Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Tiago Santos.
Sepsis is a clinical syndrome characterized by physiological, pathological, and biochemical abnormalities induced by an invading pathogen, causing dysregulated host immune response and resulting as ultimately responsible for life-threatening organ dysfunction. It is a leading cause of morbidity and mortality, affecting all age groups and representing a significant global burden.
- sepsis
- diagnostic
- treatment
- drug delivery
- nanomedicine
- nanotechnology
1. Introduction
Sepsis is a clinical syndrome characterized by physiological, pathological, and biochemical abnormalities induced by an invading pathogen, causing dysregulated host immune response and resulting as ultimately responsible for life-threatening organ dysfunction. It is a leading cause of morbidity and mortality, affecting all age groups and representing a significant global burden [1,2,3][1][2][3]. Clinical outcomes in patients admitted due to sepsis mainly depend on timely diagnosis and appropriate early therapeutic intervention.
Various consensus meetings have been held in recent decades to better define sepsis as a clinical entity. In the 1990s, sepsis was characterized by a systemic inflammatory response syndrome that, when complicated by organ dysfunction, was termed “severe sepsis” and could progress to septic shock [4,5][4][5]. Despite the limitations of these definitions and the attempts to identify precise diagnostic criteria, the definitions of sepsis, septic shock, and organ dysfunction have remained mainly unchanged. To unify these concepts, the Sepsis-3 meeting recently defined sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection, emphasizing the primacy of a nonhomeostatic host response, the potential lethality, and the need for early recognition [5]. Even a modest degree of organ dysfunction is associated with an in-hospital mortality excess of 10% [5]. Despite advancements in the understanding of sepsis as a clinical entity and its pathophysiology, it remains a common condition with no approved specific molecular therapies and significant mortality [6,7,8][6][7][8]. Controversy continues to surround nearly every variable in the management of sepsis. At the same time, clinical trials fail to show significant results in the attempts to normalize or enhance various aspects of the physiology of these patients [9].
Nanotechnology is considered one of the most promising technologies of the 21st century and refers to the design and use of technologies at the nanoscale. These structures have unique physical, chemical, and biological properties that can be of interest in the engineering of devices and diagnostic systems, as well as for treating some medical conditions. The possibility to design nanostructures with specific characteristics such as size, shape, elasticity, surface charge, and functionalization allows its application in biomedical areas ranging from drug delivery, vaccine, and antibacterial drug development to diagnosis and imaging tools [10,11][10][11].
2. Pathophysiology of Sepsis
Sepsis is fundamentally an inflammatory disease consisting of short-term hyperinflammatory and longer-term immunosuppressive phases [17][12]. In an early establishment phase, a “cytokine storm” induces an overwhelming inflammatory reaction, resulting in high fevers and refractory shock that can be followed by cardiac and pulmonary failure [17][12]. This initial phase of the disease is responsible for the highest death rates. After that, exhaustion of the immune system, immune cell dysfunction, and apoptosis cause persistent immunosuppression, resulting in organ damage and failure and late-period mortality [18][13]. The triggering event is the recognition of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). The recognition of these and other microbial-derived products by epithelial and endothelial cell populations triggers a complex intracellular signaling system that promotes inflammation. The microbially derived molecules and the signaling pathways activated regulate the intensity and direction of the inflammatory response. Simultaneously, the activation of the complement system and production of pro-inflammatory cytokines profoundly affects coagulation and vascular endothelium function. During the establishment of sepsis, the expression of adhesion molecules, including pro-coagulant and anti-coagulant proteins, is significantly altered, resulting in the transition of the epithelium from an anti-coagulant to a pro-coagulant state. The overactivation of complement system mediators is also associated with the generation of elevated levels of reactive oxygen species and the release of granular enzymes, causing significant inflammatory tissue damage. These mechanisms are believed to contribute to vasodilation, tissue damage, and multiple organ failure in acute infection (as reviewed in [19][14]). Although early systemic inflammatory response is considered a hallmark of sepsis, immunosuppression is usually observed in these patients. Surviving patients exhibit persistent inflammation/immunosuppression and catabolism syndrome [20,21][15][16]. The main features of this clinical syndrome are markedly increased C-reactive protein (CRP) concentrations, neutrophilia, and the release of immature myeloid cells [22][17]. Immature myeloid cells have defective antimicrobial activity and produce anti-inflammatory cytokines when mobilized to circulation, downregulating the inflammation and resulting in functional immunosuppression. Although the etiology of this entity is unknown, it is likely driven by DAMPs produced by injured tissues and organs [23][18].3. Nanotechnology at a Glance
Nanotechnology is a complex field encompassing solid-state physics, materials science, surface chemistry, and quantum mechanics. Nanomaterials have a characteristic dimension from 1 to 100 nm and are generally classified into organic and inorganic materials. These are designed with specific chemical, physical, and surface properties that yield the desired biological properties and functions [12][19]. Depending on the materials used, adding or subtracting a few atoms can significantly impact the size and shape of the structure and, consequently, its effects. Nanomaterials can transport drugs by adsorbing, entrapping, or binding covalently to them. Organic nanomaterials typically comprise carbonated skeletons that can either be lipid-based or synthetic polymeric materials (Figure 1). Some examples of organic materials are: protein-based, polysaccharides, chitosan, liposomes, polymeric micelles, poly(ethylenimine), poly(alkylcyanoacrylates), poly(amidoamine) dendrimers, or poly(lactic-co-glycolic acid). These materials exhibit excellent biocompatibility and low toxicity and do not elicit significant immunological responses since they mainly consist of carbon, nitrogen, and oxygen. An additional advantage of organic nanomaterials, particularly biologically derived ones, is the interaction with specific receptors/transporters [24][20]. Organic nanomaterials have great functional diversity, and their chemical and physical properties can be modulated to carry medical agents and favor binding to a particular subset of cells. Researchers can modulate the composition of organic nanoparticles through the conjugation of molecules, such as antibodies or peptides. This functionalization of the material allows interaction with a diverse range of biological moieties to achieve targeted delivery. A disadvantage of these materials is the batch-to-batch variability, limited ability for controlled modification, and poor tracking capabilities. Organic nanomaterials are currently being used to develop vaccines, immunotherapy, and diagnostics.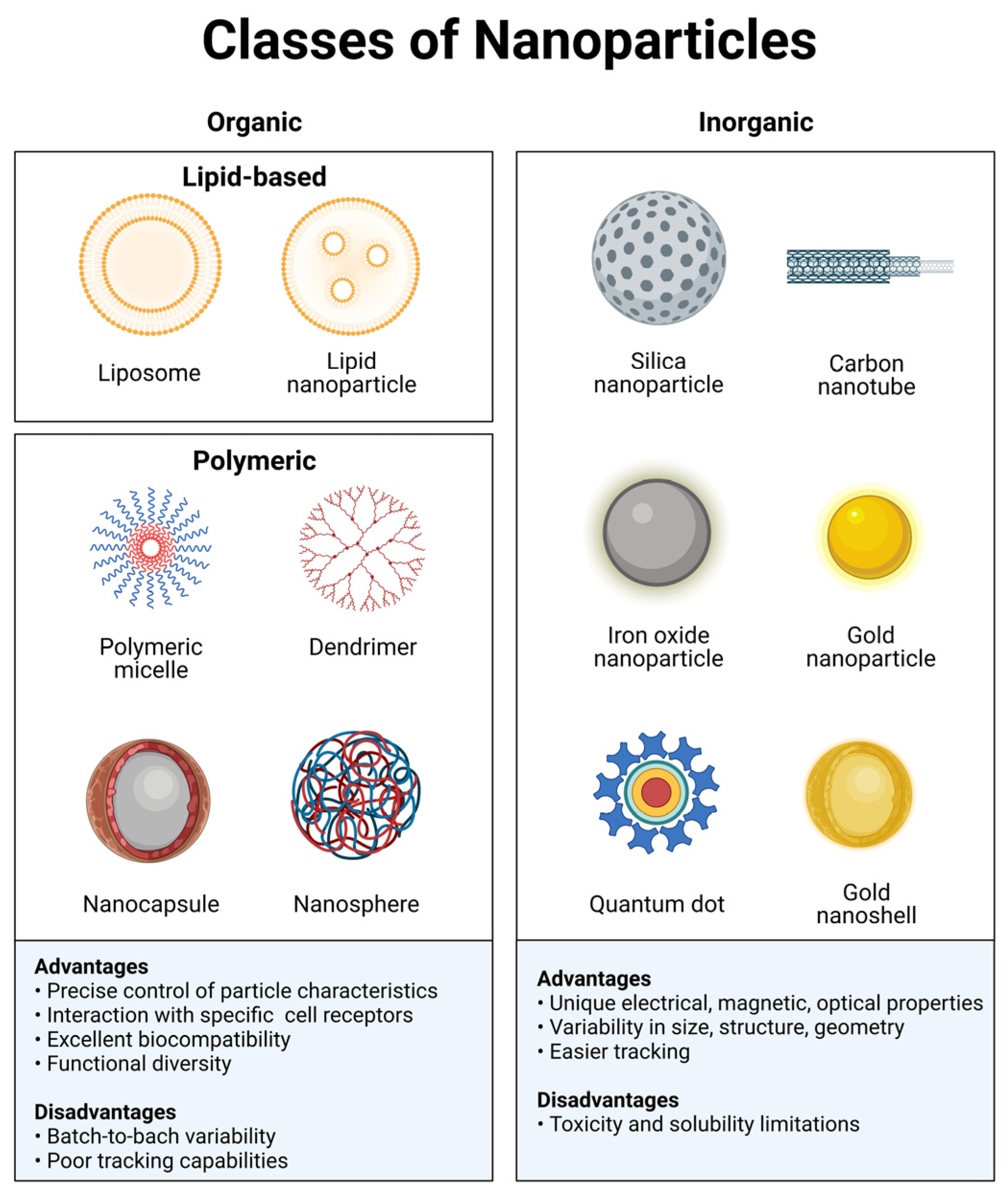
Figure 1. Classes of nanoparticles. Each class has numerous advantages and disadvantages regarding cargo, delivery, and patient response. Image created with BioRender.com.
4. Nanotechnology Applications in Sepsis
4.1. Diagnosis Devices
The gold standard technique currently employed for diagnosis and pathogen identification is microbiological cultures from biofluids. This technically simple laboratory procedure offers helpful information but is severely hindered by long incubation times, which are unsuitable for emergency diagnostics. Furthermore, a significant proportion of patients with sepsis (approximately 40%) display negative blood cultures, usually due to antibiotic administrations before sampling, low concentrations of pathogen colony-forming units, or atypical pathogens which are not recognized by standard analysis [29,30][25][26]. Polymerase-chain-reaction (PCR) detects target pathogen DNA sequences but fails to provide functional information about microbial antibiotic susceptibility. Additionally, this laboratory technique is highly sensitive, and DNA sequences from the host or contaminants that resemble the target sequence could bind the primers used and ultimately produce false positives. There is a pressing need for technologies that not only allow the rapid and accurate detection of infection but also enable the reliable identification of pathogens and their functional characteristics. This would improve the overall patient outcome by tailoring antimicrobial therapies, reducing the burden of broad-spectrum antimicrobials, and limiting the progression of multidrug-resistant organisms [29][25]. Nanotechnology-based biosensors display improved sensitivity and processing time while not requiring specialized skills. Nanosized systems also allow the detection of several relevant biomarkers in a rapid and accurate manner, aiding patient diagnosis and ultimately prognosis. This section describes different nanotechnology-based biosensors, some applications in the detection of clinically relevant biomarkers, as well as innovative approaches currently being developed.-
Electrochemical sensors: These comprise a molecular recognition system and a physicochemical transducer that transforms the chemical responses into an analytical signal [31][27]. Electrochemical sensors are small devices that exhibit small surface-to-volume ratios and simple immobilization techniques, allowing them to be more rapid, sensitive, selective, and reproducible.
-
Immunosensors: These devices use specific antibody–antigen reactions, providing a sensitive and selective tool for the quantification of various biomarkers. Due to the high affinity of the antibodies, signal amplification, high sensitivity, simple fabrication, low cost, reproducibility, and reliability, the application of immunosensors for diagnosis is a growing field of research. These devices usually utilize nanobodies, particles characterized by recombinant variable domains of heavy-chain-only antibodies. These materials exhibit excellent solubility, stability, and specificity, and display quick blood clearance and deep tissue penetration [32][28].
-
Miscellaneous nanosensors: Other diagnostic approaches that have been explored in this field use, for instance, the principles of optical and magnetic resonance properties alongside nanoparticles, allowing the detection of multiple molecules of interest ranging from protein biomarkers to pathogens [14][29].
4.2. Treatment Strategies
Unfortunately, current advancements make a single multimodal and specific medicine as an ‘antisepsis’ something beyond the bounds of possibility [44][30]; therefore, the management of sepsis is multifaceted [45,46][31][32]. Current guidelines emphasize the importance of immediate fluid resuscitation and antibiotic administration; however, despite supportive therapy and timely administration, antibiotics are often ineffective and have little impact on lowering patients’ mortality rate [47][33]. Due to the ever-evolving increase in drug-resistant pathogens and marked limitations in the development of new antibiotic drugs, the research focus has changed accordingly. Targeted drug delivery, local potency enhancement, and reduced adverse effects have become the main points of focus of antimicrobial research in recent years [14][29]. Nanotechnology provides benefits beyond tailoring physicochemical features, notably overcoming resistance and preventing its development while minimizing adverse reactions (Figure 2) [14,48][29][34]. Nanoparticle formulations can also extend the half-lives of antibiotic drugs by acting as a sustained-release system that enables a reduced frequency of drug administration while improving therapeutic indexes [49,50][35][36]. Additionally, many nanomaterials, such as silver and zinc oxide nanoparticles, possess potent inherent antimicrobial activity that can be conveniently used as a treatment adjuvant for antibiotic resistance. This feature is advantageous in inhibiting biofilm generation and targeting intracellular pathogens [51][37].
Figure 2. Examples of nanoparticle-mediated drug-delivery. Various delivery platforms can be employed, such as those described in Figure 1. Ideally, these particles can be designed to enable a targeted and controlled release of the active pharmaceutical agent, maximizing the therapeutic effect while minimizing undesired side effects. Image created using BioRender.com.
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211.
- Vincent, J.L.; Marshall, J.C.; Namendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir. Med. 2014, 2, 380–386.
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272.
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655.
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810.
- Iwashyna, T.J.; Cooke, C.R.; Wunsch, H.; Kahn, J.M. Population burden of long-term survivorship after severe sepsis in older Americans. J. Am. Geriatr. Soc. 2012, 60, 1070–1077.
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174.
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637.
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585.
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476–11521.
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381.
- Choudhary, R. Sepsis Management, Controversies, and Advancement in Nanotechnology: A Systematic Review. Cureus 2022, 14, e22112.
- Cao, C.; Yu, M.; Chai, Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019, 10, 782.
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045.
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma. Acute Care Surg. 2012, 72, 1491–1501.
- Nakamura, K.; Ogura, K.; Ohbe, H.; Goto, T. Clinical Criteria for Persistent Inflammation, Immunosuppression, and Catabolism Syndrome: An Exploratory Analysis of Optimal Cut-Off Values for Biomarkers. J. Clin. Med. 2022, 11, 5790.
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.C.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Immunol. 2018, 9, 1511.
- Rubartelli, A.; Lotze, M.T. Inside, outside, upside down: Damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007, 28, 429–436.
- Sindhwani, S.; Chan, W.C.W. Nanotechnology for modern medicine: Next step towards clinical translation. J. Intern. Med. 2021, 290, 486–498.
- Saraiva, C.; Praca, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control Release 2016, 235, 34–47.
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16.
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134.
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337.
- Kanelidis, I.; Kraus, T. The role of ligands in coinage-metal nanoparticles for electronics. Beilstein J. Nanotechnol. 2017, 8, 2625–2639.
- Claxton, A.; Papafilippou, L.; Hadjidemetriou, M.; Kostarelos, K.; Dark, P. The challenge of recognising sepsis: Future nanotechnology solutions. J. Intensive Care Soc. 2020, 21, 241–246.
- Phua, J.; Ngerng, W.; See, K.; Tay, C.; Kiong, T.; Lim, H.; Chew, M.; Yip, H.; Tan, A.; Khalizah, H.; et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit. Care 2013, 17, R202.
- Faridbod, F.; Gupta, V.K.; Zamani, H.A. Electrochemical Sensors and Biosensors. Int. J. Electrochem. 2011, 2011, 352546.
- Bao, G.; Tang, M.; Zhao, J.; Zhu, X. Nanobody: A promising toolkit for molecular imaging and disease therapy. EJNMMI Res. 2021, 11, 6.
- Pant, A.; Mackraj, I.; Govender, T. Advances in sepsis diagnosis and management: A paradigm shift towards nanotechnology. J. Biomed. Sci. 2021, 28, 6.
- Molnár, Z.; Giamarellos-Bourboulis, E.J.; Kumar, A.; Nierhaus, A. Sepsis: Diagnostic and Therapeutic Challenges. Biomed. Res. Int. 2016, 2016, 5786182.
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit. Care Med. 2020, 48, e440–e469.
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377.
- Busani, S.; Roat, E.; Serafini, G.; Mantovani, E.; Biagioni, E.; Girardis, M. The Role of Adjunctive Therapies in Septic Shock by Gram Negative MDR/XDR Infections. Can. J. Infect. Dis. Med. Microbiol. 2017, 2017, 2808203.
- Kankala, R.K.; Lin, W.Z.; Lee, C.H. Combating Antibiotic Resistance through the Synergistic Effects of Mesoporous Silica-Based Hierarchical Nanocomposites. Nanomaterials 2020, 10, 597.
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103.
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249.
- Zhu, X.; Radovic-Moreno, A.F.; Wu, J.; Langer, R.; Shi, J. Nanomedicine in the Management of Microbial Infection—Overview and Perspectives. Nano Today 2014, 9, 478–498.
More
