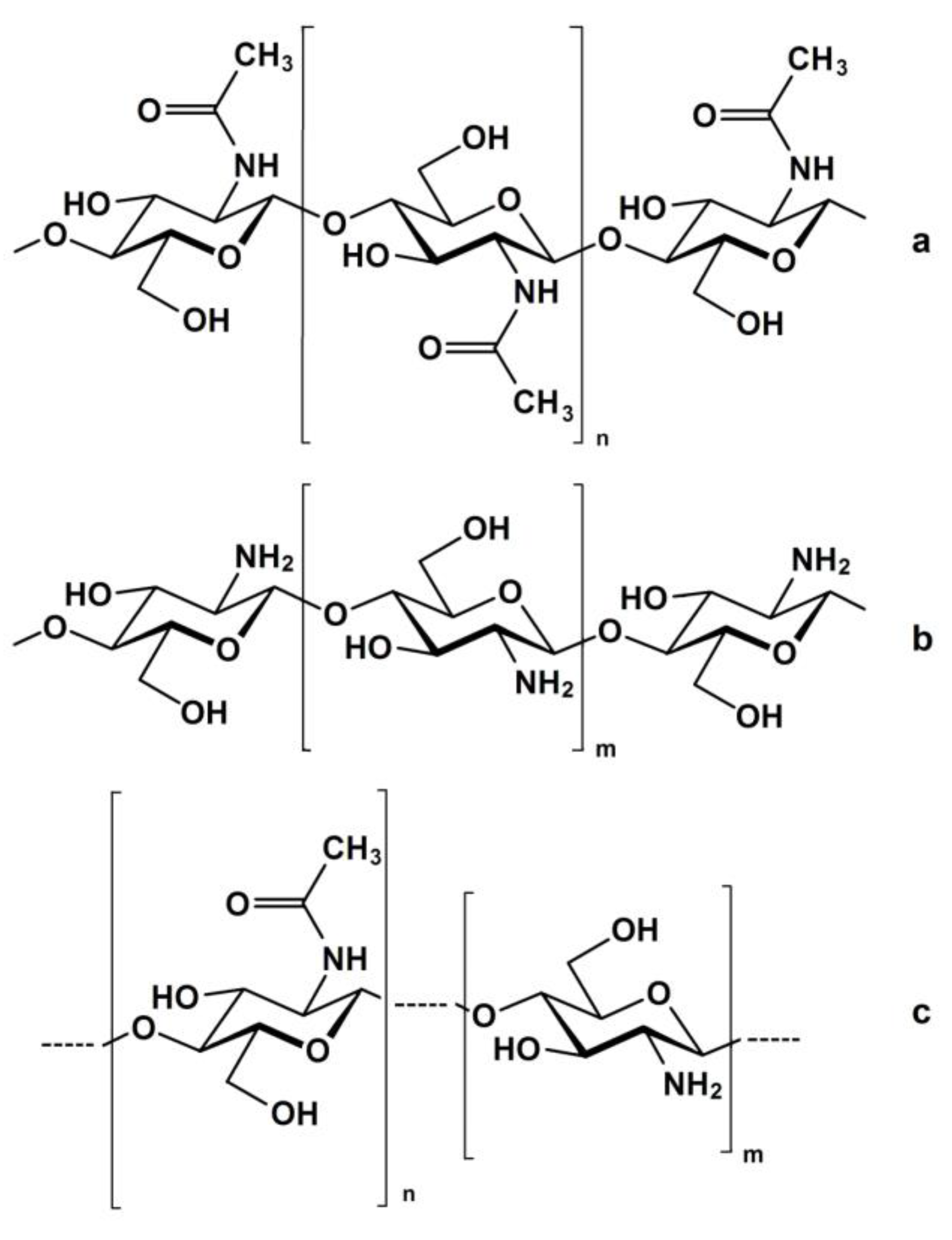
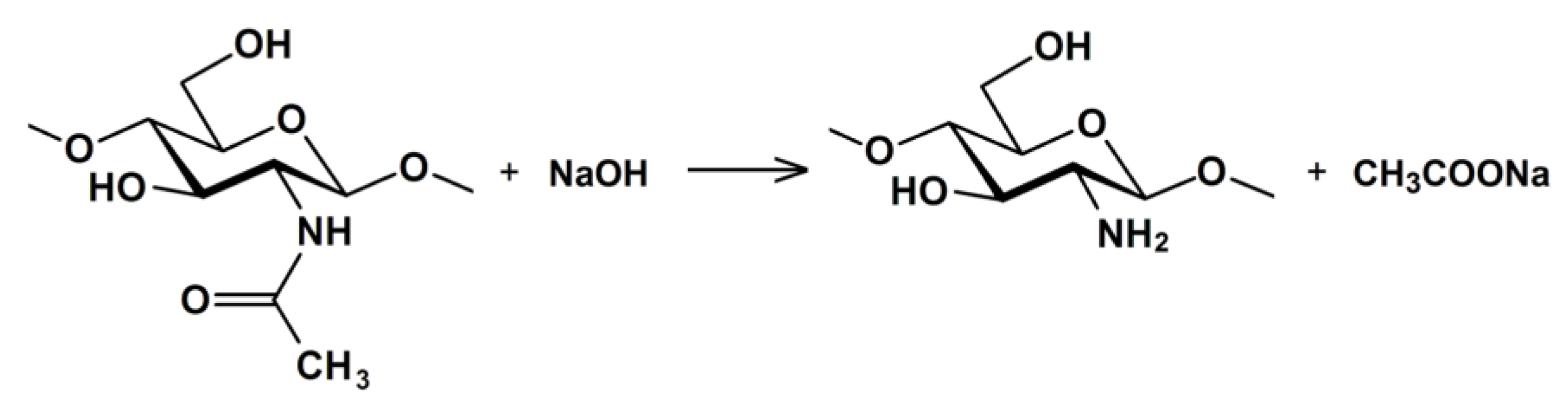
2. Kinetics of the Chitin Deacetylation Reaction
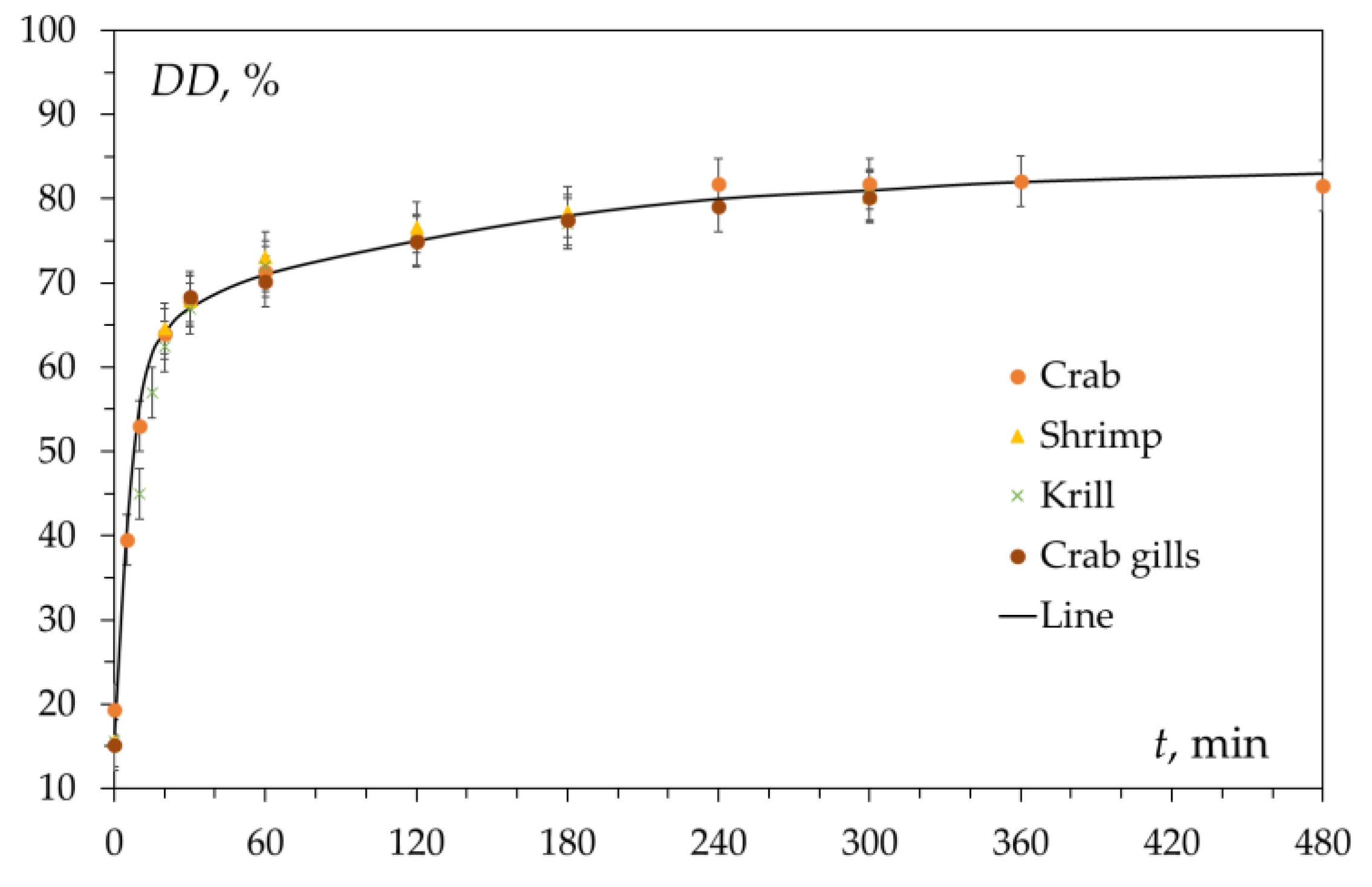
2.1. Factors Determining the Inhibition of the Deacetylation Reaction
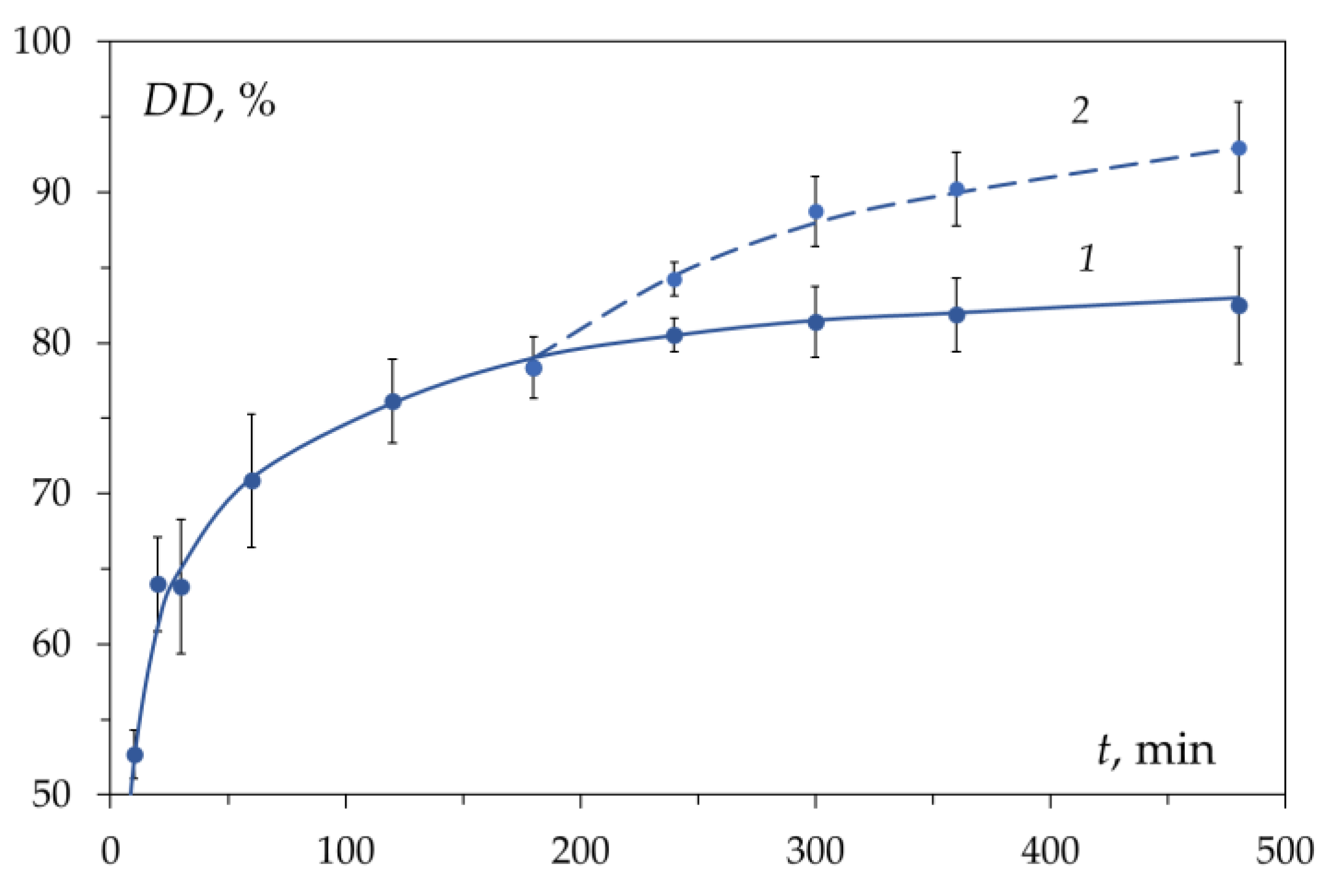
To explain the features of the kinetics of the chitin deacetylation reaction—namely, the presence of fast and slow deacetylation regions on the kinetic curve, various hypotheses were proposed: the existence of a reverse acetylation reaction [15], the presence of crystalline and amorphous regions in chitin [55], changes in the morphology of partially deacetylated chitin during washing, the decrease in alkali concentration during deacetylation [56], diffusion of hydroxide ions and reaction products and the effect of chitin particle porosity [57][58][59][60], the existence of two parallel reactions leading to the formation of different products, the formation of a reaction inhibitor or inactive intermediates (complexes, hydrates), and the effect of hydration (solvation) of reacting particles on their interaction with chitin [15][44].2.2. Reversibility of the Reaction
2.3. Crystallinity of Chitin
It has been suggested that the presence on the kinetic curve (Figure 3) of areas of fast and slow deacetylation is explained by the presence of amorphous and crystalline [61] regions in chitin. This hypothesis was put forward in [55] and continues to be used in various works [49][62][63]. It is assumed that in the amorphous regions, the deacetylation reaction proceeds rapidly (the first section of the kinetic curve), while in the crystalline regions, the reaction rate is lower due to the slow diffusion of the reacting particles (alkali molecules or hydroxide ions) to the chitin molecules. Therefore, after the complete deacetylation of amorphous regions, the deacetylation rate decreases, and the second section of the kinetic curve appears.2.4. Diffusion of Alkali
The results of studies of the kinetics of the deacetylation reaction obtained by 1H and 13C NMR spectroscopy [57][58], indicate that the main role is played by the rate of diffusion of alkali into the solid particles of chitin. Confirmed by data in the literature [64][65], the observed decrease in the deacetylation rate is mainly ascribed to diffusion limitations. It is assumed that the rate of heterogeneous deacetylation can be controlled by the effective diffusivity of OH− through the reacted (deacetylated) layer inside the polysaccharide particles [53]. According to the model proposed in [54], the remarkable decrease of the deacetylation rate is attributed to a strong reduction in the diffusivity of the reactant within the polymer preventing the degree of deacetylation reaching 100%, even in the presence of excess NaOH.2.5. Porosity of Chitin
The porosity of chitin is due to the structure of the shell of crustaceans [66]. In order to establish the effect of the porosity of chitin particles on the kinetics of deacetylation, scholars studied the deacetylation of chitin with different supramolecular structures—the pore volume, specific surface area, pore diameter, and crystallinity [46][67]. When dried in air with a gradual evaporation of water, flexible chitin fibrils approached each other, which led to the disappearance of small pores. During freeze-drying, the primary structure of chitin is preserved, therefore, chitin samples dried differently differ in pore size.3. On the Mechanism of the Chitin Deacetylation Reaction
3.1. The Reaction Mechanism in the First Section of the Kinetic Curve
When considering the mechanism that determines the course of the reaction in the first section of the kinetic curve, special attention is paid to the effect of the hydration (solvation) of particles, the formation of an active particle that causes deacetylation, and the influence of the nature and concentration of alkali on the reaction rate.3.1.1. Hydration of Chitin/Chitosan and Sodium Hydroxide
The most probable factor affecting the kinetics of the deacetylation reaction is the formation of hydrate (solvate) complexes as a result of the interaction of chitin/chitosan macromolecules and sodium hydroxide with a solvent (water). It was shown in [46] that the rate of deacetylation of wet (previously kept in water) chitin is significantly lower than the rate of deacetylation of the initial dry chitin. Apparently, the hydration of chitin macromolecules leads to a decrease in the reaction rate in the first part of the kinetic curve.3.1.2. Alkali Concentration and the Nature of the Active Particle
It has been reported in many studies that the rate of the deacetylation reaction increases with increasing NaOH concentration [63][64][68], but not monotonously. The degree of deacetylation sharply increases starting from a NaOH concentration of 20–25 wt.%. In this concentration range, there is also a maximum specific electrical conductivity (s) of sodium hydroxide solution. These experimental data can be explained by the complete hydration of sodium hydroxide ions, in which water molecules bind into stable hydrate complexes of cations and anions, leading to a decrease in the number of “free” water molecules.2.1.3. The Nature of Alkali and the Number of Hydroxide Ions Hydration
The effect of hydration of alkali ions on the kinetics of the deacetylation reaction is confirmed by experiments using NaOH and KOH solutions. The first assumption is that in the case of different cations (Na+ or K+) with different ion diameters (di) and different hydration energies (Gh), the concentration of “free” water in the system will differ. The hydration energy and size of the K+ ion are Gh = 398 kJ/mol and di = 0.102 nm; for Na+, Gh = 271 kJ/mol and di = 0.138 nm [69][70]. This difference should affect the degree of hydration of hydroxide ions competing with cations for water molecules in the hydration shell and, consequently, the concentration of “free” water, the reaction rate, and the shape of the chitin deacetylation kinetic curve. To elucidate the mechanism of the deacetylation reaction in the first section of the kinetic curve, scholars expressed the alkali content not as a percentage or molar concentration, but as the ratio of water molecules to one ion of the dissolved electrolyte. Knowing the molar concentration of alkali (Cal) and water (Cw), it is possible to calculate the number of water molecules per electrolyte ion Cw/Cal (Table 1). In this case, almost identical dependences of the deacetylation reaction rate on Cw/Cal are obtained (Figure 4).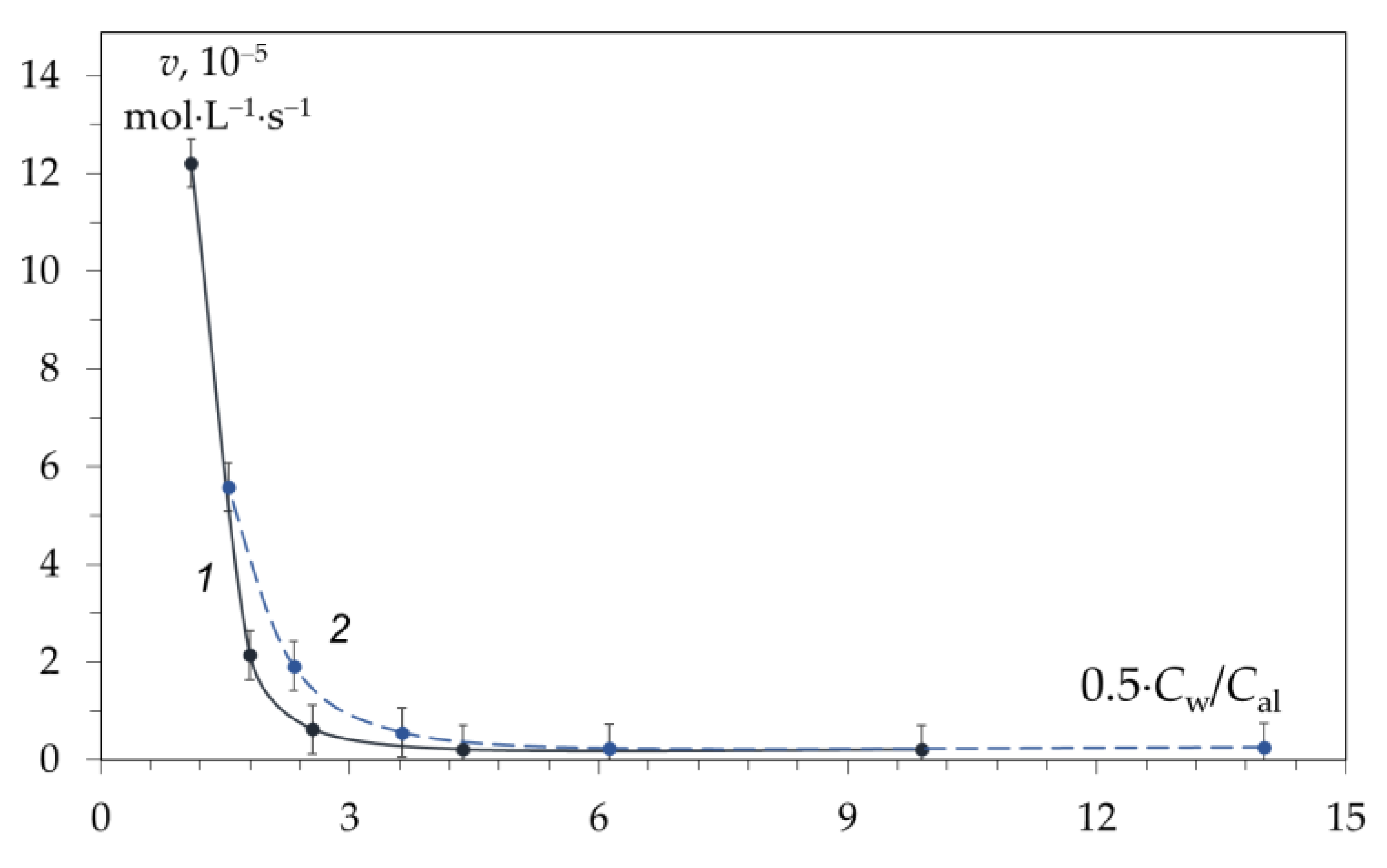 Figure 4. Dependence of the rate of the deacetylation reaction in the first section of the kinetic curve during the deacetylation time of 0–10 min at 100 oC in a solution of NaOH (1) and KOH (2) on the number of water molecules per one ion of dissolved hydroxide. Original figure.
According to the data, the deacetylation reaction begins to proceed at a noticeable rate when the number of water molecules per electrolyte ion (OH–) is less than that required for complete hydration—less than 6. Before LCH, OH– ions have complete hydration shells and are practically inactive. Upon crossing the LCH, the hydration shell is depleted, and the chitin deacetylation reaction begins. Thus, the reactivity of hydroxide ions appears at the LCH point and further increases with a decrease in the degree of hydration (Figure 4).
When discussing the mechanism of deacetylation, one should also take into account the fact that the number of ion pairs and associates of alkali molecules increases in concentrated solutions [93,99], which leads to a decrease in the concentration of “free” hydroxide ions. Thus, the actual concentration of hydroxide ions after the LCH point decreases. Consequently, the increase in the activity of hydroxide ions turns out to be greater than that observed in experiments. A similar conclusion was made in a monograph [100], where it was shown that the dependence of the activity of hydroxide ions on the water concentration is approximately 1/[H2O]2. The graph of the dependence of the activity of OH− ions on the molar concentration of water is similar to the dependence obtained (Figure 4). Thus, water does not participate in the chitin deacetylation reaction but, on the contrary, inhibits the reaction.
Figure 4. Dependence of the rate of the deacetylation reaction in the first section of the kinetic curve during the deacetylation time of 0–10 min at 100 oC in a solution of NaOH (1) and KOH (2) on the number of water molecules per one ion of dissolved hydroxide. Original figure.
According to the data, the deacetylation reaction begins to proceed at a noticeable rate when the number of water molecules per electrolyte ion (OH–) is less than that required for complete hydration—less than 6. Before LCH, OH– ions have complete hydration shells and are practically inactive. Upon crossing the LCH, the hydration shell is depleted, and the chitin deacetylation reaction begins. Thus, the reactivity of hydroxide ions appears at the LCH point and further increases with a decrease in the degree of hydration (Figure 4).
When discussing the mechanism of deacetylation, one should also take into account the fact that the number of ion pairs and associates of alkali molecules increases in concentrated solutions [93,99], which leads to a decrease in the concentration of “free” hydroxide ions. Thus, the actual concentration of hydroxide ions after the LCH point decreases. Consequently, the increase in the activity of hydroxide ions turns out to be greater than that observed in experiments. A similar conclusion was made in a monograph [100], where it was shown that the dependence of the activity of hydroxide ions on the water concentration is approximately 1/[H2O]2. The graph of the dependence of the activity of OH− ions on the molar concentration of water is similar to the dependence obtained (Figure 4). Thus, water does not participate in the chitin deacetylation reaction but, on the contrary, inhibits the reaction.
3.2. Reaction Mechanism in the Second Section of the Kinetic Curve: Inhibition of the Deacetylation Reaction
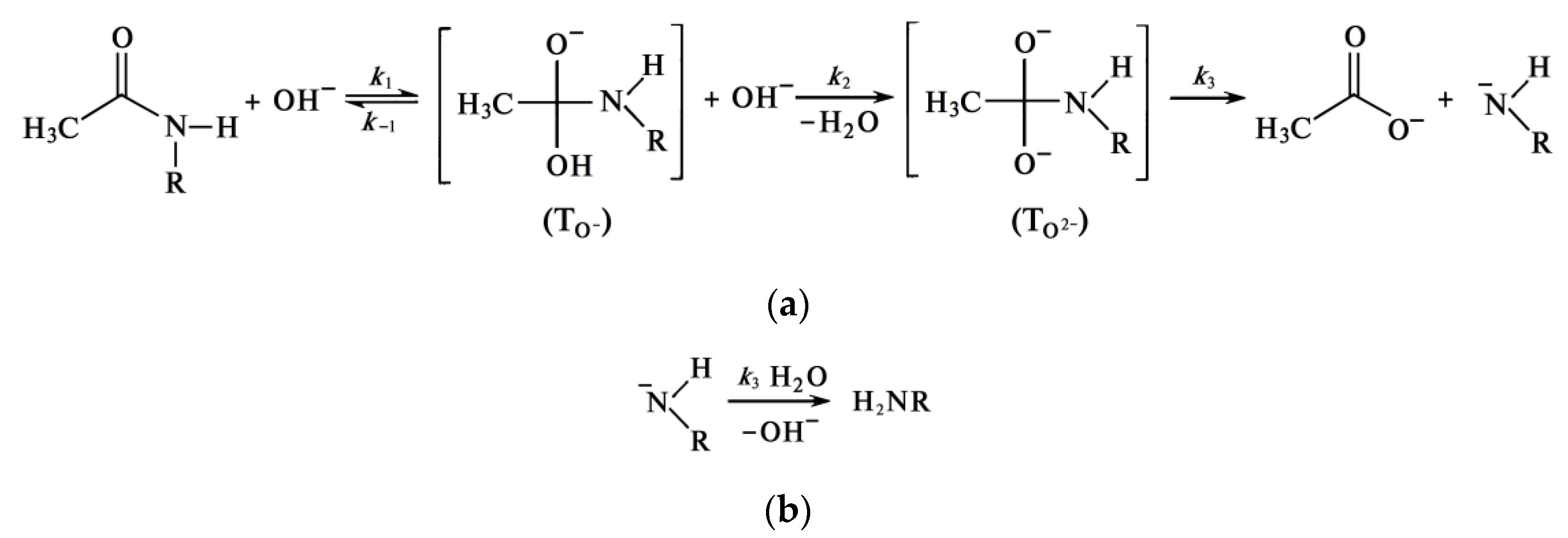
The rate of the chitin deacetylation reaction in the second section decreases as shown in many studies [47][63][71][72][73], and the kinetic curve runs almost parallel to the abscissa axis (Figure 3). As the alkali concentration increases, the reaction rate decreases. As it was determined in [43], the activation energy of this step is about 48.76 kJ/mol as it. It can be assumed that the deceleration of the deacetylation reaction is explained by the formation of a certain amount of water during the reaction. It hypothesized that this water, which is formed in close proximity to the chitin molecule, leads to chitin hydration, reducing the rate of further deacetylation. To determine the source of “free” water during deacetylation, consider the mechanism of the reaction of deacetylation. The reaction of deacetylation—the reaction of alkaline hydrolysis of acetamide bonds (deacetylation reaction) is a bimolecular second-order nucleophilic substitution of SN2, in which a strong hydroxyl ion nucleophile attacks the acetamide bond [69][74]. As a result of the overall deacetylation reaction, no water is produced, and none is consumed. The detailed description of the mechanism includes several steps. In alkali solutions, when the amide carbon is attacked by a hydroxide ion (it can be conventionally called the “first hydroxide ion”), an anionic tetrahedral intermediate (TO−) is formed [75][76][77][78]. The reaction is reversible; therefore, the resulting intermediate can either turn into the starting compound or decompose into hydrolysis products. Decomposition pathways may include interaction with various forms in solution that transfer a proton from the hydroxide ion OH− to the amide ion NHR−. In dilute solutions, water is postulated as a proton carrier [77]. At a high alkali concentration, the H+ proton is taken away by the “second” hydroxide ion OH− to form a water molecule, and the second intermediate (TO2−) is formed, which then decomposes to form an acetate ion and an amide ion. The amide ion NHR−, obtained in this way, takes away the H+ proton from the water molecule [76]. The described mechanism of cleavage of the acetamide bond in a concentrated alkali solution can be depicted by the scheme in Figure 5, where R is the residue of the chitin/chitosan molecule.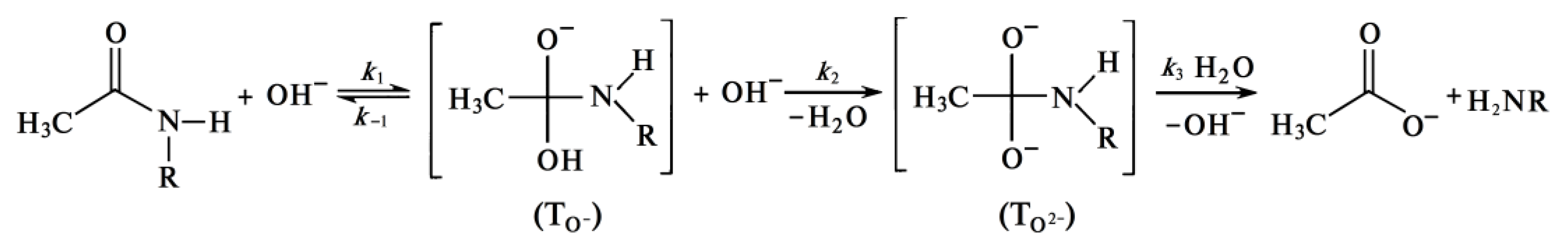
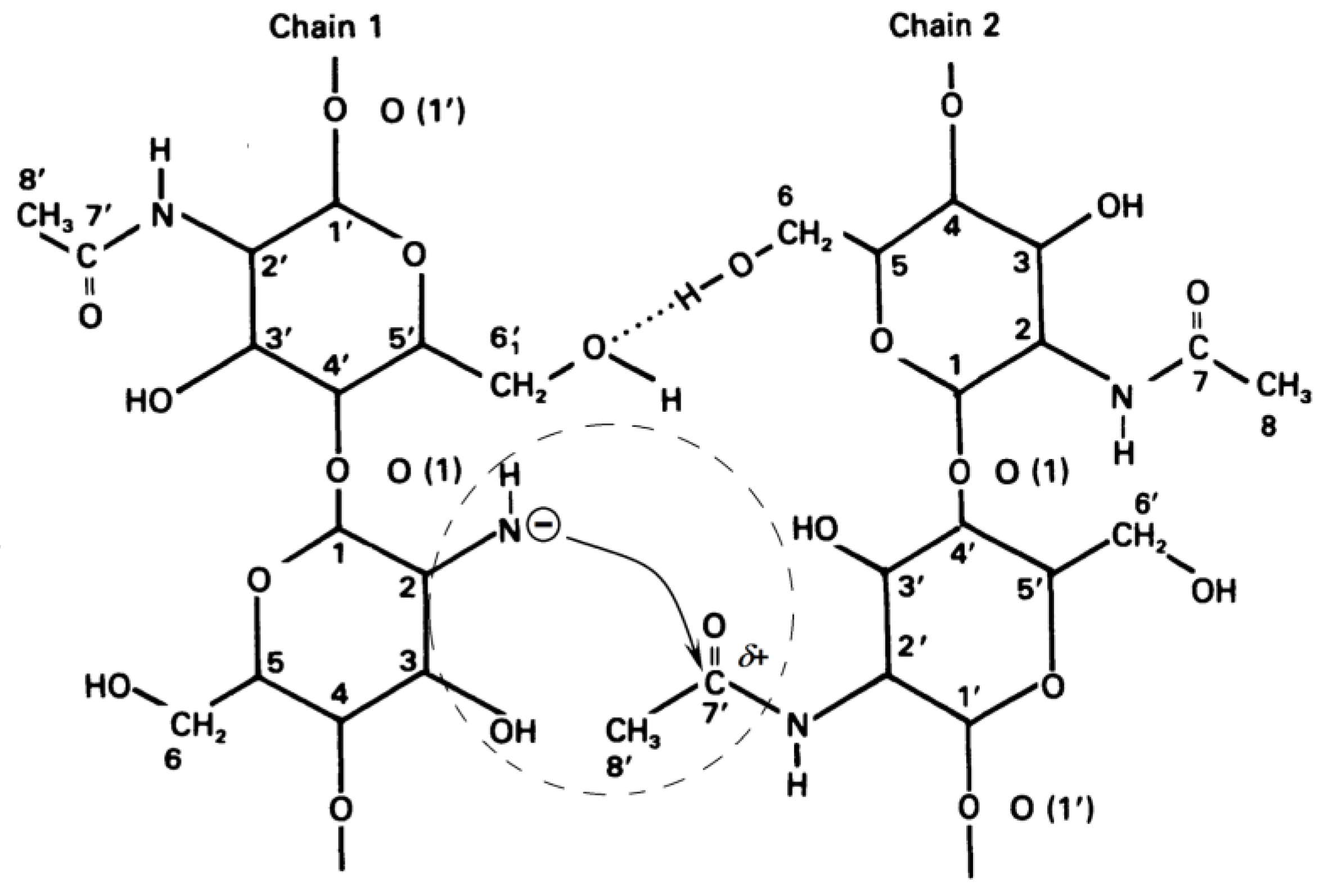
Formation of Quasi-Stable Chitosan Amide Anion
4. Conclusions
References
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226.
- Younes, I.; Rinaudo, M. Chitin and Chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174.
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306.
- Hasan, S.; Boddu, V.M.; Viswanath, D.S.; Ghost, T.K. Chitin and Chitosan: Science and Engineering; Springer: Cham, Switzerland, 2022; p. 421.
- Hou, F.; Gong, Z.; Jia, F.; Cui, W.; Song, S.; Zhang, J.; Wang, Y.; Wang, W. Insights into the relationships of modifying methods, structure, functional properties and applications of chitin: A review. Food Chem. 2023, 409, 135336.
- Wei, A.; Fu, J.; Guo, F. Mechanical properties of chitin polymorphs: A computational study. J. Mater. Sci. 2021, 56, 12048–12058.
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709.
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186.
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27.
- Muzzarelli, R.A.A. The discovery of chitin. In Chitosan in Pharmacy and Chemistry; Muzzarelli, R.A.A., Muzzarelli, C., Eds.; Atec: Grottammare, Italy, 2002; pp. 1–8.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Akpan, E.I.; Gbenebor, O.P.; Adeosun, S.O.; Cletus, O. Chapter 5—Solubility, degree of acetylation, and istribution of acetyl groups in chitosan. In Handbook of Chitin and Chitosan: Volume 1: Preparation and Properties; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–164.
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194.
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Chitin and chitosan: History, fundamentals and innovations. In Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2019; Volume 35, pp. 49–123.
- Percot, A.; Chaussard, G.; Sorlier, P.; Schatz, C.; Montembault, A.; Viton, C. Overall consideration on the evolution of the study of chitosan properties. In Advances in Chitin Science; Boucher, I., Jamieson, K., Retnakaran, A., Eds.; European Chitin Society: Montreal, QC, Canada, 2004; Volume 7, pp. 1–6.
- Xu, D.; Aihemaiti, Z.; Cao, Y.; Teng, C.; Li, X. Physicochemical stability, microrheological properties and microstructure of lutein emulsions stabilized by multilayer membranes consisting of whey protein isolate, flaxseed gum and chitosan. Food Chem. 2016, 202, 156–164.
- Abere, D.V.; Ojo, S.A.; Paredes-Epinosa, M.B.; Hakami, A. Derivation of composites of chitosan-nanoparticles from crustaceans source for nanomedicine: A mini review. Adv. Biomed. Eng. 2022, 4, 100058.
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 85, 832–848.
- Sami El-banna, F.; Mahfouz, M.E.; Leporatti, S.; El-Kemary, M.A.; Hanafy, A.N.N. Chitosan as a natural copolymer with unique properties for the development of hydrogels. Appl. Sci. 2019, 9, 2193.
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245.
- Do, N.H.N.; Truong, Q.T.; Le, P.K.; Ha, A.C. Recent developments in chitosan hydrogels carrying natural bioactive compounds. Carbohydr. Polym. 2022, 294, 119726.
- Xu, J.; Zhang, M.; Du, W.; Zhao, J.; Ling, G.; Zhang, P. Chitosan-based high-strength supramolecular hydrogels for 3D bioprinting. Int. J. Biol. Macromol. 2022, 219, 545–557.
- Meng, Q.; Zhong, S.; Wang, J.; Gao, Y.; Cui, X. Advances in chitosan-based microcapsules and their applications. Carbohydr. Polym. 2023, 300, 120265.
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825.
- Chen, S.; Tian, H.; Mao, J.; Ma, F.; Zhang, M.; Chen, F.; Yang, P. Preparation and application of chitosan-based medical electrospun nanofibers. Int. J. Biol. Macromol. 2023, 226, 410–422.
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosanin food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692.
- Rebello, S.; Sali, S.; Jisha, M.S.; Reshmy, R.; Pugazhendhi, A.; Madhavan, A.; Binod, P.; Awasthi, M.K.; Pandey, A.; Sindhu, R. Chitosan a versatile adsorbent in environmental remediation in the era of circular economy- a mini review. Sustain. Chem. Pharm. 2023, 32, 101004.
- Issahaku, I.; Tetteh, I.K.; Tetteh, A.Y. Chitosan and chitosan derivatives: Recent advancements in production and applications in environmental remediation. Environ. Adv. 2023, 11, 100351.
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594.
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of Chitosan for Biomedical Applications. In Chitosan Based Biomaterials, Volume 1: Fundamentals; Jennings, J.A., Bumgardner, J.D., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 3–30.
- Kedir, W.M.; Abdi, G.F.; Goro, M.M.; Tolesa, L.D. Pharmaceutical and drug delivery applications of chitosan biopolymer and its modified nanocomposite: A review. Heliyon 2022, 8, e10196.
- Baharlouei, P.; Rahman, A. Chitin and chitosan: Prospective biomedical applications in drug delivery, cancer treatment, and wound healing. Mar. Drugs 2022, 20, 460.
- Sharma, P.P.; Bhardwaj, S.; Sethi, A.; Goel, V.K.; Grishina, M.; Poonam; Rathi, B. Chitosan based architectures as biomedical carriers. Carbohydr. Res. 2022, 522, 108703.
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Kennedy, J.F. Chitosan-based nanocomposites as coatings and packaging materials for the postharvest improvement of agricultural product: A review. Carbohydr. Polym. 2023, 309, 120666.
- Fan, Z.; Wang, L.; Qin, Y.; Li, P. Activity of chitin/chitosan/chitosan oligosaccharide against plant pathogenic nematodes and potential modes of application in agriculture: A review. Carbohydr. Polym. 2023, 306, 120592.
- Xu, K.; Li, L.; Huang, Z.; Tian, Z.; Li, H. Efficient adsorption of heavy metals from wastewater on nanocomposite beads prepared by chitosan and paper sludge. Sci. Total Environ. 2022, 846, 157399.
- Gal, M.R.; Rahmaninia, M.; Hubbe, M.A. A comprehensive review of chitosan applications in paper science and technologies. Carbohydr. Polym. 2023, 309, 120665.
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242.
- Elwakeel, K.Z. Environmental application of chitosan resins for the treatment of water and wastewater: A Review. J. Dispers. Sci. Technol. 2010, 31, 273–288.
- Choi, C.; Nam, J.-P.; Nah, J.-W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10.
- Chauhan, S.; Thakur, A. Chitosan-based biosensors-A comprehensive Review. Mater. Today Proc. 2023.
- Philibert, T.; Lee, B.H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337.
- Ahlafi, H.; Moussout, H.; Boukhlifi, F.; Echetna, M.; Bennani, M.N.; Slimane, S.M. Kinetics of N-deacetylation of chitin extracted from shrimp shells collected from coastal area of Morocco. Mediterr. J. Chem. 2013, 2, 503–513.
- Novikov, V.Y.; Konovalova, I.N.; Dolgopyatova, N.V. The mechanism of chitin and chitosan deacetylation during long-term alkaline treatment. Appl. Biochem. Microbiol. 2022, 58, 309–314.
- Novikov, V.Y. The general relationships of chitin and chitosan chemical hydrolysis. In Advances in Chitin Science; Struszczyk, H., Domard, A., Peter, M.G., Pospieszny, H., Eds.; Publisher Institute of Plant Protection: Poznan, Poland, 2005; Volume 8, pp. 109–113.
- Chebotok, E.N.; Novikov, V.Y.; Konovalova, I.N. Kinetics of base deacetylation of chitin and chitosan as influenced by their crystallinity. Russ. J. Appl. Chem. 2007, 80, 1753–1758.
- Dolgopiatova, N.V.; Kuchina, Y.A.; Dyakina, T.N.; Volkova, T. Effect of heterogeneous deacetylation on the properties of northern shrimp chitin and chitosan. KnE Life Sci. 2020, 5, 315–324.
- Novikov, V.Y.; Rysakova, K.S.; Shumskaya, N.V.; Mukhortova, A.M.; Kesarev, K.A. King crab gills as a new source of chitin/chitosan and protein hydrolysates. Int. J. Biol. Macromol. 2023, 232, 123346.
- Narudin, N.A.H.; Rosman, N.A.; Shahrin, E.W.; Sofyan, N.; Mahadi, A.H.; Kusrini, E.; Hobley, J.; Usman, A. Extraction, characterization, and kinetics of N-deacetylation of chitin obtained from mud crab shells. Polym. Polym. Compos. 2022, 30, 09673911221109611.
- Liu, T.G.; Li, B.; Huang, W.; Lv, B.; Chen, J.; Zhang, J.X.; Zhu, L.P. Effects and kinetics of a novel temperature cycling treatment on the N-deacetylation of chitin in alkaline solution. Carbohydr. Polym. 2009, 77, 110–117.
- Bradić, B.; Bajec, D.; Pohar, A.; Novak, U.; Likozar, B. A reaction–diffusion kinetic model for the heterogeneous N-deacetylation step in chitin material conversion to chitosan in catalytic alkaline solutions. React. Chem. Eng. 2018, 3, 920–929.
- De Moura, C.M.; de Moura, J.M.; Soares, N.M.; de Almeida Pinto, L.A. Evaluation of molar weight and deacetylation degree of chitosan during chitin deacetylation reaction: Used to produce biofilm. Chem. Eng. Process. 2011, 50, 351–355.
- Jiang, C.J.; Xu, M.Q. Kinetics of heterogeneous deacetylation of β-Chitin. Chem. Eng. Technol. 2006, 29, 511–516.
- De Souza, J.R.; Giudici, R. Effect of diffusional limitations on the kinetics of deacetylation of chitin/chitosan. Carbohydr. Polym. 2021, 254, 117278.
- Kurita, K.; Sannan, T.; Iwakura, Y. Studies on chitin, 4. Evidence for formation of block and random copolymers of N-acetyl-D-glucosamine and D-glucosamine by hetero- and homogeneous hydrolysis. Makromol. Chem. 1977, 178, 3197–3202.
- Yaghobi, N.; Mirzadeh, H. Enhancement of chitin’s degree of deacetylation by multistage alkali treatments. Iran. Polym. J. 2004, 13, 131–136.
- Varum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrod, O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field nmr spectroscopy. Carbohydr. Res. 1991, 211, 17–23.
- Varum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrod, O. 13C-N.m.r. studies of the acetylation sequences in partially N-deacetylated chitins (chitosans). Carbohydr. Res. 1991, 217, 19–27.
- Lamarque, G.; Cretenet, M.; Lucas, J.; Crepet, A.; Viton, C.; Domard, A. Optimization of α- and β-chitin heterogeneous de-N-acetylation from a multi-step process: New route of de-N-acetylation by means of freeze-pump-thaw cycles. In Advances in Chitin Science; Boucher, I., Jamieson, K., Retnakaran, A., Eds.; European Chitin Society: Montreal, QC, Canada, 2004; Volume 7, pp. 66–73.
- Lamarque, G.; Cretenet, M.; Viton, C.; Domard, A. New route of deacetylation of alpha- and beta-chitins by means of freeze-pump out-thaw cycles. Biomacromolecules 2005, 6, 1380–1388.
- Sikorski, P.; Hori, R.; Wada, M. Revisit of alpha-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 2009, 10, 1100–1105.
- Lamarque, G.; Viton, C.; Domard, A. Comparative study of the second and third heterogeneous deacetylations of alpha- and beta-chitins in a multistep process. Biomacromolecules 2004, 5, 1899–1907.
- Lavertu, M.; Darras, V.; Buschmann, M.D. Kinetics and efficiency of chitosan reacetylation. Carbohydr. Polym. 2012, 87, 1192–1198.
- Methacanon, P.; Prasitsilp, M.; Pothsree, T.; Pattaraarchachai, J. Heterogeneous N-deacetylation of squid chitin in alkaline solution. Carbohydr. Polym. 2003, 52, 119–123.
- Chebotok, E.N.; Novikov, V.Y.; Konovalova, I.N. Depolymerization of chitin and chitosan in the course of base deacetylation. Russ. J. Appl. Chem. 2006, 79, 1162–1166.
- Nikolov, S.; Fabritius, H.; Petrov, M.; Friak, M.; Lymperakis, L.; Sachs, C.; Raabe, D.; Neugebauer, J. Robustness and optimal use of design principles of arthropod exoskeletons studied by ab initio-based multiscale simulations: Multiscale mechanics. J. Mech. Behav. Biomed. Mater. 2011, 4, 129–145.
- Novikov, V.; Derkach, S.; Konovalova, I. Chitosan technology from crustacean shells of the northern seas. KnE Life Sci. 2020, 5, 65–74.
- Khong, T.T.; Aachmann, F.L.; Vrum, K.M. Kinetics of de-N-acetylation of the chitin disaccharide in aqueous sodium hydroxide solution. Carbohydr. Res. 2012, 352, 82–87.
- Hummer, G.; Pratt, L.R.; Garcia, A.E. Free energy of ionic hydration. J. Phys. Chem. 1996, 100, 1206–1215.
- Pearson, R.G. Ionization potentials and electron affinities in aqueous solution. J. Am. Chem. Soc. 1986, 108, 6109–6114.
- Chen, C.H.; Wang, F.Y.; Ou, Z.P. Deacetylation of β-chitin. I. Influence of the deacetylation conditions. J. Appl. Polym. Sci. 2004, 93, 2416–2422.
- Jaworska, M.M. Kinetics of enzymatic deacetylation of chitosan. Cellulose 2012, 19, 363–369.
- Tsaih, M.L.; Chen, R.H. The effect of reaction time and temperature during heterogenous alkali deacetylation on degree of deacetylation and molecular weight of resulting chitosan. J. Appl. Polym. Sci. 2003, 88, 2917–2923.
- Khan, M.N. Experimental versus theoretical evidence for the rate-limiting steps in uncatalyzed and H+- and HO−-catalyzed hydrolysis of the amide bond. Int. J. Chem. Kinet. 2009, 41, 599–611.
- Carroll, F.A. Perspectives on Structure and Mechanism in Organic Chemistry, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 966.
- Brown, R.S. Studies in amide hydrolysis: The acid, base, and water reactions. In The Amide Linkage. Structural Significance in Chemistry, Biochemistry, and Materials Science; Greenberg, A., Breneman, C.M., Liebman, J.F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 85–114.
- Challis, B.C.; Challis, J.A. Chapter 13. Reactions of the carboxamide group. In The Chemistry of Amides; Zabicky, J., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1970; pp. 731–857.
- Xiong, Y.; Zhan, C.-G. Theoretical studies of the transition-state structures and free energy barriers for base-catalyzed hydrolysis of amides. J. Phys. Chem. A 2006, 110, 12644–12652.
- Roberts, G.A.F. Chitin Chemistry; The Macmillan Press Ltd.: Basingstoke, UK; London, UK, 1992; p. 368.
- Muzzarelli, R.A.A. Chitin nanostructures in living organisms. In Chitin. Formation and Diagenesis (Topics in Geobiology); Gupta, N.S., Ed.; Springer: Dordrecht, The Netherlands, 2011; Volume 34, pp. 1–34.
