You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
Reducing low-density lipoprotein cholesterol (LDL-C) levels is a key target for lowering cardiovascular risk and preventing atherosclerotic cardiovascular disease (ASCVD). Red yeast rice (RYR) is a nutraceutical widely used as a lipid-lowering dietary supplement. The main cholesterol-lowering components of RYR are monacolins, particularly monacolin K, which is structurally identical to lovastatin and targets the same key enzyme of cholesterol biosynthesis. RYR supplementation reduces LDL-C levels by approximately 15–34% versus placebo, with a similar effect to low-dose, first-generation statins in subjects with mild-to-moderate dyslipidemia.
- efficacy
- endothelial function
- inflammatory markers
1. Epidemiology and Natural History of Hypercholesterolemia and ASCVD
ASCVD is the leading cause of death globally, with ischemic heart disease being responsible for 16% of deaths [1], and it is a major cause of disability in developed countries [2]. A well-established, major, modifiable risk factor for the development of ASCVD is hypercholesterolemia, particularly elevated LDL-C [3][4], making lowering LDL-C levels a key target for reducing CVD risk [4].
The prevalence of hypercholesterolemia differs markedly by country. For example, in the United States, data from the National Health and Nutrition Examination Survey showed that the prevalence of hypercholesterolemia (total cholesterol [TC] ≥240 mg/dL) in adults aged ≥20 years was 11.4% during 2015–2018, with the greatest prevalence in adults aged 40–59 (15.7%), and no significant differences in hypercholesterolemia by race or Hispanic ethnicity [5]. For elevated LDL-C (≥130 mg/dL), the age-adjusted prevalence in the United States was 29.4% for 2015–2016 [6]. In contrast, in the Asia Pacific region, the prevalence of TC ≥240 mg/dL ranged from 9.0 to 44.9% across countries and survey years (2006–2015) [7], while in the United Kingdom, the crude prevalence of primary hypocholesterolemia and mixed dyslipidemia was estimated at 23.5% in 2019 [8]. Globally, the overall burden of high LDL-C levels is increasing when measured in terms of the total numbers of disability-adjusted life years (DALYs), deaths, years lived with disabilities, and years of life lost [9]. The total number of DALYs reached 98.6 million in 2019, with age-standardized DALY rates specifically due to high LDL-C highest in Eastern Europe, North Africa, the Middle East, Oceania, and Central Asia, and lowest in Australasia, Western Europe, Andean Latin America, and the high-income Asia Pacific [9].
2. Purpose and Outcome of Treatment
The management of LDL-C forms part of a comprehensive ASCVD risk-reduction strategy, which is based on individual CVD risk profiles and preferences [4][10]. Given that guideline recommendations for the management of dyslipidemia are based on ASCVD risk calculators, which are strongly dominated by age [4][10][11], many individuals do not reach the designated level of risk for intervention until middle age or older, leading to a delay in the initiation of therapy. However, by this time, such individuals may have been exposed to LDL-C levels for decades.
Prolonged reduction in LDL-C levels is associated with a substantially decreased risk of ASCVD [3]. A meta-analysis of data from 170,000 participants in 26 clinical trials of lipid-lowering drugs demonstrated a reduction of approximately one-fifth in the risk of major ASCVD events per 1 mmol/L decrease in LDL-C in the secondary prevention setting, with a similar benefit in primary prevention [12]. Further meta-analyses have confirmed that the greater the absolute LDL-C reduction, the greater the ASCVD risk reduction [10]. Mendelian randomization studies indicate that, alongside absolute LDL-C level, the duration of exposure to higher LDL-C is significantly associated with ASCVD risk, demonstrating a cumulative impact of LDL-C throughout an individual’s lifetime [13][14]. One such study examined data from >300,000 participants and showed that the prolonged exposure to lower LDL-C mediated by nine polymorphisms in six different genes (i.e., beginning from early life) was associated with a three-fold greater risk reduction per unit lower LDL-C versus intervention with a statin started later in life [13]. Overall, this indicates a rationale for early and prolonged lipid-lowering interventions to achieve lifetime risk reduction.
The recommended LDL-C goals in the ESC/EAS guidelines for the management of dyslipidemia are based on the patient’s ASCVD risk, with goals of <116 mg/dL (<3.0 mmol/L) and <100 mg/dL (<2.6 mmol/L) for patients at low and moderate risk, respectively [10]. Tighter control of LDL-C is recommended for patients at high and very high risk, with targets of a ≥50% reduction in LDL-C from baseline and an absolute LDL-C <70 mg/dL (<1.8 mol/L) for high risk and <55 mg/dL (<1.4 mmol/L), or even <40 mg/dL (<1.0 mmol/L), in selected patients for very high risk [10].
Dietary modifications—avoiding trans fats, lowering intake of saturated fats and cholesterol, and increasing fiber intake—all lower LDL-C by levels 5–10% [10]. Additional lowering of LDL-C by nutraceuticals such as RYR might help people meet their LDL-C goals, particularly individuals with elevated LDL-C levels who do not qualify for treatment with statins because they have a low global CV risk [10] and those who are unwilling to use statins [15].
3. Metabolism of the Bioactive Components and Mechanism of Action of RYR
The main cholesterol-lowering effects of RYR are provided by monacolins, its main bioactive components. Monacolin K, the most abundant monacolin in RYR [16], occurs in two forms: the lactone (inactive) and hydroxyl acid (active) [16][17][18]. The proportion of the acid form has been reported to vary from 5% to 100%, depending on pH: at low pH, the lactone form predominates, whereas at neutral and acidic pH, the acid form predominates [16][17][19]. The lactone form of monacolin K is structurally identical to lovastatin [16][17]; therefore, similar to other statins, monacolin K is a reversible inhibitor of β-hydroxyβ-methylglutaryl coenzyme A reductase (HMG-CoAR), the enzyme catalyzing the rate-limiting step of cholesterol biosynthesis (Figure 1) [19]. Lovastatin is a prodrug that must be hydrolyzed to the acid form [16][17][19] because only this form bonds to key amino acid residues in the binding pocket of HMG-CoAR (differing from those bound by the lactone form), stabilizing the interaction between the two molecules [20]. By contrast, the acid form is naturally present in RYR, and this can result in differences in bioavailability and clinical profiles between RYR and lovastatin. In addition to inhibition of HMG-CoAR, statins have demonstrated actions at the transcription level, with increases in nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα), mediated by the Rho-signaling pathway, potentially explaining increases in high-density lipoprotein cholesterol (HDL-C) levels with statin therapy [21]. Statins also influence the expression of a wide range of other genes, including transcription factors involved in inflammation, proliferation, and differentiation [22][23], and cholesterol transporters in a range of tissues, including the liver, intestine, adipose tissue, and skin, and apolipoprotein A1 (apo-A1) in the liver (Figure 2) [24]. This leads to an increased transfer of cholesterol to apo-A1, leading to a greater production of HDL.
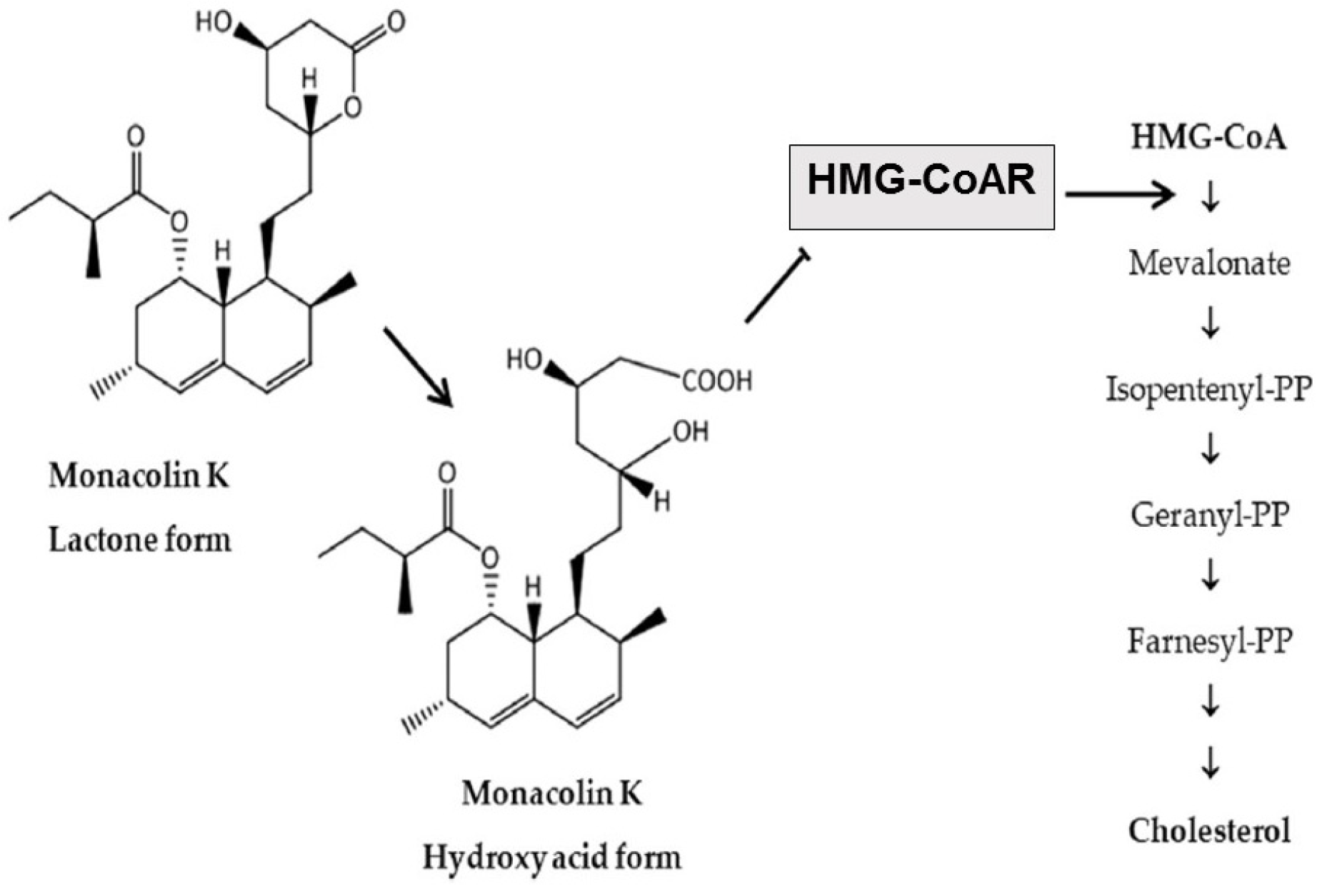
Figure 1. Main cholesterol-lowering mechanism of action of RYR [16]. RYR = red yeast rice. HMG-CoA = β-hydroxy β-methylglutaryl coenzyme A; HMG-CoAR = β-hydroxy β-methylglutaryl coenzyme A reductase; PP = pyrophosphate.
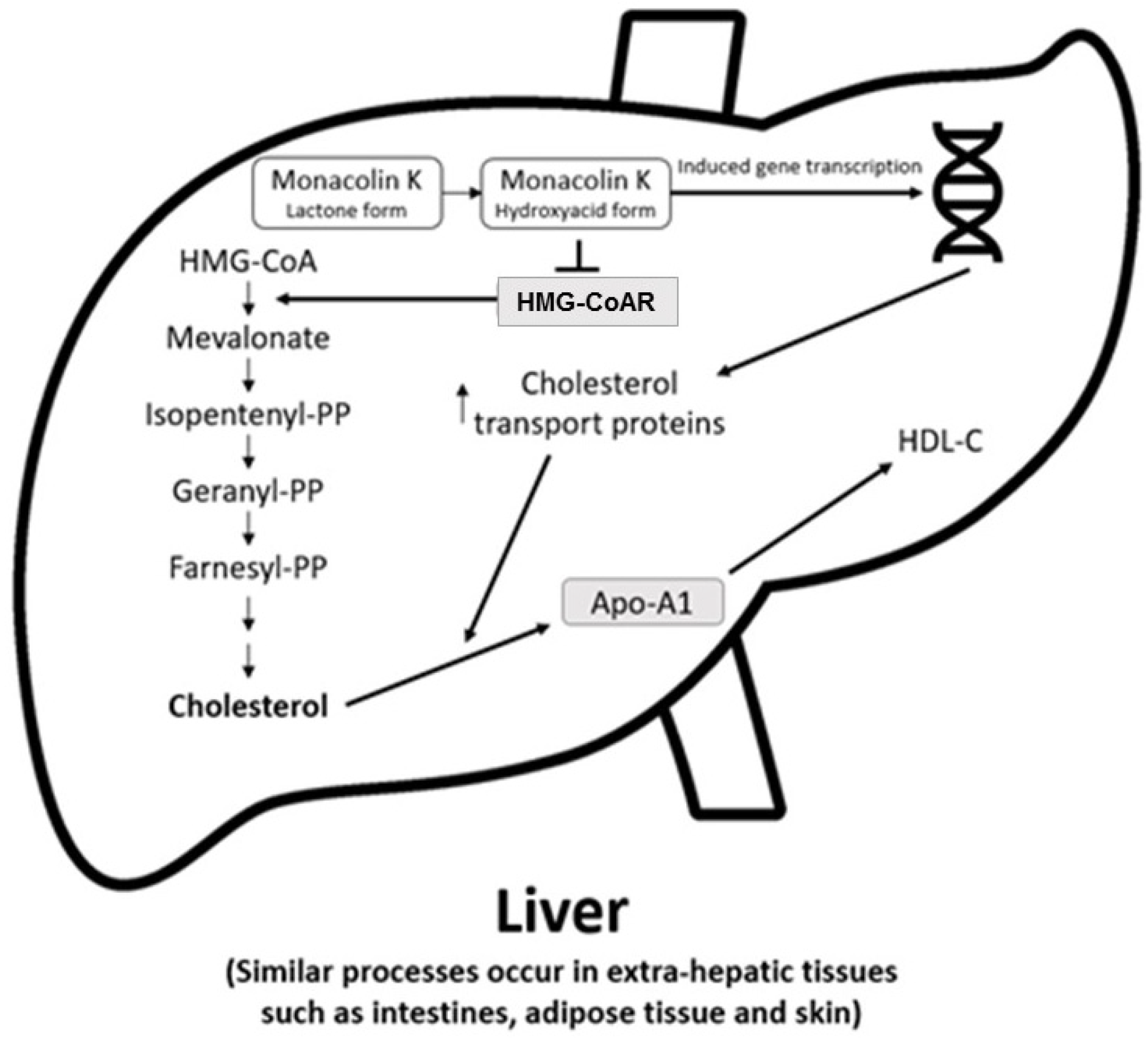
Figure 2. Actions of RYR on cholesterol synthesis, transport, and gene expression [24]. Apo-A1 = apolipoprotein A1; HDL-C = high-density lipoprotein cholesterol; HMG-CoA = β-hydroxy β-methylglutaryl coenzyme A; HMG-CoAR = β-hydroxy β-methylglutaryl coenzyme A reductase; PP = pyrophosphate.
Similarly to lovastatin, CYP3A4 is involved in the metabolism of monacolins which are substrates of P-glycoprotein. Their bioavailability and safety could therefore be affected by interactions with other medicinal products and foods, although these potential effects are yet to be fully characterized [17][25]. Plasma concentrations of the hydroxy acid and lactone forms of monacolin K after ingestion of 2400 mg RYR are much lower than those of the corresponding forms of lovastatin after taking a 20 mg conventional tablet [26]; this is expected to reduce the risk of adverse effects.
RYR supplements differ widely in their monacolin K content, resulting in a daily dose range of approximately 0.1–14.5 mg [27][28][29]. This is partly because the relative abundances of monacolin K and other monacolin subtypes in RYR, including monacolins J, L, and X, are affected by the fermentation conditions and yeast strain used during their production [18][28][29]. In addition, RYR contains non-monacolin components, including sugars (25–73% mainly starch), proteins (14–31%), water (2–7%), fatty acids (1–5%), and other bioactive components such as pigments, sterols, isoflavones, and citrinin (discussed below) [19][30]. The contributions of components other than monacolins to the cholesterol-lowering properties of RYR supplements are difficult to assess due to their varying compositions and very small concentration.
A study in healthy volunteers taking an RYR product reported that the pharmacokinetic properties of both forms of monacolin K (lovastatin and lovastatin acid) were linear across the range of 1–4 capsules taken as a single dose, with no significant accumulation after multiple dosing [25].
Asian ethnicity is associated with a higher statin exposure than in non-Asians at a given dose, partly due to genetically determined differences in transport mechanisms [31]. The approved maximum doses of most statins are therefore lower in some Asian countries (e.g., Japan) than elsewhere [31]. The greater exposure in Asians than Caucasians is more marked for some statins (e.g., rosuvastatin) [32] than for others (e.g., atorvastatin) [33].
4. Effects of RYR on Lipids
4.1. RYR versus Placebo
Randomized trials have investigated a variety of RYR and monacolin K daily doses versus placebo for primary and secondary CVD prevention in subjects with dyslipidemia [34][35][36][37][38][39][40][41][42][43][44][45]. RYR treatment of at least 4 weeks’ duration demonstrated significant reductions in LDL-C of approximately 15–34% from baseline (p < 0.05); significant differences versus placebo were also observed. In several of these studies, the daily dose of monacolin K (where stated) was less than 10 mg/day [34][38][39][43][44][45], below the 10–80 mg/day recommended for the structural analog lovastatin. Significant decreases were also seen for TC compared with baseline and/or placebo [34][35][36][37][38][39][40][41][42][43][44][45], while effects on HDL-C and triglycerides were generally small and inconsistent [34][35][36][37][38][39][40][41][42][43][44][45]. Several of these studies also reported significant decreases in apolipoprotein B levels from baseline with RYR (−27% to −19%; all p < 0.001) [35][36][37][39][42][45], and small, mostly non-significant increases in apo-A1 [35][36][37][39][42].
4.2. RYR versus Other Statin Preparations
Clinical trials comparing RYR with other statin preparations [46][47][48][49][50][51][52][53][54][55][56] have reported reductions in LDL-C from baseline of up to 33.4%, generally similar to the statin comparator in each study. However, most of these studies were performed in China and are not of high quality. In addition, genetically determined differences in transport mechanisms in East Asians mean that RYR seems to be more effective in Chinese people [31], and this might contribute to the impressive results versus other statins. The finding that RYR, even at a low dose, has the same effect as high-intensity statins, is therefore not reliable. These trials included two studies with a formulation (Armolipid Plus®, Meda-Mylan, Monza, Italy) providing monacolin K 3 mg/day [49][50]. Certain studies have also reported that RYR significantly reduced TC [49][50][51][52][53][55][56] and triglycerides [46][47][48][52][55][56], while effects on HDL-C were generally small and inconsistent [47][48][49][50][52][53][54][55][56].
4.3. RYR versus Placebo and Other Statins Meta-Analyses
A 2021 meta-analysis of 15 high-quality randomized controlled trials (mostly using doses of 600–2400 mg twice daily) involving 1012 subjects showed that RYR significantly decreased LDL-C versus placebo (mean difference [MD] −35.82 mg/dL; 95% confidence interval [CI] −43.36, −28.29 [p < 0.00001]), with no significant difference versus other statins (simvastatin 20 mg/d, pravastatin 40 mg/d, or rosuvastatin 10 mg/d) with an MD of 1.89 mg/dL (95% CI −7.93, 11.71 [p = 0.71]) [57]. RYR significantly increased HDL-C versus placebo (MD 3.47 mg/dL; 95% CI 0.94, 6.00 [p = 0.007]) to a similar degree as other statins (MD 2.50 mg/dL; 95% CI −4.21, 9.22 [p = 0.46]). While RYR significantly reduced TC versus placebo (MD −37.43 mg/dL; 95% CI −47.08, −27.79 [p < 0.00001]), it was less effective than other statins (MD 12.24 mg/dL; 95% CI 2.19, 22.29 [p = 0.02]). For triglycerides, significant decreases were seen for RYR versus placebo (MD −20.65 mg/dL; 95% CI −35.60, −5.70 [p = 0.007]) and versus other statins (MD −19.90 mg/dL; 95% CI −32.22, −7.58 [p = 0.002]) [57].
An earlier meta-analysis of 20 randomized trials involving 6663 subjects showed that RYR (1200–4800 mg/d) was more effective than a placebo at reducing LDL-C (MD −1.02 mmol/L; 95% CI −1.20, −0.83 [p < 0.00001]) and TC (MD −1.00 mmol/L; 95% CI −1.23, −0.77 [p < 0.00001]) [58]. The effect of RYR was similar to that of low-intensity/low-dose statins on LDL-C (MD 0.03 mmol/L; 95% CI −0.36, 0.41 [p = 0.89]) and TC (MD −0.05 mmol/L; 95% CI −0.28, 0.18 [p = 0.67]) [58]. Finally, a network meta-analysis of 47 randomized controlled trials involving 4824 subjects, evaluating three different Chinese RYR supplements (most commonly Xuezhikang 600 mg twice daily), showed that RYR significantly reduced LDL-C, TC, and triglycerides versus placebo [59]. No significant differences in the levels of these parameters were observed with RYR versus simvastatin, although a ranking analysis suggested that Xuezhikang (surface under the cumulative ranking [SUCRA] value 82.6%) was more likely than simvastatin (SUCRA value 74.9%) to be effective in reducing LDL-C [59].
5. Effects of RYR on Inflammatory and Vascular Remodeling Biomarkers and Endothelial Function
Statins have been shown to reduce levels of the inflammatory biomarker high-sensitivity C-reactive protein (hs-CRP) [60], and this is also true of RYR, with reductions in hs-CRP versus placebo observed in subjects with mild hypercholesterolemia (−23.77%, 95% CI −30.54, −17.01) [40] and in those with coronary heart disease (CHD) (−50.0% versus −25.4%, p < 0.05) [36]. Significantly greater reductions in hs-CRP have also been reported for RYR versus simvastatin (p < 0.05) in subjects with unstable angina pectoris and statin-induced elevated liver enzymes [48].
Additionally, compared with a placebo, RYR treatment resulted in more favorable decreases of −28.05% (95% CI −35.18, −20.93) and −27.19% (95% CI −36.21, −18.15) in the vascular remodeling biomarkers matrix metalloproteinase (MMP)-2 and MMP-9, respectively, in subjects with mild hypercholesterolemia [40]. Moreover, 6 weeks’ treatment with RYR (Xuezhikang, 1200 mg/d) has been reported to significantly improve endothelial function (p < 0.05) measured by both pre-prandial and post-prandial flow-mediated vasodilation in subjects with CHD, while no change was seen with a placebo [36].
6. Beneficial Effects of Exposure to RYR on ASCVD Risk and Events
6.1. RYR versus Placebo
The effects of RYR compared with placebo on ASCVD risk and events have been evaluated in clinical studies and meta-analyses in varying frail subject populations with a history of MI and/or CHD.
In a randomized Chinese study of almost 5000 subjects with a previous MI and average LDL-C levels at baseline, those taking RYR (Xuezhikang) daily for a mean of 4.5 years experienced a lower relative risk of non-fatal MI and death from CHD, ASCVD mortality, and total mortality by 45%, 30%, and 33%, respectively, compared with those taking a placebo [61]. Similar reductions in ASCVD events versus placebo were observed in subgroups from this trial of hypertensive subjects [62] and elderly (aged ≥ 65 years) hypertensive subjects treated with RYR [63].
Meta-analyses in subjects with a history of MI and/or CHD, including those with metabolic syndrome (diabetes or hypertension plus dyslipidemia) showed that, compared with subjects taking a placebo, those taking RYR had a lower risk of ASCVD endpoints including non-fatal MI (risk ratio (RR) 0.42, 95% CI 0.34, 0.52), revascularization (RR 0.58, 95% CI 0.48, 0.71), and sudden death (RR 0.71, 95% CI 0.53, 0.94) [64], as well as major ASCVD events (RR 0.54, 95% CI 0.43, 0.66) and mortality (RR 0.62, 95% CI 0.49, 0.78) [65].
6.2. RYR versus Other Statins
A real-world, retrospective, population-based cohort study of Taiwan’s National Health Insurance Program in individuals without a history of stroke reported a lower risk of stroke in subjects taking RYR than age- and sex-matched controls who received the monacolin K analog lovastatin (hazard ratio [HR] 0.65, 95% CI 0.59, 0.71) [66]. However, hypertension and diabetes were significantly more common in the lovastatin cohort at baseline, which may have increased the baseline risk of stroke in the lovastatin versus RYR cohort [66].
In a comparison of RYR and simvastatin in 90 Chinese subjects with unstable angina pectoris and elevated liver enzymes associated with simvastatin, a significantly lower proportion of subjects receiving RYR or who continued taking simvastatin experienced ASCVD endpoints versus those who stopped taking simvastatin [48].
7. Beneficial Effects of RYR-Berberine Combinations
In order to lower the dose of RYR and improve tolerability while potentially increasing the cholesterol-lowering effect, combinations of RYR with other nutraceutical compounds that have different lipid-lowering mechanisms of action have been investigated.
One of the most extensively studied RYR nutraceutical combinations (Armolipid Plus®) includes berberine, a plant alkaloid that enhances the hepatic uptake of cholesterol, for which the largest pharmacovigilance datasets are available [67][68][69]. Armolipid Plus® contains 3 mg monacolin K, 500 mg berberine, and 10 mg policosanols (aliphatic alcohols derived from sugar cane, reported to have cholesterol-lowering effects [70]). A meta-analysis of 12 randomized control trials involving 1050 subjects with up to 12 months’ follow-up showed that this preparation significantly decreased body-mass index (MD −0.25 kg/m2; p = 0.008), LDL-C (MD −26.67 mg/dL; p < 0.001), TC (MD −25.07 mg/dL; p < 0.001), triglycerides (MD −11.47 mg/dL; p < 0.001), fasting glucose (MD −3.52 mg/dL; p < 0.001), and hs-CRP versus placebo (−0.61 mg/L; p = 0.022), and significantly increased HDL-C (MD 1.84 mg/dL; p < 0.001) versus placebo [68]. For further details of these trials please see the meta-analysis publication [68].
Other RYR combinations with antioxidants, including coenzyme Q10, have also demonstrated lipid-lowering effects and decreases in hs-CRP compared with placebo [71][72]. In a crossover study in 25 subjects with moderate hypercholesterolemia, 4 weeks’ treatment with RYR containing 10 mg monacolins combined with antioxidants showed more favorable percentage changes from baseline than a placebo in TC (−18.35% versus −5.39%), LDL-C (−22.36% versus −1.38%), non-HDL-C (−22.83% versus −7.15%), and hs-CRP (−2.33% versus 2.11%) [71].
In a separate study in 40 subjects with moderate hypercholesterolemia, 6 months’ treatment with RYR containing 10 mg monacolins plus 30 mg coenzyme Q10 significantly reduced LDL-C versus placebo (−26.3% versus +3.4%, p < 0.05) [72]. Such combinations also demonstrated improvements compared with placebo in endothelial function assessed via pulse volume displacement (p < 0.05) and arterial stiffness assessed via pulse wave velocity (p < 0.05) in subjects with moderate hypercholesterolemia [71]. The potential benefits of these lipid-lowering and vascular remodeling effects from RYR combinations on CV risks and outcomes remain to be elucidated [68].
8. Convenience and Preference, and Health Economic Impact
Specific studies relating to the cost-effectiveness, convenience, and patient preference for RYR have not been identified. However, depending on the country, high-quality, highly purified RYR products could cost more than generic statins [19]. Additionally, while lipid-lowering drugs may be reimbursed by healthcare systems, the cost of RYR supplements is likely to fall on consumers unless they are classified as medicines, and this may limit their use [73]. Conversely, economic inequalities and geographic location markedly affect the proportion of people who take ASCVD medications, including lipid-lowering drugs [74][75]. For example, one study reported that statin use for secondary prevention was 66.5% in high-income countries compared with only 3.3% in low-income countries [75]. Statin use was also higher in urban versus rural areas within countries (19.9% versus 12% overall; p < 0.0001), with the greatest variation in the lowest-income countries [75]. Thus, RYR may be more easily accessible than pharmacologic interventions in some lower- or middle-income countries.
An additional aspect contributing to preference for RYR is that many individuals experience a real or perceived intolerance to statins [73], often relating to toxicity concerns [76]. Indeed, a key factor related to non-adherence to lipid-lowering drugs in patients with dyslipidemia is presenting with adverse events (AEs) or expressing concerns about them [77]. Adherence to lipid-lowering treatment is also associated with cost, with treatment persistence over 2 years found to be greater for nutraceuticals than for statins in 628 subjects with moderate hypercholesterolemia (odds ratio 1.29, 95% CI 1.14, 1.38) who were fully responsible for paying for treatment [78].
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 August 2022).
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2017, 13, 965–1005.
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472.
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337.
- Carroll, M.D.; Fryar, C.D. Total and High-Density Lipoprotein Cholesterol in Adults: United States, 2015–2018. Centers for Disease Control and Prevention. 2020. Available online: https://www.cdc.gov/nchs/products/databriefs/db363.htm (accessed on 27 April 2023).
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596.
- Lin, C.-F.; Chang, Y.-H.; Chien, S.-C.; Lin, Y.-H. Epidemiology of dyslipidemia in the Asia Pacific region. Int. J. Gerontol. 2018, 12, 2–6.
- Bilitou, A.; Were, J.; Farrer, A.; Rabe, A.; Ming, S.W.Y.; Haq, I.; Dunton, K. Prevalence and patient outcomes of adult primary hypercholesterolemia and dyslipidemia in the UK: Longitudinal retrospective study using a primary care dataset from 2009 to 2019. Clinicoecon. Outcomes Res. 2022, 14, 189–203.
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021.
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188.
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019, 139, e1082–e1143.
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681.
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A.; Flack, J.M. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A mendelian randomization analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639.
- Ference, B.A.; Majeed, F.; Penumetcha, R.; Flack, J.M.; Brook, R.D. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1l1, HMGCR, or both: A 2 × 2 factorial Mendelian randomization study. J. Am. Coll. Cardiol. 2015, 65, 1552–1561.
- Penson, P.E.; Banach, M. Nutraceuticals for the control of dyslipidaemias in clinical practice. Nutrients 2021, 13, 2957.
- Banach, M.; Bruckert, E.; Descamps, O.S.; Ellegård, L.; Ezhov, M.; Föger, B.; Fras, Z.; Kovanen, P.T.; Latkovskis, G.; März, W.; et al. The role of red yeast rice (RYR) supplementation in plasma cholesterol control: A review and expert opinion. Atheroscler. Suppl. 2019, 39, e1–e8.
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018, 16, e05368.
- Song, J.; Luo, J.; Ma, Z.; Sun, Q.; Wu, C.; Li, X. Quality and authenticity control of functional red yeast rice-a review. Molecules 2019, 24, 1944.
- Cicero, A.F.G.; Fogacci, F.; Zambon, A. Red yeast rice for hypercholesterolemia: JACC focus seminar. J. Am. Coll. Cardiol. 2021, 77, 620–628.
- Mannino, G.; Iovino, P.; Lauria, A.; Genova, T.; Asteggiano, A.; Notarbartolo, M.; Porcu, A.; Serio, G.; Chinigò, G.; Occhipinti, A.; et al. Bioactive triterpenes of protium heptaphyllum gum resin extract display cholesterol-lowering potential. Int. J. Mol. Sci. 2021, 22, 2664.
- Martin, G.; Duez, H.; Blanquart, C.; Berezowski, V.; Poulain, P.; Fruchart, J.C.; Najib-Fruchart, J.; Glineur, C.; Staels, B. Statin-induced inhibition of the Rho-signaling pathway activates PPARalpha and induces HDL apoA-I. J. Clin. Investig. 2001, 107, 1423–1432.
- Bjarnadottir, O.; Kimbung, S.; Johansson, I.; Veerla, S.; Jonsson, M.; Bendahl, P.O.; Grabau, D.; Hedenfalk, I.; Borgquist, S. Global transcriptional changes following statin treatment in breast cancer. Clin. Cancer. Res. 2015, 21, 3402–3411.
- Dichtl, W.; Dulak, J.; Frick, M.; Alber, H.F.; Schwarzacher, S.P.; Ares, M.P.; Nilsson, J.; Pachinger, O.; Weidinger, F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 58–63.
- Ahmadi, Y.; Ghorbanihaghjo, A.; Argani, H. The effect of statins on the organs: Similar or contradictory? J. Cardiovasc. Thorac. Res. 2017, 9, 64–70.
- Chen, C.H.; Uang, Y.S.; Wang, S.T.; Yang, J.C.; Lin, C.J. Interaction between red yeast rice and CYP450 enzymes/P-glycoprotein and its implication for the clinical pharmacokinetics of lovastatin. Evid. Based Complement. Altern. Med. 2012, 2012, 127043.
- Li, Z.; Seeram, N.P.; Lee, R.; Thames, G.; Minutti, C.; Wang, H.J.; Heber, D. Plasma clearance of lovastatin versus Chinese red yeast rice in healthy volunteers. J. Altern. Complement. Med. 2005, 11, 1031–1038.
- Cohen, P.A.; Avula, B.; Khan, I.A. Variability in strength of red yeast rice supplements purchased from mainstream retailers. Eur. J. Prev. Cardiol. 2017, 24, 1431–1434.
- Gordon, R.Y.; Cooperman, T.; Obermeyer, W.; Becker, D.J. Marked variability of monacolin levels in commercial red yeast rice products: Buyer beware! Arch. Intern. Med. 2010, 170, 1722–1727.
- Marcheluzzo, S.; Faggian, M.; Zancato, M.; Peron, G. Analysis of monacolins and berberine in food supplements for lipid control: An overview of products sold on the Italian market. Molecules 2021, 26, 2222.
- Zhu, B.; Qi, F.; Wu, J.; Yin, G.; Hua, J.; Zhang, Q.; Qin, L. Red yeast rice: A systematic review of the traditional uses, chemistry, pharmacology, and quality control of an important Chinese folk medicine. Front. Pharmacol. 2019, 10, 1449.
- Tomlinson, B.; Chan, P.; Liu, Z.M. Statin intolerance-an Asian perspective. J. Atheroscler. Thromb. 2020, 27, 485–488.
- Lee, E.; Ryan, S.; Birmingham, B.; Zalikowski, J.; March, R.; Ambrose, H.; Moore, R.; Lee, C.; Chen, Y.; Schneck, D. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin. Pharmacol. Ther. 2005, 78, 330–341.
- Gandelman, K.; Fung, G.L.; Messig, M.; Laskey, R. Systemic exposure to atorvastatin between Asian and Caucasian subjects: A combined analysis of 22 studies. Am. J. Ther. 2012, 19, 164–173.
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am. J. Clin. Nutr. 1999, 69, 231–236.
- Zhao, S.-P.; Liu, L.; Cheng, Y.-C.; Li, Y.-L. Effect of xuezhikang, a cholestin extract, on reflecting postprandial triglyceridemia after a high-fat meal in patients with coronary heart disease. Atherosclerosis 2003, 168, 375–380.
- Zhao, S.P.; Liu, L.; Cheng, Y.C.; Shishehbor, M.H.; Liu, M.H.; Peng, D.Q.; Li, Y.L. Xuezhikang, an extract of cholestin, protects endothelial function through antiinflammatory and lipid-lowering mechanisms in patients with coronary heart disease. Circulation 2004, 110, 915–920.
- Lin, C.-C.; Li, T.-C.; Lai, M.-M. Efficacy and safety of monascus purpureus went rice in subjects with hyperlipidemia. Eur. J. Endocrinol. 2005, 153, 679–686.
- Becker, D.J.; Gordon, R.Y.; Halbert, S.C.; French, B.; Morris, P.B.; Rader, D.J. Red yeast rice for dyslipidemia in statin-intolerant patients: A randomized trial. Ann. Intern. Med. 2009, 150, 830–839.
- Bogsrud, M.P.; Ose, L.; Langslet, G.; Ottestad, I.; Strøm, E.C.; Hagve, T.A.; Retterstøl, K. HypoCol (red yeast rice) lowers plasma cholesterol—A randomized placebo controlled study. Scand. Cardiovasc. J. 2010, 44, 197–200.
- Cicero, A.F.; Derosa, G.; Parini, A.; Maffioli, P.; D’Addato, S.; Reggi, A.; Giovannini, M.; Borghi, C. Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr. Res. 2013, 33, 622–628.
- Verhoeven, V.; Lopez Hartmann, M.; Remmen, R.; Wens, J.; Apers, S.; Van Royen, P. Red yeast rice lowers cholesterol in physicians—A double blind, placebo controlled randomized trial. BMC Complement. Altern. Med. 2013, 13, 178.
- Moriarty, P.M.; Roth, E.M.; Karns, A.; Ye, P.; Zhao, S.P.; Liao, Y.; Capuzzi, D.M.; Bays, H.E.; Zhang, F.; Liu, S.; et al. Effects of Xuezhikang in patients with dyslipidemia: A multicenter, randomized, placebo-controlled study. J. Clin. Lipidol. 2014, 8, 568–575.
- Heinz, T.; Schuchardt, J.P.; Möller, K.; Hadji, P.; Hahn, A. Low daily dose of 3 mg monacolin K from RYR reduces the concentration of LDL-C in a randomized, placebo-controlled intervention. Nutr. Res. 2016, 36, 1162–1170.
- Wang, T.J.; Lien, A.S.; Chen, J.L.; Lin, C.H.; Yang, Y.S.; Yang, S.H. A randomized clinical efficacy trial of red yeast rice (monascus pilosus) against hyperlipidemia. Am. J. Chin. Med. 2019, 47, 323–335.
- Minamizuka, T.; Koshizaka, M.; Shoji, M.; Yamaga, M.; Hayashi, A.; Ide, K.; Ide, S.; Kitamoto, T.; Sakamoto, K.; Hattori, A.; et al. Low dose red yeast rice with monacolin K lowers LDL cholesterol and blood pressure in japanese with mild dyslipidemia: A multicenter, randomized trial. Asia Pac. J. Clin. Nutr. 2021, 30, 424–435.
- Kou, W.; Lu, Z.; Guo, J. Effect of xuezhikang on the treatment of primary hyperlipidemia. Zhonghua Nei Ke Za Zhi 1997, 36, 529–531.
- Chen, L.L.; Liu, J. The effects of Xuezhikang on hypercholesterolemia. Her. Med. 2002, 21, 31–32.
- Cui, F.; Zhang, Y.; Wei, Q.; Liu, C.; Wang, J.; Zhang, M. A novel medical treatment for lipid control in patients with unstable angina pectoris and statin-induced liver dysfunction. Acta Cardiol. Sin. 2015, 31, 66–71.
- Ruscica, M.; Gomaraschi, M.; Mombelli, G.; Macchi, C.; Bosisio, R.; Pazzucconi, F.; Pavanello, C.; Calabresi, L.; Arnoldi, A.; Sirtori, C.R.; et al. Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with armolipid plus. J. Clin. Lipidol. 2014, 8, 61–68.
- Marazzi, G.; Campolongo, G.; Pelliccia, F.; Quattrino, S.; Vitale, C.; Cacciotti, L.; Massaro, R.; Volterrani, M.; Rosano, G. Comparison of low-dose statin versus low-dose statin + armolipid plus in high-intensity statin-intolerant patients with a previous coronary event and percutaneous coronary intervention (ADHERENCE trial). Am. J. Cardiol. 2017, 120, 893–897.
- Gheith, O.; Sheashaa, H.; Abdelsalam, M.; Shoeir, Z.; Sobh, M. Efficacy and safety of monascus purpureus went rice in subjects with secondary hyperlipidemia. Clin. Exp. Nephrol. 2008, 12, 189–194.
- Shang, X. Clinical observation of xuezhikang and atorvastatin on dyslipidemia and hemorheology in patients with coronary heart disease. Guangxi Med. 2007, 8, 1158–1159.
- Halbert, S.C.; French, B.; Gordon, R.Y.; Farrar, J.T.; Schmitz, K.; Morris, P.B.; Thompson, P.D.; Rader, D.J.; Becker, D.J. Tolerability of red yeast rice (2400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am. J. Cardiol. 2010, 105, 198–204.
- Xue, Y.; Tao, L.; Wu, S.; Wang, G.; Qian, L.; Li, J.; Liao, L.; Tang, J.; Ji, K. Red yeast rice induces less muscle fatigue symptom than simvastatin in dyslipidemic patients: A single center randomized pilot trial. BMC Cardiovasc. Disord. 2017, 17, 127.
- Li, B.; Hu, S.-Y.; Wu, X.; Xu, H.-L.; Zhang, H.-M.; Wang, L. Anti-oxidant and anti-inflammatory effects of xuezhikang capsule on patients with coronary heart diseases. Prog. Mod. Biomed. 2011, 12, 2289–2291.
- Liu, L.T.; Wu, M.; Wang, H.X. Clinical study on the treatment of abnormal blood lipids complicated with carotid atherosclerosis with lipid-reducing red rice minute powder: A randomized controlled trial. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011, 31, 1196–1200.
- Li, P.; Wang, Q.; Chen, K.; Zou, S.; Shu, S.; Lu, C.; Wang, S.; Jiang, Y.; Fan, C.; Luo, Y. Red yeast rice for hyperlipidemia: A meta-analysis of 15 high-quality randomized controlled trials. Front. Pharmacol. 2021, 12, 819482.
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.; Gerdes, V.E. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423.
- Xu, G.; Lin, M.; Dai, X.; Hu, J. Comparing the effectiveness of Chinese patent medicines containing red yeast rice on hyperlipidaemia: A network meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 2022, 5, e00314.
- Kandelouei, T.; Abbasifard, M.; Imani, D.; Aslani, S.; Razi, B.; Fasihi, M.; Shafiekhani, S.; Mohammadi, K.; Jamialahmadi, T.; Reiner, Z.; et al. Effect of statins on serum level of hs-CRP and CRP in patients with cardiovascular diseases: A systematic review and meta-analysis of randomized controlled trials. Mediat. Inflamm. 2022, 2022, 8732360.
- Lu, Z.; Kou, W.; Du, B.; Wu, Y.; Zhao, S.; Brusco, O.A.; Morgan, J.M.; Capuzzi, D.M.; Li, S. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am. J. Cardiol. 2008, 101, 1689–1693.
- Li, J.J.; Lu, Z.L.; Kou, W.R.; Chen, Z.; Wu, Y.F.; Yu, X.H.; Zhao, Y.C. Impact of Xuezhikang on coronary events in hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). Ann. Med. 2010, 42, 231–240.
- Li, J.J.; Lu, Z.L.; Kou, W.R.; Chen, Z.; Wu, Y.F.; Yu, X.H.; Zhao, Y.C. Beneficial impact of Xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J. Clin. Pharmacol. 2009, 49, 947–956.
- Sungthong, B.; Yoothaekool, C.; Promphamorn, S.; Phimarn, W. Efficacy of red yeast rice extract on myocardial infarction patients with borderline hypercholesterolemia: A meta-analysis of randomized controlled trials. Sci. Rep. 2020, 10, 2769.
- Yuan, R.; Yuan, Y.; Wang, L.; Xin, Q.; Wang, Y.; Shi, W.; Miao, Y.; Leng, S.X.; Chen, K.; Cong, W. Red yeast rice preparations reduce mortality, major cardiovascular adverse events, and risk factors for metabolic syndrome: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 744928.
- Chang, C.-C.; Sun, M.-F.; Chou, Y.-C.; Yeh, C.-C.; Hu, C.-J.; Cherng, Y.-G.; Chen, T.-L.; Liao, C.-C. Decreased risk of stroke in people using red yeast rice prescriptions (LipoCol Forte®): A total population-based retrospective cohort study. Evid. Based Complement. Altern. Med. 2022, 2022, 8160425.
- Banach, M.; Katsiki, N.; Latkovskis, G.; Rizzo, M.; Pella, D.; Penson, P.E.; Reiner, Z.; Cicero, A.F.G. Postmarketing nutrivigilance safety profile: A line of dietary food supplements containing red yeast rice for dyslipidemia. Arch. Med. Sci. 2021, 17, 856–863.
- Cicero, A.F.G.; Kennedy, C.; Knežević, T.; Bove, M.; Georges, C.M.G.; Šatrauskienė, A.; Toth, P.P.; Fogacci, F. Efficacy and safety of armolipid plus®: An updated prisma compliant systematic review and meta-analysis of randomized controlled clinical trials. Nutrients 2021, 13, 638.
- Pirro, M.; Mannarino, M.R.; Bianconi, V.; Simental-Mendía, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88.
- Castano, G.; Mas, R.; Fernandez, J.; Lopez, E.; Illnait, J.; Fernandez, L.; Mesa, M. Effects of policosanol on borderline to mildly elevated serum total cholesterol levels: A prospective, double-blind, placebo-controlled, parallel-group, comparative study. Curr. Ther. Res. Clin. Exp. 2003, 64, 522–537.
- Cicero, A.F.; Morbini, M.; Parini, A.; Urso, R.; Rosticci, M.; Grandi, E.; Borghi, C. Effect of red yeast rice combined with antioxidants on lipid pattern, hs-CRP level, and endothelial function in moderately hypercholesterolemic subjects. Ther. Clin. Risk Manag. 2016, 12, 281–286.
- Cicero, A.F.; Morbini, M.; Rosticci, M.; D’Addato, S.; Grandi, E.; Borghi, C. Middle-term dietary supplementation with red yeast rice plus Coenzyme Q10 improves lipid pattern, endothelial reactivity and arterial stiffness in moderately hypercholesterolemic subjects. Ann. Nutr. Metab. 2016, 68, 213–219.
- Baumgartner, S.; Bruckert, E.; Gallo, A.; Plat, J. The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management. Atherosclerosis 2020, 311, 116–123.
- Murphy, A.; Palafox, B.; O’Donnell, O.; Stuckler, D.; Perel, P.; AlHabib, K.F.; Avezum, A.; Bai, X.; Chifamba, J.; Chow, C.K.; et al. Inequalities in the use of secondary prevention of cardiovascular disease by socioeconomic status: Evidence from the PURE observational study. Lancet Glob. Health 2018, 6, e292–e301.
- Yusuf, S.; Islam, S.; Chow, C.K.; Rangarajan, S.; Dagenais, G.; Diaz, R.; Gupta, R.; Kelishadi, R.; Iqbal, R.; Avezum, A.; et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE study): A prospective epidemiological survey. Lancet 2011, 378, 1231–1243.
- Toth, P.P.; Patti, A.M.; Giglio, R.V.; Nikolic, D.; Castellino, G.; Rizzo, M.; Banach, M. Management of statin intolerance in 2018: Still more questions than answers. Am. J. Cardiovasc. Drugs 2018, 18, 157–173.
- Lopes, J.; Santos, P. Determinants of non-adherence to the medications for dyslipidemia: A systematic review. Patient Prefer. Adherence 2021, 15, 1853–1871.
- Cicero, A.F.; Derosa, G.; Parini, A.; Baronio, C.; Borghi, C. Factors associated with 2-year persistence in fully non reimbursed lipid-lowering treatments. Atherosclerosis 2014, 235, 81–83.
More
