Grasslands form a large and essential part of terrestrial ecosystems in terms of occupied areas as well as for both biodiversity maintenance and functional importance. Among different grassland types, coastal grasslands are unique in that they are habitats where the influence of marine and terrestrial determining factors combine. Most importantly, interactions between plant and microbial diversity were recognized as the main driving force in carbon storage. There have been more attempts to make functional generalizations of grassland existence related to changes or gradients of environmental factors. For example, the belowground characteristics of plants—the presence of clonal growth organs, vegetative buds, fine root spread—have been related to the degree of water availability in grasslands. There has been an increasing awareness of the fact that precisely functional interactions involving plants and their diversity are important drivers of plant distribution and multiple ecosystem services in grasslands.
- arbuscular mycorrhiza
- ecosystem services
- coastal grasslands
- resilience
- mycorrhizal symbiosis
1. Mycorrhizal vs. Non-Mycorrhizal Plants
2. Mycorrhizal Fungal Community Structure
3. Mycorrhizal Symbiosis in Resource Acquisition
4. Common Mycorrhizal Networks
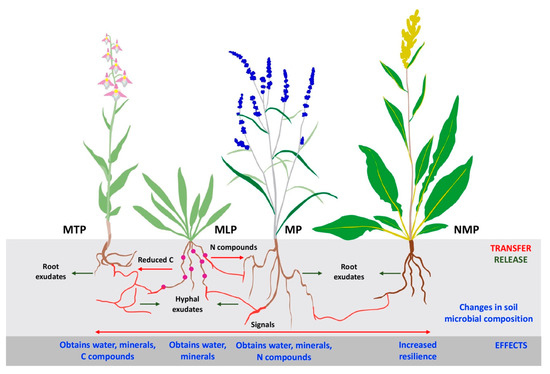
5. Mycorrhizal Symbiosis in Environmental Resilience
References
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142.
- Berger, F.; Gutjahr, C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994.
- Sportes, A.; Hériché, M.; Boussageon, R.; Noceto, P.-A.; van Tuinen, D.; Wipf, D.; Courty, P.E. A historical perspective on mycorrhizal mutualism emphasizing arbuscular mycorrhizas and their emerging challenges. Mycorrhiza 2021, 31, 637–653.
- Druva-Lusite, I.; Ievinsh, G. Diversity of arbuscular mycorrhizal symbiosis in plants from coastal habitats. Environ. Exp. Biol. 2010, 8, 17–34.
- Mason, E. Note on the presence of mycorrhiza in the roots of salt marsh plants. New Phytol. 1928, 27, 193–195.
- Hildebrandt, U.; Janetta, K.; Ouziad, F.; Renne, B.; Nawrath, K.; Bothe, H. Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza 2001, 10, 175–183.
- Soudzilovskaia, N.A.; Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.; Merckx, V.; Tedersoo, L. FungalRoot: Global online database of plant mycorrhizal associations. New Phytol. 2020, 227, 955–966.
- Karlsons, A.; Druva-Lusite, I.; Osvalde, A.; Necajeva, J.; Andersone-Ozola, U.; Samsone, I.; Ievinsh, G. Adaptation strategies of rare plant species to heterogeneous soil conditions on a coast of a lagoon lake as revealed by analysis of mycorrhizal symbiosis and mineral constituent dynamics. Environ. Exp. Biol. 2017, 15, 113–126.
- Öpik, M.; Moora, M.; Liira, J.; Zobel, M. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J. Ecol. 2006, 94, 778–790.
- Bothe, H. Arbuscular mycorrhiza and salt tolerance of plants. Symbiosis 2012, 58, 7–16.
- Rosendahl, S.; Stukenbrock, E.H. Community structure of arbsicualr mycorrhizal fungi in undisturbed vegetation revealed by analyses of LSU rDNA sequences. Mol. Ecol. 2004, 13, 3179–3186.
- Stukenbrock, E.H.; Rosendahl, S. Distribution of dominant arbuscular mycorrhizal fungi among five plant speccies in undisturbed vegetation of a coastal grassland. Mycorrhiza 2005, 15, 497–503.
- Cui, X.; Hu, J.; Wang, J.; Yang, J.; Lin, X. Reclamation negatively influences arbuscular mycorrhizal fungal community structure and diversity in coastal saline-alkaline land in Eastern China as revealed by Illumina sequencing. Appl. Soil Ecol. 2016, 98, 140–149.
- Guo, X.; Gong, J. Differential effects of abiotic factors and host plant traits on diversity and community composition of root-colonizing arbuscular mycorrhizal fungi in a salt-stressed ecosystem. Mycorrhiza 2014, 24, 79–94.
- Kawahara, A.; Ezawa, T. Characterization of arbuscular mycorrhizal fungal communities with respect to zonal vegetation in a coastal dune ecosystem. Oecologia 2013, 173, 533–543.
- Munkvold, L.; Kjøller, R.; Vestberg, M.; Rosendahl, S.; Jakobsen, I. High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol. 2004, 164, 357–364.
- Selosse, M.-A.; Richard, F.; He, X.; Simard, S.W. Mycorrhizal networks: Des liaisons dangereuses? Trends Ecol. Evol. 2006, 21, 621–628.
- Barto, E.K.; Weidenhamer, J.D.; Cipollini, D.; Rillig, M.C. Fungal superhighways: Do common mycorrhizal networks enhance below ground communication? Trends Plant Sci. 2012, 17, 633–637.
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585.
- Mariotte, P.; Meugnier, C.; Johnson, D.; Thébault, A.; Spiegelberger, T.; Buttler, A. Arbuscular mycorrhizal fungi reduce the differences in competitiveness between dominant and subordinate plant species. Mycorrhiza 2013, 23, 267–277.
- Friede, M.; Unger, S.; Hellmann, C.; Beyschlag, W. Conditions Promoting Mycorrhizal Parasitism Are of Minor Importance for Competitive Interactions in Two Differentially Mycotrophic Species. Front. Plant Sci. 2016, 7, 1465.
- Simard, S.W.; Durall, D.M. Mycorrhizal networks: A review of their extent, function, and importance. Can. J. Bot. 2004, 82, 1140–1165.
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60.
- Gorzelak, M.A.; Asay, A.K.; Pickles, B.J.; Simard, S.W. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 2015, 7, plv050.
- Molina, R.; Horton, T.R. Mycorrhiza specificity: Its role in the development and function of common mycelial networks. In Mycorrhizal Networks; Horton, T.R., Ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 2015; pp. 1–39.
- Gilbert, L.; Johnson, D. Plant–plant communication through common mycorrhizal networks. In Advances in Botanical Research; Becard, G., Ed.; How Plants Communicate with their Biotic Environment; Academic Press: London, UK, 2017; Volume 82, pp. 83–97.
- Bidartondo, M.I. The evolutionary ecology of myco-heterotrophy. New Phytol. 2005, 167, 335–352.
- Wang, Y.; He, X.; Yu, F. Non-host plants: Are they mycorrhizal networks players? Plant Divers. 2022, 44, 127–134.
- Boyno, G.; Demir, S. Plant-mycorrhizal communication and mycorrhizae in inter-plant communication. Symbiosis 2022, 86, 155–168.
- Song, Y.; Zeng, R.S.; Xu, J.F.; Li, J.; Shen, X.; Yihdego, W.G. Interplant Communication of Tomato Plants through Underground Common Mycorrhizal Networks. PLoS ONE 2010, 5, e13324.
- Wu, P.; Wu, Y.; Liu, C.-C.; Liu, L.-W.; Ma, F.-F.; Wu, X.-Y.; Wu, M.; Hang, Y.-Y.; Chen, J.-Q.; Shao, Z.-Q.; et al. Identification of Arbuscular Mycorrhiza (AM)-Responsive microRNAs in Tomato. Front. Plant Sci. 2016, 7, 429.
- Couzigou, J.-M.; Lauressergues, D.; André, O.; Gutjahr, C.; Bruno Guillotin, B.; Bécard, G.; Combier, J.-P. Positive gene regulation by a natural protective miRNA enables arbuscular mycorrhizal symbiosis. Cell Host Microbe 2017, 21, 106–112.
- Šečić, E.; Kogel, K.-H.; Ladera-Carmona, M.J. Biotic stress-associated microRNA families in plants. J. Plant Physiol. 2021, 263, 153451.
- Zhang, L.; Zou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular mycorrhizal fungi conducting the hyposphere bacterial orchestra. Trends Plant Sci. 2022, 27, 402–411.
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379.
- Aliasgharzadeh, N.; Rastin, S.N.; Towfighi, H.; Alizadeh, A. Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza 2001, 11, 119–122.
- Sonjak, S.; Udovič, M.; Wraber, T.; Likar, M.; Regvar, M. Diversity of halophytes and identification of arbuscular mycorrhizal fungi colonising their roots in an abandoned and sustained part of Sečovlje salterns. Soil Biol. Biochem. 2009, 41, 1847–1856.
- Füzy, A.; Biró, B.; Tóth, T. Effect of saline soil parameters on endomycorrhizal colonisation of dominant halophytes in four Hungarian sites. Span. J. Agric. Res. 2010, 8, 144.
- Füzy, A.; Biró, B.; Tóth, T.; Hildebrandt, U.; Bothe, H. Drought, but not salinity, determines the apparent effectiveness of halophytes colonized by arbuscular mycorrhizal fungi. J. Plant Physiol. 2008, 165, 1181–1192.
- Carvalho, L.M.; Correia, P.M.; Caçador, I.; Martins-Loução, M.A. Effects of salinity and flooding on the infectivity of salt marsh arbuscular mycorrhizal fungi in Aster tripolium L. Biol. Fertil. Soils 2003, 38, 137–143.
- Tian, C.; Feng, G.; Li, X.; Zhang, F. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl. Soil Ecol. 2004, 26, 143–148.
- Borde, M.; Dudhane, M.; Jite, P. Growth photosynthetic activity and antioxidant responses of mycorrhizal and non-mycorrhizal bajra (Pennisetum glaucum) crop under salinity stress condition. Crop. Prot. 2011, 30, 265–271.
- Estrada, B.; Aroca, R.; Barea, J.M.; Ruiz-Lozano, J.M. Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci. 2013, 201–202, 42–51.
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31.
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 96, 114–121.
- Rahman, M.; Ali, M.; Alam, F.; Banu, M.; Anik, M.; Bhuiyan, M. Effect of Arbuscular Mycorrhizal Fungi on Germination, Nodulation and Sporulation of Lentil (Lens culinaris) at Different NaCl Levels. Bangladesh J. Microbiol. 2017, 34, 73–81.
- Elhindi, K.M.; El-Din, A.S.; Elgorban, A. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci. 2017, 24, 170–179.
- Dashtebani, F.; Hajiboland, R.; Aliasgharzad, N. Characterization of salt-tolerance mechanisms in mycorrhizal (Claroideoglomus etunicatum) halophytic grass, Puccinellia distans. Acta Physiol. Plant. 2014, 36, 1713–1726.
- Hammer, E.C.; Nasr, H.; Pallon, J.; Olsson, P.A.; Wallander, H. Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 2011, 21, 117–129.
- Shokri, S.; Maadi, B. Effects of Arbuscular Mycorrhizal Fungus on the Mineral Nutrition and Yield of Trifolium alexandrinum Plants under Salinity Stress. J. Agron. 2009, 8, 79–83.
- Camprubí, A.; Abril, M.; Estaún, V.; Calvet, C. Contribution of arbuscular mycorrhizal symbiosis to the survival of psammophilic plants after sea water flooding. Plant Soil 2012, 351, 97–105.
- Hartmond, U.; Schaesberg, N.V.; Graham, J.H.; Syvertsen, J.P. Salinity and flooding stress effects on mycorrhizal and non-mycorrhizal citrus rootstock seedlings. Plant Soil 1987, 104, 37–43.
- Rutto, K.L.; Mizutani, F.; Kadoya, K. Effect of root-zone flooding on mycorrhizal and non-mycorrhizal peach (Prunus persica Batsch) seedlings. Sci. Hortic. 2002, 94, 285–295.
- Zheng, F.-L.; Liang, S.-M.; Chu, X.-N.; Yang, Y.-L.; Wu, Q.-S. Mycorrhizal fungi enhance flooding tolerance of peach through inducing proline accumulation and improving root architecture. Plant Soil Environ. 2020, 66, 624–631.
- Fougnies, L.; Renciot, S.; Müller, F.; Plenchette, C.; Prin, Y.; De Faria, S.M.; Bouvet, J.M.; Sylla, S.N.; Dreyfus, B.; Bâ, A.M. Arbuscular mycorrhizal colonization and nodulation improve flooding tolerance in Pterocarpus officinalis Jacq. seedlings. Mycorrhiza 2007, 17, 159–166.
- Wang, Y.; Bao, X.; Li, S. Effects of Arbuscular Mycorrhizal Fungi on Rice Growth Under Different Flooding and Shading Regimes. Front. Microbiol. 2021, 12, 756752.
- Miller, S.P.; Sharitz, R.R. Manipulation of flooding and arbuscular mycorrhiza formation influences growth and nutrition of two semiaquatic grass species. Funct. Ecol. 2000, 14, 738–748.
- Miller, S.P. Arbuscular mycorrhizal colonization of semi-aquatic grasses along a wide hydrologic gradient. New Phytol. 2000, 145, 145–155.
- Ellis, J.R. Post Flood Syndrome and Vesicular-Arbuscular Mycorrhizal Fungi. J. Prod. Agric. 1998, 11, 200–204.
- Vallino, M.; Fiorilli, V.; Bonfante, P. Rice flooding negatively impacts root branching and arbuscular mycorrhizal colonization, but not fungal viability. Plant Cell Environ. 2014, 37, 557–572.
- Neto, D.; Carvalho, L.M.; Cruz, C.; Martins-Loução, M.A. How do mycorrhizas affect C and N relationships in flooded Aster tripolium plants? Plant Soil 2006, 279, 51–63.
- Wang, Y.; Huang, Y.; Qiu, Q.; Xin, G.; Yang, Z.; Shi, S. Flooding Greatly Affects the Diversity of Arbuscular Mycorrhizal Fungi Communities in the Roots of Wetland Plants. PLoS ONE 2011, 6, e24512.
- Wang, Y.; Li, Y.; Bao, X.; Björn, L.O.; Li, S.; Olsson, P.A. Response differences of arbuscular mycorrhizal fungi communities in the roots of an aquatic and a semiaquatic species to various flooding regimes. Plant Soil 2016, 403, 361–373.
- Huang, G.-M.; Srivastava, A.K.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Exploring arbuscular mycorrhizal symbiosis in wetland plants with a focus on human impacts. Symbiosis 2021, 84, 311–320.
- Deepika, S.; Kothamasi, D. Soil moisture—A regulator of arbuscular mycorrhizal fungal community assembly and symbiotic phosphorus uptake. Mycorrhiza 2015, 25, 67–75.
- Cheng, S.; Zou, Y.-N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Elucidating the Mechanisms Underlying Enhanced Drought Tolerance in Plants Mediated by Arbuscular Mycorrhizal Fungi. Front. Microbiol. 2021, 12, 809473.
- Wang, Y.; Zou, Y.-N.; Shu, B.; Wu, Q.-S. Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Sci. Hortic. 2023, 308, 111591.
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398.
