Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by NEILA ANNABI-TRABELSI.
Solar salterns and salt marshes are unique ecosystems with special physicochemical features and characteristic biota. There are very few studies focused on the impacts of pollution on these economic and ecological systems. Unfortunately, diversified pollution (metals, Polycyclic Aromatic Hydrocarbons, etc.) has been detected in these complex ecosystems. These hypersaline environments are under increasing threat due to anthropogenic pressures.
- solar salterns
- biota
- pollution
- bioremediation
1. Introduction
Solar salterns have considerable economic, ecological, and scientific value. They are distributed globally along tropical and subtropical coasts in arid and semiarid regions [1,2][1][2]. They are mainly distributed in Mediterranean regions, where the climate is characterized by long dry periods in the summer, during which evaporation of seawater in ponds is accentuated [3]. Multi-pond salterns are human-controlled semi-artificial coastal systems designed to harvest NaCl from seawater for human consumption. In this system, seawater is pumped through a series of separate shallow ponds (Figure 1) that are typically less than 0.5 m in depth [4], in which it is gradually driven to ponds of greater salinities, ranging from seawater to sodium chloride saturation and sometimes even beyond [5]. Salterns are well known as continuous or semi-continuous systems because each set of ponds is characterized by a distinct range of salinity and biogeochemical attributes [6,7][6][7]. These thalassohaline environments are operated in repeated cycles of feeding with natural saltwater, increasing salt concentration due to water evaporation, and, finally, salt precipitation. Hence, they have certain attributes of semi-closed chemostats [6].
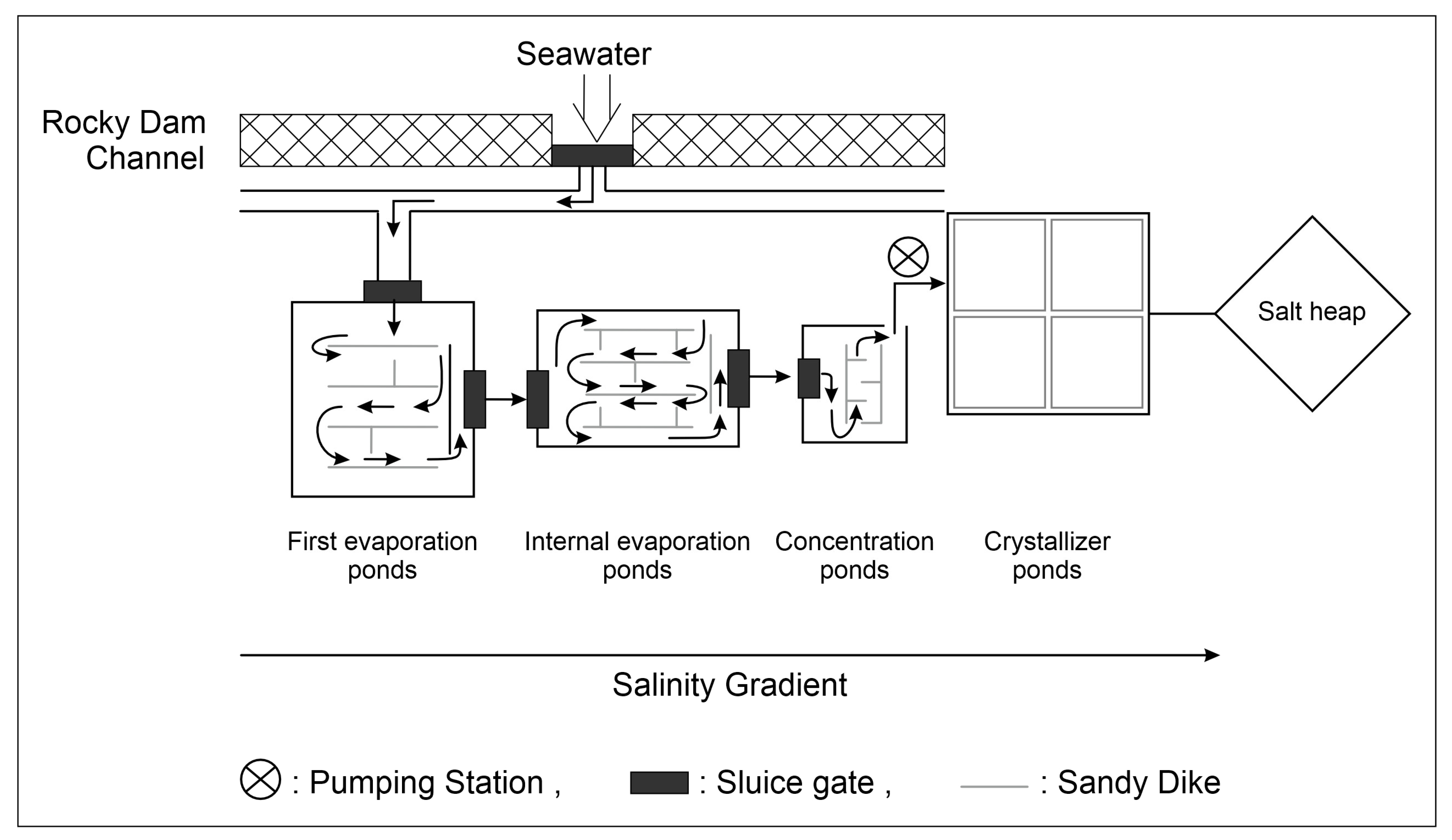
Figure 1. Water flow in solar salterns: the direction of the water circulation is indicated by the arrows.
2. Physicochemical Parameters
Salterns are hypersaline extreme ecosystems with unique abiotic features, including a wide range of salinities, low oxygen, and intense ultraviolet radiation [1,7][1][7]. The physicochemical properties of seawater change due to evaporation in the flow-through multi-pond system. Although the pH of seawater is slightly alkaline, owing to carbonate buffering systems, the pH of saline water in salterns is generally close to neutral in ponds with biota [3,8,9,10,11,12][3][8][9][10][11][12]. The pH of ponds in salterns can be regulated via the salinity, temperature, and amount of carbonate ions [2]. It was shown to gradually decrease, along the distinct ponds, from 8.3 for seawater to 5.8 for magnesium chloride solutions as measured in the Sfax solar saltern in Tunisia [13] and exhibits little seasonal variation [14]. The nutrient concentrations found in solar salterns depend on a variety of parameters. Geographic factors influencing nutrients include the proximity to rivers, urban pollution, the nutrient status of the incoming seawater, and climate change [15]. The nature and extent of the fauna and flora, the season of productivity, and management practices also influence nutrient concentration [15]. According to Kobbi-Rebai [16], the internal recycling processes such as the release from sediment and the mineralization of organic matter are the key drivers of phosphorous concentrations in ponds. However, these concentrations are mainly impacted by the seawater inflow in the first pond [16]. Total phosphorus (TP) would be one of the most suitable chemical parameters from which to propose a methodology for the determination of trophic status in solar salterns [17]. Among solar salterns, both oligotrophic [18,19][18][19] and eutrophic systems [11,20,21,22,23,24][11][20][21][22][23][24] have been described. However, nutrients are concentrated in first ponds and decrease with increases in the salt concentration [22]. Nitrate and nitrite concentrations are influenced by halophilic bacteria activity [25]. Extremely halophilic denitrifying bacteria in hypersaline environments reduce nitrate to nitrite, nitrous oxide, and even dinitrogen [26].
3. Biota
The biota of the salterns are not only of great scientific interest, but also have a direct impact on the quality and the quantity of the salt produced via evaporation and precipitation [6,27][6][27]. Solar salterns are inhabited by highly specialized extremophiles (Figure 2). Many ecological changes happen throughout the salinity gradient. Biodiversity decreases with the increase in salinity [9,11,28,29,30[9][11][28][29][30][31],31], with the zonal biological community structure ranging from marine to extreme halophilic communities [9,32,33,34][9][32][33][34]. Archaea and Bacteria are the two components of the microbial community in solar salterns [28,35,36][28][35][36]. These organisms are generally members of the archaeal Halobacteria class and in the bacterial family of Salinibacteraceae (Rhodothermia class) [37,38][37][38]. Nevertheless, relatively high species richness is noted within each class [39]. Analysis of prokaryotic communities’ compositions in solar salterns in Mexico, Spain, Tunisia, and Turkey showed that the most highly represented genera were Haloquadratum, followed by Halorubrum, Haloarcula, and Halonotius [40,41,42,43][40][41][42][43]. In the crystallizer ponds, which have very low prokaryotic diversity with Archaea making up the dominant fraction [44[44][45],45], the most abundant Archaea observed were Haloquadratum walsbyi and Halorubrum sp. [5]. Salinibacter phylotypes and other Bacteroidetes are highly abundant in the microbial community [44,46,47][44][46][47]. However, in lower salinity ponds, a more diverse assemblage of Archaea and Bacteria was detected [47].
The phytoplankton community of Dinophyta and Diatomeae found in lower salinities was replaced by a community of Dunaliella and Cyanobacteria adapted to higher salt concentrations [9,11,22,48,49,50][9][11][22][48][49][50]. A shift was observed from Diatomeae to Dinophyta dominance along the salinity gradient (from 38 to 86 psu), as exemplified in the Sfax solar saltern in Tunisia [51]. This salinity gradient was negatively correlated with the amount of Diatomeae, while it was positively correlated with the amount of Dinophyta [51]. The Chlorophyta Dunaliella salina and the Cyanobacteria Aphanothece sp. and Phormidium sp. dominated the phytoplanktonic community in the saltiest ponds from 190 to 476 psu [9]. Dunaliella salina is the most ubiquitous eukaryotic microorganism in hypersaline environments [15]. The phytoplankton community in the crystallizer ponds (TS > 300 psu) was entirely composed by D. salina (for the Sfax solar saltern, see [9,30][9][30]). Dunaliella salina release enzymes and nitrogen compounds into the water which favor the growth of halophilic bacteria and, in turn, accelerate evaporation [31]. According to Elloumi et al. [9], the Ciliophora community is dominated by Oligotrichida (Strobilidium sp., Strombidium sp., Tintinnides sp.) at salinities from 41 psu to 46.9 psu. The Prostomatida Urotricha sp. became the dominant taxon at salinity values of 45.6–146.8 psu, but from 184 to 203.2 psu the Ciliophora shifted to a dominance of Heterotrichida (Fabrea salina and Blepharisma sp.), Hymenostomata (Uronema sp.), and Gymnostomatida (Encheylodon sp.).
Among zooplankton groups, Artemiidae (Branchiopoda) and Copepoda are the most abundant groups in hypersaline ecosystems [14,52,53,54,55,56][14][52][53][54][55][56]. In keeping with their fluctuations in abundance along the salinity gradient in the Sfax solar saltern, Copepoda species are split into thalassophilic species (species whose abundance decreases with increasing salinity) and halophilic species (species whose abundance increases with increasing salinity) [12,16,34,51,56][12][16][34][51][56]. At salinities from 157 to 312 psu, the euryhaline Artemia spp. is the only zooplanktonic taxon [14,54,56,57][14][54][56][57].
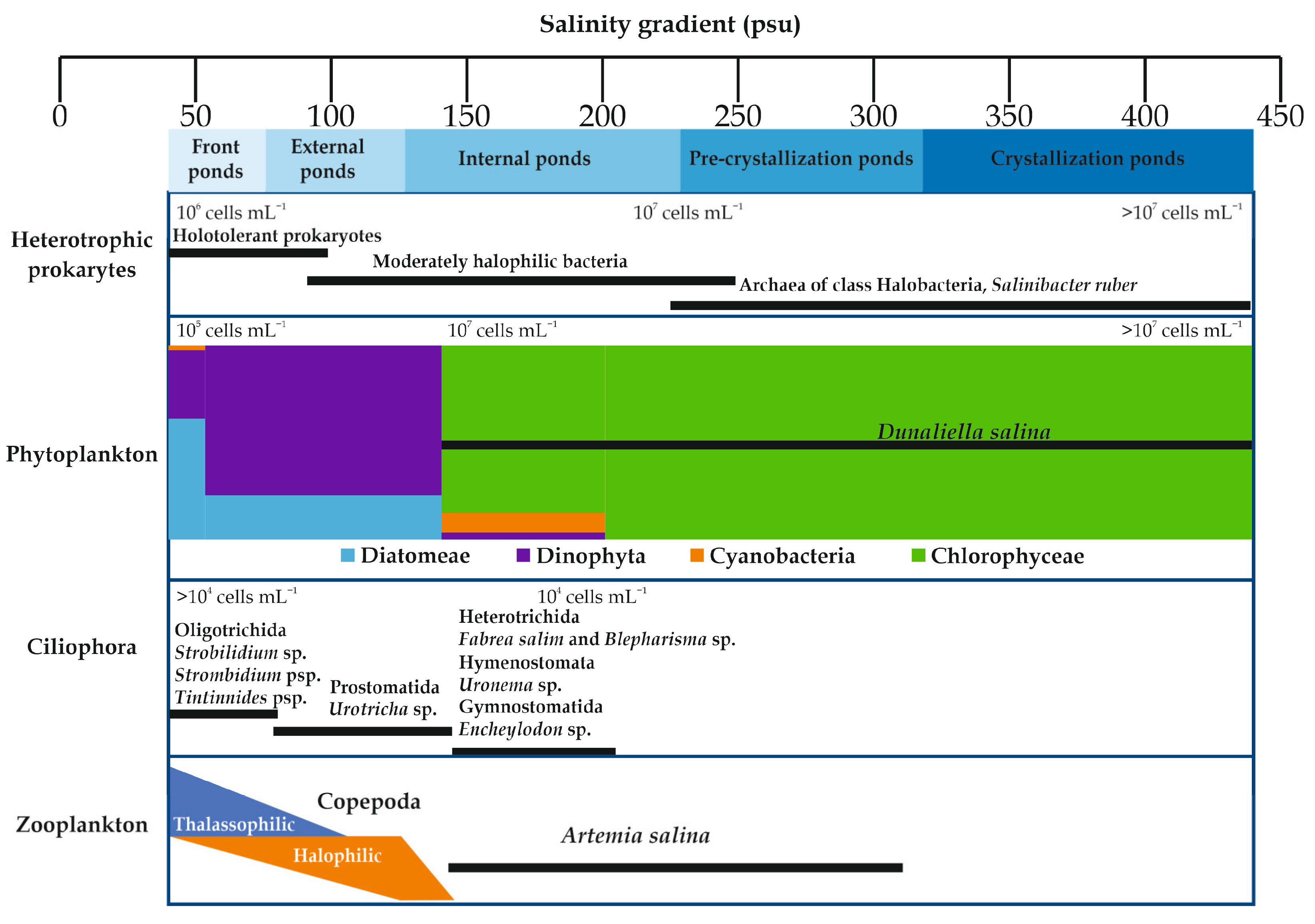

Figure 2. Distribution of the dominant living beings (plankton and heterotrophic prokaryotes) along the salinity gradient in solar salterns.
4. Pollution Diversity in Solar Salterns
Hypersaline environments, including solar salterns and salt marshes, are often polluted with various contaminants [59,60][58][59]. They are fragile econiches and are very susceptible to disturbances [61][60]. Knowledge of pollution existing in solar salterns is not generalizable to every site and the completion of analyses of a great number of sites remains to be achieved (Table 1).
The metal (Cd, Cu, Pb, and Zn) concentrations in the salt marsh sediments of the Karnaphuli River coast (Bangladesh) are related to contamination from domestic and industrial discharges [62][61] (Table 1). The metal concentrations in the sediments were: 105.0 ppm for Zn, 26.70 ppm for Pb, 45.79 ppm for Cu, and 0.43 ppm for Cd [62][61]. The Ribandar solar salterns (India) are fed by the Mandovi Estuary and are, in turn, vulnerable to metal effluent influxes from ferromanganese ore mining activity, barge traffic, and sewage disposal, affecting the water and sediment quality in the salt pan and its inhabitant organisms [63][62]. Sediment quality indicates that the Ribandar solar saltern sediments were moderately contaminated by Co, Fe, Mn, Ni, Pb, and Zn during the salt-making seasons [63][62]. The concentrations of heavy metals in the sediments ranged from a minimum of 1.7 ± 0.1–2.6 ± 0.7 ppm Pb to a maximum of 44 ± 21.6–62.8 ± 23.6 ppm Zn [63][62] (Table 1).
In the Pomorie brine salterns (the Black Sea, Bulgaria), the distribution of Pb and Cu in the three constituents of the brine system, the salt solution, colloidal particles, and biota (Halobacterium salinarium and microalgae Dunaliela salina), showed the highest percentages in biota, at 45% and 48%, respectively [64][63]. However, Cd and Bi have not been detected in biota and are uniformly distributed between the salt solution and colloidal particles [64][63] (Table 1).
A comparison of the concentrations of trace metals in the sediments of three salt marshes (Tinto, Odiel, and Piedras) in Huelva, Spain showed that the Tinto sediments were the most polluted, with high amounts of As (600 ppm), Cu (3300 ppm), and Zn (2500 ppm) [65][64] (Table 1). On the other hand, the Piedras estuary had not been affected by anthropogenic inputs and was the least polluted estuary [65][64].
As an example of pollution and dysfunction in the hypersaline ecosystem, wresearchers present the case of the Sfax solar saltern in Tunisia. Since the 1950s, there has been rapid industrialization and urbanization along the coastal area near the solar saltern. Thus, the treated domestic wastewater (pH 8.3) and the untreated industrial wastewater released diversified pollutants. For example, near the Sfax solar saltern (Tunisia), the phosphate treatment plant, SIAPE, and the gypsum water produced from the lixiviation of SIAPE phosphogypsum deposits are sources of heavy metals which are rapidly precipitated in marine sediments [66,67,68,69][65][66][67][68]. Such a mixture of effluents, which has a pH of 3, facilitated the dissolution of heavy metals, thus enhancing the movement of the bioavailable ionic forms of these metals in seawater.
The brine metal concentrations in the Sfax solar saltern vary from 0.065 to 1.57 mg L−1 for Zn, 0.002–0.034 mg L−1 for Cd, 0.006–0.064 mg L−1 for Cu, and 0.002–0.128 mg L−1 for Pb. The analysed metals are present in the following order: Zn > Pb > Cu > Cd. The computed enrichment factors (EFs) showed significant brine contamination due the impact of industrial particulate fallout highly enriched with heavy metals [70][69]. In fact, concentrations of trace metals in surface sediment samples have shown Fe varying from 8750 to 8889 ppm of dry weight, Zn from 39.92 to 574.89 ppm of dry weight, Pb from 18.98 to 233.46 ppm of dry weight, Ni from 17.47 to 160.92 ppm of dry weight, Cu from 13 to 98 ppm of dry weight, and Cd from 4.86 to 37.42 ppm of dry weight [69][68]. The highest metal concentrations have been found in sites frequently subject to local pollutant sources and in sites often saturated by high-tide marine water which drains industrial waste from the port area. This agrees with the findings of Amdouni [71][70], who reported the presence of a variety of metals (Al, Cd, Cu, Pb, and Zn) in crystallization ponds.
According to Amdouni [72][71], the concentrations of trace elements in brines are affected by the evaporation phenomenon in the same way as those of the major elements. Initially, the evaporation effect is very limited and the behavior of trace elements is under the direct influence of the biological activities which colonize the first ponds of the saline saltern. Thus, the evolution of trace element concentration seems to be controlled only by the evaporation–salt precipitation antagonist effect in the more concentrated brine where biological activity is absent or very limited. However, the concentrations of trace metals such as mercury, copper, zinc, lead, and cadmium in cysts and biomasses of Artemia (Branchiopoda) originating from the Sfax solar saltern are lower than those recorded in other strains, i.e., those from other localities that are already commercialized and used in larval fish feeding [73][72]. The lowest bioaccumulation of trace elements in Artemia was observed at the highest salinity (190 psu) [74][73]. In fact, the bioavailability of elements often decreases with increasing salinity due to trace element complexation [75][74]. Compared to studies carried out at various solar salterns around the world, the presence of Ni seems to be a characteristic novelty, and the order of abundance of the metal concentration is not entirely similar either [62,63][61][62].
Solar salterns and salt marshes may be also contaminated by aliphatic and aromatic hydrocarbons. A hydrocarbon analysis performed in the Sfax solar saltern allowed for the detection of aliphatic hydrocarbons and n-alkanes [76][75]. The total aliphatic hydrocarbon concentrations varied from 92.5 mg. L−1 in the first pond, which has marine characteristics, to 661.1 mg. L−1 in the crystallizer pond [76][75]. The use of n-alkane distribution indices coupled with environmental factors permitted for the assertion of the assumption that a major proportion of the hydrocarbons resulted from eukaryotic and prokaryotic communities and a low proportion of the hydrocarbons might be petrogenic [76][75]. Hydrocarbon extraction and analysis from the Sfax coastal region near the solar saltern showed that the sediments are contaminated by petrogenic aliphatic and aromatic hydrocarbons [77,78,79][76][77][78]. The transportation of oil, shipping and industrial activities, urban runoff, and waste water discharge are the main sources of hydrocarbon contamination in the Sfax coastal zone [77][76].
Table 1. Contaminants recorded in solar salterns.
| Contaminants | Solar Saltern | Fraction | References | ||
|---|---|---|---|---|---|
| Trace metals | Sfax solar saltern (Tunisia) | Surface sediments | [69,80] | [68][79] | |
| Water | [70,72] | [69][71] | |||
| Biota: | Artemia salina | [73] | [72] | ||
| Ribandar solar saltern (India) | Surface sediments | [63] | [62] | ||
| Porteresia Bed, Karnafully coastal area (Bangladesh) | Surface sediments | [62] | [61] | ||
| The Black Sea brine Pomorie salterns, Burgas (Bulgaria) | Water | [64] | [63] | ||
| Biota | Halobacterium salinarium | and microalgae | Dunaliela salina | [64] | [63] |
| Colloidal particles | [64] | [63] | |||
| Tinto, Odiel, and Piedras salt marshes in Huelva (Spain). |
Surface sediments | [65] | [64] | ||
| Hydrocarbons | Sfax solar saltern (Tunisia) | Surface sediments | [76] | [75] | |
| Water | [76] | [75] |
References
- Oren, A. Saltern Evaporation Ponds as Model Systems for the Study of Primary Production Processes under Hypersaline Conditions. Aquat. Microb. Ecol. 2009, 56, 193–204.
- Chung, D.; Kim, H.; Choi, H.S. Fungi in Salterns. J. Microbiol. 2019, 57, 717–724.
- Zafrilla, B.; Martínez-Espinosa, R.M.; Alonso, M.A.; Bonete, M.J. Biodiversity of Archaea and Floral of Two Inland Saltern Ecosystems in the Alto Vinalopó Valley, Spain. Saline Syst. 2010, 6, 10.
- Pedrós-Alió, C. Trophic Ecology of Solar Salterns. In Halophilic Microorganisms; Ventosa, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 33–48. ISBN 978-3-642-05664-2.
- Benlloch, S.; Lopez-Lopez, A.; Casamayor, E.O.; Ovreas, L.; Goddard, V.; Daae, F.L.; Smerdon, G.; Massana, R.; Joint, I.; Thingstad, F.; et al. Prokaryotic Genetic Diversity throughout the Salinity Gradient of a Coastal Solar Saltern. Environ. Microbiol. 2002, 4, 349–360.
- Javor, B. Industrial Microbiology of Solar Salt Production. J. Ind. Microbiol. Biotechnol. 2002, 28, 42–47.
- Cantrell, S.A.; Dianese, J.C.; Fell, J.; Gunde-Cimerman, N.; Zalar, P. Unusual Fungal Niches. Mycologia 2011, 103, 1161–1174.
- Abid, O.; Sellami-Kammoun, A.; Ayadi, H.; Drira, Z.; Bouain, A.; Aleya, L. Biochemical Adaptation of Phytoplankton to Salinity and Nutrient Gradients in a Coastal Solar Saltern, Tunisia. Estuar. Coast. Shelf Sci. 2008, 80, 391–400.
- Elloumi, J.; Guermazi, W.; Ayadi, H.; Bouain, A.; Aleya, L. Abundance and Biomass of Prokaryotic and Eukaryotic Microorganisms Coupled with Environmental Factors in an Arid Multi-Pond Solar Saltern (Sfax, Tunisia). J. Mar. Biol. Assoc. UK 2009, 89, 243–253.
- Guermazi, W.; Ayadi, H.; Aleya, L. Correspondence of the Seasonal Patterns of the Brine Shrimp, Artemia Salina (Leach, 1819) (Anostraca) with Several Environmental Factors in an Arid Solar Saltern (Sfax, Southern Tunisia). Crustaceana 2009, 82, 327–348.
- Madkour, F.F.; Gaballah, M.M. Phytoplankton assemblage of a solar saltern in Port Fouad, Egypt. Oceanologia 2012, 54, 687–700.
- Thabet, R.; Leignel, V.; Ayadi, H.; Tastard, E. Interannual and Seasonal Effects of Environmental Factors on the Zooplankton Distribution in the Solar Saltern of Sfax (South-Western Mediterranean Sea). Cont. Shelf Res. 2018, 165, 1–11.
- Amdouni, R. Chemical Study of Free Brines in the Solar Salt Works of Sfax Saline (SE. Tunisia). In Proceedings of the 8th World Salt Symposium, The Hague, The Netherlands, 7–11 May 2000; Geertman: Arnhem, The Netherlands, 2002; pp. 501–506.
- Toumi, N.; Ayadi, H.; Abid, O.; Carrias, J.-F.; Sime-Ngando, T.; Boukhris, M.; Bouain, A. Zooplankton Distribution in Four Ponds of Different Salinity: A Seasonal Study in the Solar Salterns of Sfax (Tunisia). Hydrobiologia 2005, 534, 1–9.
- Javor, B. Solar Salterns. In Hypersaline Environments; Brock/Springer Series in Contemporary Bioscience; Springer: Berlin/Heidelberg, Germany, 1989; pp. 189–204. ISBN 978-3-642-74372-6.
- Kobbi-Rebai, R.; Annabi-Trabelsi, N.; Khemakhem, H.; Ayadi, H.; Aleya, L. Impacts of Restoration of an Uncontrolled Phosphogypsum Dumpsite on the Seasonal Distribution of Abiotic Variables, Phytoplankton, Copepods, and Ciliates in a Man-Made Solar Saltern. Environ. Monit. Assess. 2013, 185, 2139–2155.
- Romero, I.; Pachés, M.; Martínez-Guijarro, R. Selection of an Indicator to Assess a Highly Modified Saline Ecosystem. Sci. Total Environ. 2019, 693, 133656.
- Sammy, N. Biological Systems in North—Western Australian Solar Salt Fields. In Proceedings of the 6th World Salt Symposium; The Salt Institute: Alexandria, VA, USA, 1983; Volume 1, pp. 207–215.
- Litchfield, C.; Gillevet, P. Microbial Diversity and Complexity in Hypersaline Environments: A Preliminary Assessment. J. Ind. Microbiol. Biotechnol. 2002, 28, 48–55.
- Carpelan, L.H. Hydrobiology of the Alviso Salt Ponds. Ecology 1957, 38, 375.
- Landry, J.C.; Jaccard, J. Chimie Des Eaux Libres Des Marais Salants de Salin-de-Giraud (Sud de La France). Géologie Méditerranéenne 1982, 9, 329–348.
- Khemakhem, H.; Elloumi, J.; Moussa, M.; Aleya, L.; Ayadi, H. The Concept of Ecological Succession Applied to Phytoplankton over Four Consecutive Years in Five Ponds Featuring a Salinity Gradient. Estuar. Coast. Shelf Sci. 2010, 88, 33–44.
- Khemakhem, H.; Elloumi, J.; Ayadi, H.; Aleya, L.; Moussa, M. Modelling the Phytoplankton Dynamics in a Nutrient-Rich Solar Saltern Pond: Predicting the Impact of Restoration and Climate Change. Environ. Sci. Pollut. Res. 2013, 20, 9057–9065.
- Shenbaga Devi, A.; Santhanam, P.; Ananth, S.; Dinesh Kumar, S. Distribution of Phytoplankton in Selected Salt Pans of Tamil Nadu, Southeast Coast of India. In Basic and Applied Phytoplankton Biology; Santhanam, P., Begum, A., Pachiappan, P., Eds.; Springer: Singapore, 2019; pp. 251–276. ISBN 978-981-10-7937-5.
- Amat Doménech, F. Bioecología de Artemia (Crustácea, Branchiopoda) en la Laguna de la Mata, Torrevieja, Alicante; Instituto de Cultura “Juan Gil-Albert”, Diputación de Alicante: Alicante, Spain, 1991; ISBN 978-84-7784-996-4.
- Hochstein, L.I.; Tomlinson, G.A. Denitrification by Extremely Halophilic Bacteria. FEMS Microbiol. Lett. 1985, 27, 329–331.
- Davis, J.S. Structure, Function, and Management of the Biological System for Seasonal Solar Saltworks. Glob. Nest J. 2000, 2, 217–226.
- Benlloch, S.; Acinas, S.G.; Martínez-Murcia, A.J.; Rodríguez-Valera, F. Description of Prokaryotic Biodiversity along the Salinity Gradient of a Multipond Solar Saltern by Direct PCR Amplification of 16S RDNA. In Coastal Lagoon Eutrophication and ANaerobic Processes (C.L.E.AN.); Caumette, P., Castel, J., Herbert, R., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 19–31. ISBN 978-94-010-7279-3.
- Joint, I.; Henriksen, P.; Garde, K.; Riemann, B. Primary Production, Nutrient Assimilation and Microzooplankton Grazing along a Hypersaline Gradient. FEMS Microbiol. Ecol. 2002, 39, 245–257.
- Ayadi, H.; Abid, O.; Elloumi, J.; Bouaïn, A.; Sime-Ngando, T. Structure of the Phytoplankton Communities in Two Lagoons of Different Salinity in the Sfax Saltern (Tunisia). J. Plankton Res. 2004, 26, 669–679.
- Mohebbi, F. The Brine Shrimp Artemia and Hypersaline Environments Microalgal Composition: A Mutual Interaction. Int. J. Aquat. Sci. 2010, 1, 19–27.
- Javor, B.J. Planktonic Standing Crop and Nutrients in a Saltern Ecosystem. Limnol. Oceanogr. 1983, 28, 153–159.
- Oren, A. A Hundred Years of Dunaliella Research: 1905–2005. Saline Syst. 2005, 1, 2.
- Kobbi-Rebai, R.; Annabi-Trabelsi, N.; Al-Jutaili, S.; Al-Enezi, Y.; Subrahmanyam, M.N.V.; Ali, M.; Belmonte, G.; Ayadi, H. Abundance and Reproduction Variables of Two Species of Harpacticoid Copepods along an Increasing Salinity Gradient. Aquat. Ecol. 2020, 54, 387–400.
- Pedrós-Alió, C. Diversity of Microbial Communities: The Case of Solar Salterns. In Adaptation to Life at High Salt Concentrations in Archaea, Bacteria, and Eukarya; Gunde-Cimerman, N., Oren, A., Plemenitaš, A., Eds.; Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 9, pp. 71–90. ISBN 978-1-4020-3632-3.
- López-López, A.; Yarza, P.; Richter, M.; Suárez-Suárez, A.; Antón, J.; Niemann, H.; Rosselló-Móra, R. Extremely Halophilic Microbial Communities in Anaerobic Sediments from a Solar Saltern. Environ. Microbiol. Rep. 2010, 2, 258–271.
- Antón, J.; Rosselló-Mora, R.; Rodríguez-Valera, F.; Amann, R. Extremely Halophilic Bacteria in Crystallizer Ponds from Solar Salterns. Appl. Environ. Microbiol. 2000, 66, 3052–3057.
- Del Mora-Ruiz, M.R.; Cifuentes, A.; Font-Verdera, F.; Pérez-Fernández, C.; Farias, M.E.; González, B.; Orfila, A.; Rosselló-Móra, R. Biogeographical Patterns of Bacterial and Archaeal Communities from Distant Hypersaline Environments. Syst. Appl. Microbiol. 2018, 41, 139–150.
- Viver, T.; Orellana, L.; González-Torres, P.; Díaz, S.; Urdiain, M.; Farías, M.E.; Benes, V.; Kaempfer, P.; Shahinpei, A.; Ali Amoozegar, M.; et al. Genomic Comparison between Members of the Salinibacteraceae Family, and Description of a New Species of Salinibacter (Salinibacter altiplanensis sp. nov.) Isolated from High Altitude Hypersaline Environments of the Argentinian Altiplano. Syst. Appl. Microbiol. 2018, 41, 198–212.
- Baati, H.; Guermazi, S.; Amdouni, R.; Gharsallah, N.; Sghir, A.; Ammar, E. Prokaryotic Diversity of a Tunisian Multipond Solar Saltern. Extremophiles 2008, 12, 505–518.
- Dillon, J.G.; Carlin, M.; Gutierrez, A.; Nguyen, V.; McLain, N. Patterns of Microbial Diversity along a Salinity Gradient in the Guerrero Negro Solar Saltern, Baja CA Sur, Mexico. Front. Microbiol. 2013, 4, 399.
- Fernández, A.B.; León, M.J.; Vera, B.; Sánchez-Porro, C.; Ventosa, A. Metagenomic Sequence of Prokaryotic Microbiota from an Intermediate-Salinity Pond of a Saltern in Isla Cristina, Spain. Genome Announc. 2014, 2, e00045-14.
- Çınar, S.; Mutlu, M.B. Comparative Analysis of Prokaryotic Diversity in Solar Salterns in Eastern Anatolia (Turkey). Extremophiles 2016, 20, 589–601.
- Antón, J.; Peña, A.; Santos, F.; Martínez-García, M.; Schmitt-Kopplin, P.; Rosselló-Mora, R. Distribution, Abundance and Diversity of the Extremely Halophilic Bacterium Salinibacter Ruber. Saline Syst. 2008, 4, 15.
- Boujelben, I.; Gomariz, M.; Martínez-García, M.; Santos, F.; Peña, A.; López, C.; Antón, J.; Maalej, S. Spatial and Seasonal Prokaryotic Community Dynamics in Ponds of Increasing Salinity of Sfax Solar Saltern in Tunisia. Antonie Van Leeuwenhoek 2012, 101, 845–857.
- Antón, J.; Oren, A.; Benlloch, S.; Rodríguez-Valera, F.; Amann, R.; Rosselló-Mora, R. Salinibacter Ruber Gen. Nov., Sp. Nov., a Novel, Extremely Halophilic Member of the Bacteria from Saltern Crystallizer Ponds. Int. J. Syst. Evol. Microbiol. 2002, 52, 485–491.
- Øvreås, L.; Daae, F.L.; Torsvik, V.; Rodriguez-Valera, F. Characterization of Microbial Diversity in Hypersaline Environments by Melting Profiles and Reassociation Kinetics in Combination with Terminal Restriction Fragment Length Polymorphism (T-RFLP). Microb. Ecol. 2003, 46, 291–301.
- Estrada, M.; Henriksen, P.; Gasol, J.M.; Casamayor, E.O.; Pedrós-Alió, C. Diversity of Planktonic Photoautotrophic Microorganisms along a Salinity Gradient as Depicted by Microscopy, Flow Cytometry, Pigment Analysis and DNA-Based Methods. FEMS Microbiol. Ecol. 2004, 49, 281–293.
- Carré-Mlouka, A. Shaping Microbial Communities in Changing Environments: The Paradigm of Solar Salterns. In Extreme Environments; CRC Press: Boca Raton, FL, USA, 2021; p. 19.
- Hinzano, S.M.; Okalo, F.A.; Ngarari, M.M.; Opiyo, M.A.; Ogello, E.O.; Fulanda, A.M.; Odiwour, D.O.; Nyonje, B. Phytoplankton Distribution along a Salinity Gradient in Two Kenyan Saltworks (Tana and Kurawa). West. Indian Ocean J. Mar. Sci. 2022, 21, 113–124.
- Annabi-Trabelsi, N.; Kobbi-Rebai, R.; Al-Enezi, Y.; Ali, M.; Subrahmanyam, M.N.V.; Belmonte, G.; Ayadi, H. Factors Affecting Oithona nana and Oithona similis along a Salinity Gradient. Mediterr. Mar. Sci. 2021, 22, 552.
- Alonso, M. Anostraca, Cladocera and Copepoda of Spanish Saline Lakes. Hydrobiologia 1990, 197, 221–231.
- Torrentera, L.; Dodson, S.I. Ecology of the Brine Shrimp Artemia in the Yucatan, Mexico, Salterns. J. Plankton Res. 2004, 26, 617–624.
- Guermazi, W.; Elloumi, J.; Ayadi, H.; Bouain, A.; Aleya, L. Coupling Changes in Fatty Acid and Protein Composition of Artemia salina with Environmental Factors in the Sfax Solar Saltern (Tunisia). Aquat. Living Resour. 2008, 21, 63–73.
- Ghannay, S.; Khemakhem, H.; Ayadi, H.; Elloumi, J. Spatial Distribution and Community Structure of Phytoplankton, Ciliates and Zooplankton Coupled to Environmental Factors in the Sousse Saltern (Sahel of Tunisia). Afr. J. Mar. Sci. 2015, 37, 53–64.
- Ladhar, C.; Tastard, E.; Casse, N.; Denis, F.; Ayadi, H. Strong and Stable Environmental Structuring of the Zooplankton Communities in Interconnected Salt Ponds. Hydrobiologia 2015, 743, 1–13.
- Mitchell, B.D.; Geddes, M.C. Distribution of the Brine Shrimps Parartemia zietziana Sayce and Artemia salina (L.) along a Salinity and Oxygen Gradient in a South Australian Saltfield. Freshw. Biol. 1977, 7, 461–467.
- Le Borgne, S.; Paniagua, D.; Vazquez-Duhalt, R. Biodegradation of Organic Pollutants by Halophilic Bacteria and Archaea. Microb. Physiol. 2008, 15, 74–92.
- Fathepure, B.Z. Recent Studies in Microbial Degradation of Petroleum Hydrocarbons in Hypersaline Environments. Front. Microbiol. 2014, 5, 173.
- Naik, M.M.; Dubey, S.K. (Eds.) Marine Pollution and Microbial Remediation, 1st ed.; Springer: Singapore, 2017; ISBN 978-981-10-1044-6.
- Siddique, M.A.M.; Aktar, M. Heavy Metals in Salt Marsh Sediments of Porteresia Bed along the Karnafully River Coast, Chittagong. Soil Water Res. 2012, 7, 117–123.
- Pereira, F.; Kerkar, S.; Krishnan, K.P. Bacterial Response to Dynamic Metal Concentrations in the Surface Sediments of a Solar Saltern (Goa, India). Environ. Monit. Assess. 2013, 185, 3625–3636.
- Bozhkov, O.; Tzvetkova, C.; Russeva, E. Distribution and Determination of Pb, Cd, Bi and Cu in the Sea Brine System: Solution—Colloidal Particles—Biota. Ann. Chim. 2006, 96, 435–442.
- Mesa, J.; Mateos-Naranjo, E.; Pajuelo, E.; Caviedes, M.Á.; Rodríguez-Llorente, I.D. Heavy Metal Pollution Structures Soil Bacterial Community Dynamics in SW Spain Polluted Salt Marshes. Water. Air Soil Pollut. 2016, 227, 466.
- Azri, C.; Maalej, A.; Tlili, A.; Medhioub, K. Characterization of the Atmospheric Pollution Level in Sfax (Tunisia): Influence of Sources and Meteorological Factors. Technol. Sci. Méthodes Génie Urbain Génie Rural. 2002, 1, 78–92.
- Gargouri, D.; Azri, C.; Serbaji, M.M.; Jedoui, Y.; Montacer, M. Heavy Metal Concentrations in the Surface Marine Sediments of Sfax Coast, Tunisia. Environ. Monit. Assess. 2011, 175, 519–530.
- Nedia, G.; Chafai, A.; Moncef, S.M.; Chokri, Y. Spatial Distribution of Heavy Metals in the Coastal Zone of “Sfax-Kerkennah” Plateau, Tunisia. Environ. Prog. Sustain. Energy 2011, 30, 221–233.
- Bahloul, M.; Baati, H.; Amdouni, R.; Azri, C. Assessment of Heavy Metals Contamination and Their Potential Toxicity in the Surface Sediments of Sfax Solar Saltern, Tunisia. Environ. Earth Sci. 2018, 77, 27.
- Baati, H.; Bahloul, M.; Amdouni, R.; Azri, C. Behavior Assessment of Moderately Halophilic Bacteria in Brines Highly Enriched with Heavy Metals: Sfax Solar Saltern (Tunisia), A Case Study. Geomicrobiol. J. 2022, 39, 341–351.
- Amdouni, R. Etude Geochimique Des Saumures Libres, Des Sediments et Des Sels Dans Les Marais Salants de La Saline de Sfax (Tunisie). Ph.D. Thesis, Université Paris Diderot Paris 7, Paris, France, 1990.
- Amdouni, R. Behaviour of Trace Elements during the Natural Evaporation of Sea Water: Case of Solar Salt Works of Sfax Saline (S. E. of Tunisia). Glob. Nest J. 2009, 11, 96–105.
- Aloui, N.; Amorri, M.; Azaza, M.; Chouba, L. Study of Trace Metals (Hg, Cd, Pb, Cu, and Zn) in Cysts and Biomass of Artemia salina (Linnaeus, 1758) (Branchiopoda, Anostraca) from the Salt Work of Sfax (Tunisia). Crustaceana 2012, 85, 1–10.
- Pais-Costa, A.J.; Sánchez, M.I.; Taggart, M.A.; Green, A.J.; Hortas, F.; Vinagre, P.A.; Marques, J.C.; Martinez-Haro, M. Trace Element Bioaccumulation in Hypersaline Ecosystems and Implications of a Global Invasion. Sci. Total Environ. 2021, 800, 149349.
- Nieto, J.M.; Sarmiento, A.M.; Olías, M.; Canovas, C.R.; Riba, I.; Kalman, J.; Delvalls, T.A. Acid Mine Drainage Pollution in the Tinto and Odiel Rivers (Iberian Pyrite Belt, SW Spain) and Bioavailability of the Transported Metals to the Huelva Estuary. Environ. Int. 2007, 33, 445–455.
- Elloumi, J.; Guermazi, W.; Ayadi, H.; Bouaïn, A.; Aleya, L. Detection of Water and Sediments Pollution of An Arid Saltern (Sfax, Tunisia) by Coupling the Distribution of Microorganisms with Hydrocarbons. Water. Air Soil Pollut. 2008, 187, 157–171.
- Zaghden, H.; Kallel, M.; Louati, A.; Elleuch, B.; Oudot, J.; Saliot, A. Hydrocarbons in Surface Sediments from the Sfax Coastal Zone, (Tunisia) Mediterranean Sea. Mar. Pollut. Bull. 2005, 50, 1287–1294.
- Zaghden, H.; Kallel, M.; Elleuch, B.; Oudot, J.; Saliot, A. Sources and Distribution of Aliphatic and Polyaromatic Hydrocarbons in Sediments of Sfax, Tunisia, Mediterranean Sea. Mar. Chem. 2007, 105, 70–89.
- Zaghden, H.; Kallel, M.; Elleuch, B.; Oudot, J.; Saliot, A.; Sayadi, S. Evaluation of Hydrocarbon Pollution in Marine Sediments of Sfax Coastal Areas from the Gabes Gulf of Tunisia, Mediterranean Sea. Environ. Earth Sci. 2014, 72, 1073–1082.
- Cherif, F.; Ben Hmid, R.; Frikha, I.; Omar, T.; Choura, M. Assessment of Heavy Metal Contamination in the Subsurface Sediment of the Southern Coastal Zone of Sfax, Tunisia. J. Coast. Conserv. 2020, 24, 52.
More
